Abstract
Sodium currents were recorded from frog skeletal muscle by using fire-polished micropipettes to electrically isolate and voltage clamp a small patch of sarcolemma. Sodium current amplitude served as an assay for the number of functional sodium channels in the patch. With the pipette as a light guide, these channels were irradiated with ultraviolet (UV) light directed through a quartz fiber into the back end of the pipette. The UV light emerging from the pipette tip caused localized destruction of the sodium channels in the patch, reducing sodium current 3- to 5-fold during a 30-90 s irradiation. If sodium channels could diffuse laterally in the membrane, current from the patch should recover with time as fresh channels enter from neighboring areas. No such recovery was observed during observation for 1 hr after irradiation. Our results set an upper limit of 10(-12) cm2/s for the diffusion coefficient--1/1000th that of rhodopsin, a membrane protein in the cell membrane of retinal rods. It is suggested that sodium channels are anchored in the sarcolemma.
Full text
PDF
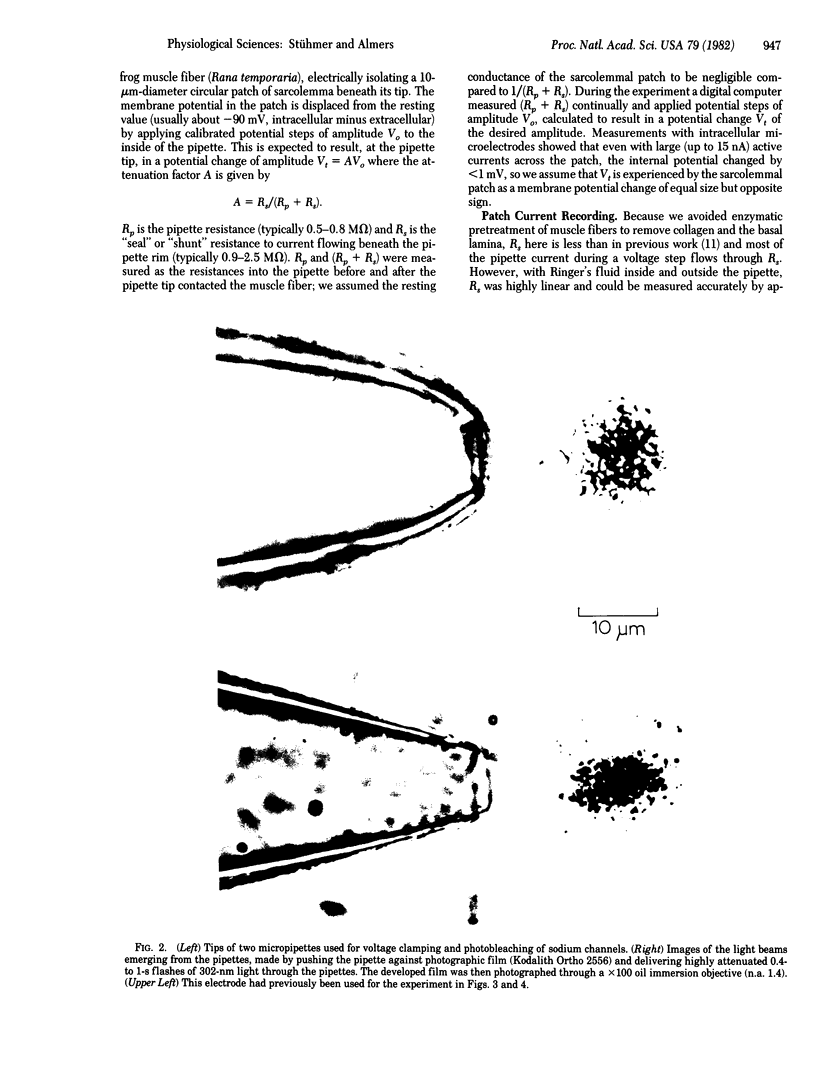
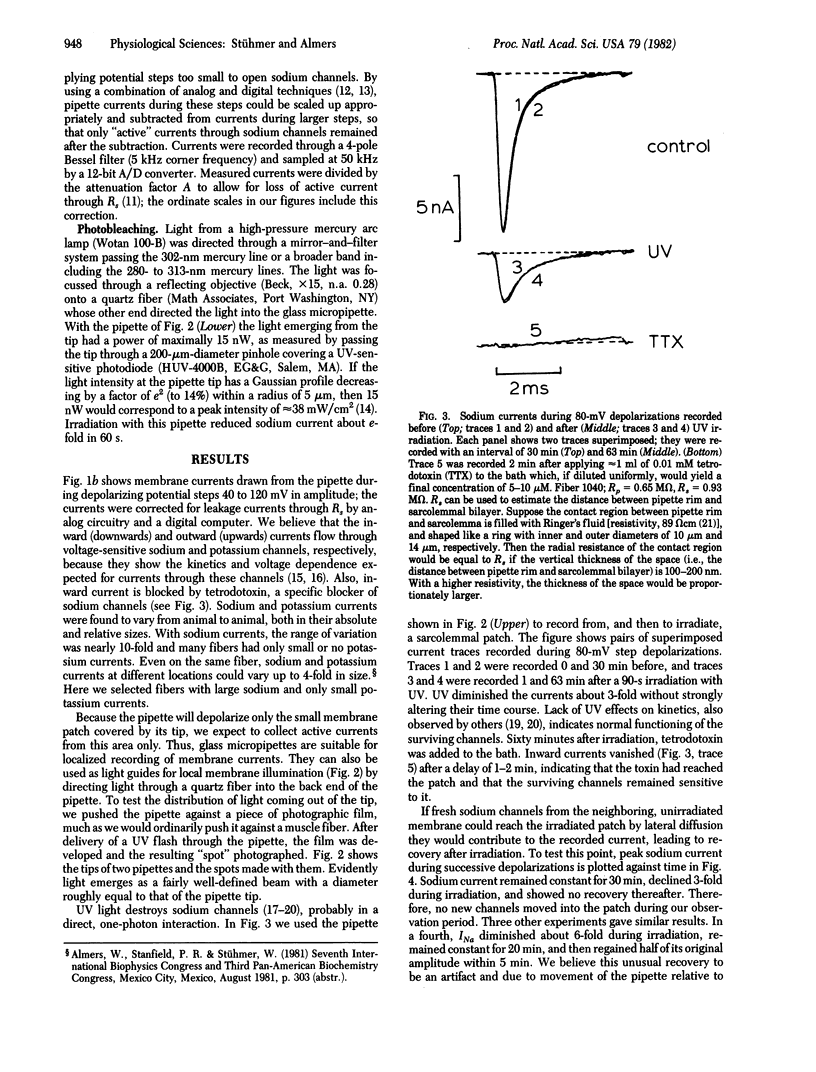

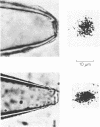
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTH J., von MURALT A., STAMPFLI R. The photochemical action of ultra-violet light on isolated single nerve fibres. Helv Physiol Pharmacol Acta. 1950;8(2):110–127. [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Evidence for the presence of potassium channels in the paranodal region of acutely demyelinated mammalian single nerve fibres. J Physiol. 1981;313:415–437. doi: 10.1113/jphysiol.1981.sp013674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona D. R., Mills J. W. Distribution of Na+-pump sites in transporting epithelia. Fed Proc. 1979 Feb;38(2):134–143. [PubMed] [Google Scholar]
- Dragsten P., Henkart P., Blumenthal R., Weinstein J., Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Wei T. Y. Diffusion rates of cell surface antigens of mouse-human heterokaryons. I. Analysis of the population. J Cell Biol. 1977 Nov;75(2 Pt 1):475–482. doi: 10.1083/jcb.75.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Dragsten P. R., Spector I. Immobilization of concanavalin A receptors during differentiation of neuroblastoma cells. Nature. 1981 Apr 30;290(5809):781–783. doi: 10.1038/290781a0. [DOI] [PubMed] [Google Scholar]
- Fox J. M. Selective blocking of the nodal sodium channels by ultraviolet radiation. I. Phenomenology of the radiation effect. Pflugers Arch. 1974;351(4):287–301. doi: 10.1007/BF00593315. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Coppersmith J., Catterall W. A. Size characteristics of the solubilized saxitoxin receptor of the voltage-sensitive sodium channel from rat brain. J Biol Chem. 1980 Nov 25;255(22):10572–10575. [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Venosa R. A., Shrager P., Horowicz P. Density and distribution of tetrodotoxin receptors in normal and detubulated frog sartorius muscle. J Gen Physiol. 1976 Apr;67(4):399–416. doi: 10.1085/jgp.67.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C. Elektrophysiologische Analyse der Wirkung von UV-Licht auf die isolierte markhaltige Nervenfaser. Pflugers Arch. 1956;262(3):244–255. doi: 10.1007/BF00369705. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P. Ultraviolet photoalteration of ion channels in voltage-clamped lobster giant axons. J Membr Biol. 1975;20(1-2):13–30. doi: 10.1007/BF01870625. [DOI] [PubMed] [Google Scholar]
- Poo M. M., Poo W. J., Lam J. W. Lateral electrophoresis and diffusion of Concanavalin A receptors in the membrane of embryonic muscle cell. J Cell Biol. 1978 Feb;76(2):483–501. doi: 10.1083/jcb.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver J. L., Stirling C. Ouabain binding to renal tubules of the rabbit. J Cell Biol. 1978 Feb;76(2):278–292. doi: 10.1083/jcb.76.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A. Impedance of a Small Electrically Isolated Area of the Muscle Cell Surface. J Gen Physiol. 1961 Jul 1;44(6):1073–1088. doi: 10.1085/jgp.44.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey C. L., Cone R. A., Edidin M. A. Lateral diffusion of rhodopsin in photoreceptor cells measured by fluorescence photobleaching and recovery. Biophys J. 1981 Feb;33(2):225–232. doi: 10.1016/S0006-3495(81)84883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]