Abstract
Neutrophil gelatinase-associated lipocalin (NGAL) is upregulated systemically and by renal tubular cells in response to inflammation and ischemia. Recent interests in NGAL have focused on its ability to predict worsening renal function. However, as an iron-regulatory glycoprotein, the relationship between systemic NGAL levels and indices of anemia has not been examined. In 130 patients with chronic systolic heart failure, we examined the relationship between plasma NGAL levels and indices of anemia independent of underlying renal function and systemic markers of inflammation and oxidant stress. Plasma NGAL levels were significantly elevated in patients with anemia versus without anemia (121 [98, 197] versus 72 [57, 98] ng/mL, p<0.001). Plasma NGAL levels were inversely correlated with indices of anemia including red blood cell count (r= −0.38, p<0.0001), hemoglobin (r= −0.41, p<0.0001), and red cell distribution width (r= 0.25, p=0.007), even in those with relatively preserved renal function (estimated glomerular filtration rate ≥60 ml/min/1.73m2; n=83, p<0.05 for all). Higher plasma NGAL levels were associated with presence of anemia independent of estimated glomerular filtration rate, plasma high-sensitive C-reactive protein, and myeloperoxidase levels (odds ratio 2.38, 95% confidence interval 1.02 – 6.20, p=0.045). Hence, systemic NGAL levels are independently associated with indices of anemia.
Neutrophil gelatinase-associated lipocalin (NGAL) is a small, 25 kDa glycoprotein member of the lipocalin superfamily. In response to diverse cellular stresses, including inflammation and ischemia, NGAL is rapidly released by a variety of cell types, including renal tubular cells, liver hepatocytes, and endothelial and smooth muscle cells 1–7. Although the clinical utility of NGAL recently has centered on its role as a marker of acute kidney injury and active chronic kidney disease, NGAL was originally identified as a bacteriostatic agent released from secondary granules of activated human neutrophils 8, 9, capable of interfering with bacterial iron uptake by sequestering bacterial ferric siderophores 10, 11. Subsequently, NGAL has been studied as an iron regulatory glycoprotein involved in iron trafficking and regulation of iron-dependent genes 11–15.
As part of the non-transferrin bound iron pool, NGAL circulates in both siderophore:iron-associated and siderophore:iron-free forms. Siderophore:iron-associated NGAL delivers iron into cells following 24p3R or megalin receptor-mediated uptake and trafficking into acidic endosomes, where iron is released and subsequently accumulates in the cytoplasm. Delivery of iron is believed to regulate iron-responsive genes involved in promotion of cellular survival, proliferation, and differentiation, including enhanced expression of heme oxygenase-1, ribonuclease reductase, and many cyclin genes and stress-related proteins 13. In parallel, siderophore:iron-free NGAL is proposed to scavenge excess free intracellular and extracellular iron, limiting labile iron-mediated cytotoxicity 13, 16.
Such pathways point toward an acute compensatory, protective role for NGAL in response to diverse cellular stresses, including inflammatory and oxidative stress 1–4. However, recent reports have implicated NGAL upregulation as a mechanism contributing to anemia in the setting of chronic inflammation. In experimental models, systemic and medullary NGAL has been demonstrated to induce inhibition of erythropoiesis through induction of apoptosis and arrest of differentiation of erythroid progenitor cells 17–21. In addition, NGAL has been found to redirect systemic iron to renal proximal tubule cells in response to renal tubular cell injury 13, 22. In the systemic inflammatory states of both chronic and advanced heart failure, iron deficiency has been described as a common cause of anemia 23, 24.
In the chronic systolic heart failure setting, we test the hypothesis that elevated systemic NGAL levels are associated with systemic indices of anemia after adjusting for underlying renal function and systemic markers of inflammation and oxidant stress.
METHODS
Study Population
We examined the hematologic and inflammatory determinants of systemic NGAL levels in stable, ambulatory patients with chronic systolic heart failure in a single-center, prospective study cohort well-characterized with comprehensive echocardiographic evaluation. All subjects provided informed consent as approved by the Cleveland Clinic Institutional Review Board. Inclusion criteria were as follows: 18 to 75 years of age with a diagnosis of heart failure for at least 3 months, a left ventricular ejection fraction ≤35% at the time of enrollment, New York Heart Association functional class I–IV symptoms. Exclusion criteria included a history of mitral stenosis or mitral valve surgery, severe mitral regurgitation (>3+), or severe aortic stenosis (peak velocity >4 m/s) or aortic regurgitation 25.
Echocardiography
All subjects underwent comprehensive echocardiographic evaluation of cardiac structure as well as systolic and diastolic performance by an experienced sonographer. Comprehensive transthoracic echocardiography was performed using commercially available HDI 5000 (Phillips Medical Systems, N.A., Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines using previously described techniques25, 26. Classification of diastolic stage was determined according to the following modifications of the recommendations set forth by the American Society of Echocardiography 27. LV mass was calculated according to previously published recommendations 28. All ventricular volume and mass measurements were indexed to body surface area. Measurements were averaged over three cycles.
Laboratory Testing
All samples were collected into ethylenediaminetetraacetic acid-plasma collecting tubes on ice simultaneously at the time of echocardiographic evaluation, processed and immediately frozen in aliquots at −80°C until analyzed. All laboratory analyses were performed with investigators blinded to cardio-renal indices and clinical outcomes data. Plasma NGAL levels were measured by a research enzyme-linked immunosorbent assay (Cat. No. KIT 036, BioPorto Diagnostics, Gentofte Denmark). The minimum detection limit of the assay was 20 ng/mL. Intra-assay and inter-assay coefficients of variation were <5% at 65 ng/mL. Plasma high-sensitivity C-reactive protein (hsCRP) levels were determined by the particle enhanced immunonephelometry assay (Dade Behring, Inc., Deerfield IL). The minimum detection limit of the assay was 0.10 ng/mL. Intra-assay and inter-assay CVs were <5%. Plasma myeloperoxidase (MPO) levels were determined by an enzyme-linked immunosorbent assay (CardioMPO II test, Cleveland Heart Labs, Cleveland OH). The minimum detection limit of the assay was 30 pM. Intra-assay and inter-assay CVs were <5%. Plasma amino-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were assayed using a commercially available immunoassay based on electrochemiluminescence technology (Roche Elecsys® NT-proBNP assay, Roche Diagnostics, Indianapolis IN). The minimum detection limit of the assay was 5 pg/mL. Intra-assay and inter-assay CVs were <3%. Complete blood count with differential analysis was performed at the Cleveland Clinic Reference Laboratory utilizing a Sysmex XE-2100 automated hematology analyzer and leukocyte differential counter (Sysmex America, Inc., Mundelein IL). Indices of anemia included hemoglobin, hematocrit, and red blood cell distribution width. Anemia was defined as hemoglobin <12g/dL for men and <11g/dL for women. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine levels using the standard 4-variable Modification of Diet in Renal Disease equation 29.
Statistical Analyses
Continuous variables were summarized as mean ± standard deviation if normally distributed, and as median and interquartile range if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Spearman’s rank correlation method was used as a nonparametric measure of association for correlations between NGAL levels and clinical and echocardiographic indices. The Wilcoxon rank-sum or Kruskal-Wallis tests were used to compare differences in NGAL levels across categorical variables. Multivariable logistic regression analysis was performed and evaluated by the Likelihood Ratio test to calculate the odds ratios of presence of anemia associated with elevated NGAL levels following adjustment for indices of underlying renal function, inflammation, and oxidant stress. Multivariable linear regression analysis using the Standard Least Squares fitting method was performed to determine the association between NGAL and continuous hemoglobin levels following adjustment for indices of renal function, inflammation and oxidant stress. Natural logarithmic transformations were applied to non-normally distributed variables. All p-values reported are from two-sided tests and a p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP 9.0 (SAS Institute, Cary, NC).
RESULTS
Table 1 illustrates the baseline characteristics of our chronic systolic heart failure cohort. Mean and median plasma NGAL levels were 93 ± 63 ng/mL and 77 (inter-quartile range 58–108) ng/mL, respectively. Higher systemic NGAL levels were associated with advanced age (r= 0.18, p=0.035), higher plasma NT-proBNP (r= 0.24, p=0.008), hsCRP (r= 0.32, p<0.001), MPO (r= 0.30, p<0.001), absolute neutrophil count (r= 0.42, p<0.0001), and lower eGFR (r= −0.53, p<0.0001), but did not differ according to gender, ethnicity, history of hypertension or diabetes mellitus, or any medication use (p>0.10 for all).
Table 1.
Baseline Subject Characteristics (n=130).
| Variable | Value |
|---|---|
| Demographics: | |
| Age (years) | 57 ± 13 |
| Male gender, n (%) | 98 (75%) |
| Body mass index (kg/m2) | 28 ± 5 |
| African American, n (%) | 22 (17%) |
| Caucasian, n (%) | 108 (83%) |
| Heart failure history: | |
| Ischemic etiology, n (%) | 57 (44%) |
| NYHA class III or IV, n (%) | 43 (33%) |
| Co-morbidities: | |
| Hypertension, n (%) | 70 (55%) |
| Diabetes mellitus, n (%) | 37 (29%) |
| Echocardiographic indices: | |
| LV mass index (g/m2) | 157 ± 46 |
| LV end-diastolic volume index (mL/m2) | 110 ± 35 |
| LV ejection fraction (%) | 26 ± 6 |
| Diastolic stage III, n (%) | 45 (35%) |
| Medications: | |
| Angiotensin converting enzyme inhibitors or angiotensin receptor blockers, n (%) | 119 (94%) |
| Beta-blockers, n (%) | 83 (65%) |
| Spironolactone, n (%) | 32 (27%) |
| Loop diuretics, n (%) | 98 (77%) |
| Digoxin, n (%) | 74 (62%) |
| Anemia Indices: | |
| Red blood cell (M/uL) | 4.5 ± 0.6 |
| Hemoglobin (g/dL) | 13.7 ± 1.7 |
| Hematocrit (%) | 40.7 ± 4.8 |
| Red cell distribution width (%) | 14.7 ± 2.3 |
| Laboratory data: | |
| eGFR (mL/min/1.73m2) | 72 ± 25 |
| NT-proBNP (pg/mL) | 1241 [540–3292] |
| MPO (pM) | 307 [256–430] |
| hsCRP (ng/mL) | 3.3 [1.5–7.3] |
| NGAL (ng/mL) | 77 [58–108] |
Abbreviation: NYHA, New York Heart Association; LV, left ventricular; eGFR, estimated glomerular filtration rate; NT-proBNP, aminoterminal pro-B-type natriuretic peptide; MPO, myeloperoxidase; hsCRP, high-sensitivity C-reactive protein; NGAL, neutrophil gelatinase-associated lipocalin.
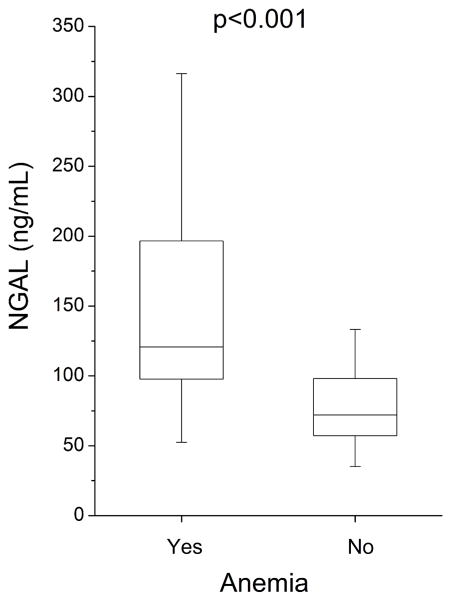
The prevalence of anemia in our chronic systolic heart failure cohort was 12.6% (7.4% in women, 14.1% in men). Plasma NGAL levels were significantly elevated in patients with anemia versus without anemia (121 [inter-quartile range 98–197] versus 72 [inter-quartile range 57–98] ng/mL, p<0.001; Figure 1). Plasma NGAL levels were inversely correlated with indices of anemia in the entire cohort as well as in those with relatively preserved renal function (estimated glomerular filtration rate ≥60 ml/min/1.73m2, Table 2).
Figure 1.
Plasma NGAL Levels Stratified According to Presence of Anemia for Chronic Systolic Heart Failure Subjects (n=130). Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin.
Table 2.
Univariate Correlation between Plasma Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Anemia Indices for the Overall Chronic Systolic Heart Failure cohort (n=130) and Subjects with Relatively Preserved Renal Function (estimated glomerular filtration rate ≥60 ml/min/1.73m2; n=83).
| Overall (n=130) | Estimated glomerular filtration rate ≥60 ml/min/1.73 m2 (n=83) | |||
|---|---|---|---|---|
| Variable | Spearman’s r | p-value | Spearman’s r | p-value |
| Red blood cell (M/uL) | −0.38 | <0.0001 | −0.16 | 0.152 |
| Hemoglobin (g/dL) | −0.41 | <0.0001 | −0.34 | 0.002 |
| Hematocrit (%) | −0.37 | <0.0001 | −0.26 | 0.022 |
| Red cell distribution width (%) | 0.25 | 0.007 | 0.24 | 0.030 |
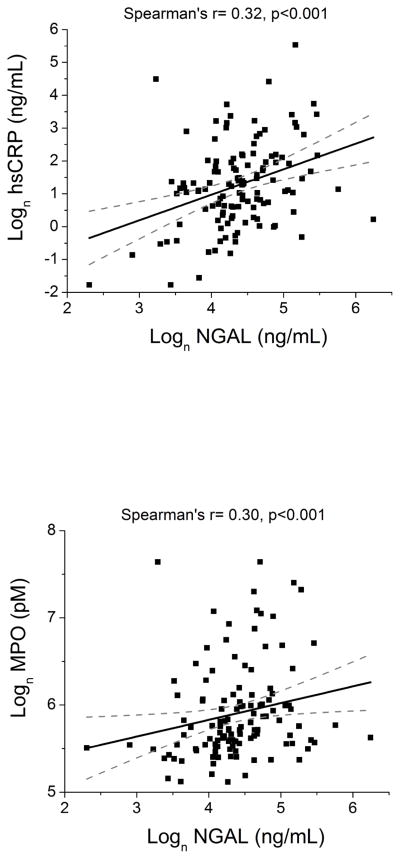
Higher plasma NGAL levels were directly associated with indices of systemic inflammation, leukocyte activation, and oxidant stress. Specifically, higher plasma NGAL levels modestly correlated with elevated plasma hsCRP (r= 0.32, p<0.001) and plasma MPO levels (r= 0.30, p<0.001) (Figure 2). Plasma NGAL levels were also directly correlated with absolute neutrophil count (r= 0.42, p<0.0001). These relationships were preserved in patients with relatively preserved renal function (p<0.01 for all).
Figure 2.
Correlations between Plasma NGAL and Indices of Systemic Inflammation, Leukocyte Activation and Oxidant Stress for Chronic Systolic Heart Failure Subjects (n=130). Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; hsCRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase.
In multivariable logistic regression analysis, higher plasma NGAL levels were associated with presence of anemia even after adjusting for eGFR, and hsCRP, and MPO levels (odds ratio: 2.38, 95% confidence interval 1.02 – 6.20, p=0.045, Table 3). In multivariable linear regression analysis, higher plasma NGAL levels were associated with lower hemoglobin levels following adjustment for age, gender, eGFR, hsCRP, MPO, absolute neutrophil count, and NT-proBNP levels (Std β = −0.35, p=0.012, Table 4).
Table 3.
Multivariable Logistic Regression Analysis for Presence of Anemia within the Overall Chronic Systolic Heart Failure Cohort (n=130).
| Neutrophil Gelatinase-Associated Lipocalin* | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Unadjusted | 3.33 (1.78 – 6.97) | <0.0001 |
| Adjusted for Age, Gender, eGFR | 2.24 (1.05 – 5.32) | 0.037 |
| Adjusted for Age, Gender, hsCRP | 3.60 (1.78 – 8.27) | <0.001 |
| Adjusted for Age, Gender, MPO | 3.80 (1.90 – 8.79) | <0.0001 |
| Adjusted for Age, Gender, NT-proBNP | 2.19 (1.09 – 5.07) | 0.026 |
| Adjusted for Age, Gender, hsCRP, MPO and NT-proBNP | 2.92 (1.29 – 7.79) | 0.008 |
| Adjusted for Age, Gender, eGFR, hsCRP, and MPO | 2.67 (1.11 – 7.47) | 0.028 |
Abbreviations: eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase; NT-proBNP, aminoterminal pro-B-type natriuretic peptide.
Odds ratio per 1 standard deviation increment (1 standard deviation of Ln NGAL = 0.58 ng/mL)
Table 4.
Multivariable Linear Regression Analysis for the Relationship between Hemoglobin Levels and Clinical Predictors within the Overall Chronic Systolic Heart Failure Cohort (n=130).
| Variable | Standard β | p-value |
|---|---|---|
| NGAL (ng/mL) | −0.35 | 0.012 |
| Age (years) | −0.17 | 0.133 |
| Gender (male) | 0.13 | 0.243 |
| eGFR (mL/min/1.73m2) | 0.22 | 0.093 |
| hsCRP (ng/mL) | −0.12 | 0.300 |
| MPO (pM) | −0.06 | 0.637 |
| Absolute neutrophil count (K/μL) | 0.22 | 0.074 |
| NT-proBNP (pg/mL) | −0.16 | 0.217 |
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase; NT-proBNP, aminoterminal pro-B-type natriuretic peptide.
DISCUSSION
The mechanistic links between heart failure, renal insufficiency and anemia remain poorly defined. Neutrophil gelatinase-associated lipocalin (NGAL) recently has emerged as an important factor in iron homeostasis and erythrocyte growth regulation that may contribute to anemia when chronically elevated. In chronic systolic heart failure patients, we report for the first time an association between elevated systemic NGAL levels and anemia indices that remains following adjustment for underlying renal function and systemic inflammatory markers.
NGAL was originally identified as a product of activated human neutrophils, capable of acting as a bacteriostatic agent by sequestering bacterial ferric siderophores and interfering with bacterial iron uptake 9, 11, and regulated by the redox- and pro-inflammatory cytokine-sensitive transcription factor, nuclear factor-κB (NF-κB) 1, 2, 5. Therefore, it is consistent to find within our chronic systolic heart failure cohort that systemic NGAL levels are associated with markers of systemic inflammation, including high-sensitivity C-reactive protein, myeloperoxidase, and absolute neutrophil count. These relationships remained present in patients with relatively preserved renal function, which may imply the likely association between systemic NGAL and anemia related to heart failure.
In this study, we utilized surrogate measures of iron deficiency, such as low mean corpuscular hemoglobin concentration (representing a relative deficiency of hemoglobin incorporation into the erythrocytes) and red cell distribution width (representing enhanced erythropoiesis) 30. In the setting of both chronic and advanced heart failure, iron deficiency has been described as a common cause of anemia 23, 24. In an effort to shuttle systemic iron to renal tubular cells to support cell proliferation and prevent apoptosis, elevated systemic NGAL levels may transiently contribute to iron deficiency anemia. In animal models, NGAL has been demonstrated to be able to strip iron from transferrin and redirect iron from the liver and spleen to the proximal tubule of the kidney 13, 22. With recent interests in intravenous iron therapy as potential treatment modality for heart failure31, further investigations regarding the relative contributions of NGAL in the development of relative iron deficiency anemia in heart failure are warranted.
Based on our understanding of the mechanistic role of NGAL, it is also conceivable that elevated systemic NGAL levels may also contribute to anemia as a direct inhibitor of erythrocyte maturation 17. Local synthesis of NGAL by immature medullary erythroid stem cell progenitors constitutes an autocrine regulatory pathway promoted by interleukin-1 that induces inhibition of erythropoiesis through induction of apoptosis and arrest of differentiation 18, 19. In addition, in studies examining exogenous administration of recombinant NGAL in mice, systemic NGAL has been shown to traffic to receptors on the surface of marrow cells where it acts to inhibit the recovery of suppressed hematopoietic function 19–21. In this fashion, chronic elevation of systemic NGAL levels, as a compensatory defense against systemic inflammatory and oxidative stress, may deleteriously act to suppress medullary hematopoietic function. Along with the ability of NGAL to enhance and prolong the proteolytic activity of matrix metalloproteinase-9, these findings appear to imply a natural history in which NGAL levels are beneficial when elevated acutely, but potentially deleterious when elevated chronically. Recent reports have even suggested that chronic elevations of NGAL may not simply be a marker of, but also a contributor to, progressive renal dysfunction in chronic kidney disease through aberrant tubular cell proliferation32. In addition, although acutely beneficial in ischemia-reperfusion models, NGAL contributes to worsening of antibody-induced nephritis via promotion of inflammation and apoptosis33. NGAL injection was found to nephritis and blockade has been hypothesized as a novel therapeutic approach33. The effects of acutely and chronically elevated systemic and local NGAL levels need to be further investigated.
LIMITATIONS
There are several limitations of our current analysis. We did not collect serial systemic NGAL levels or serial indices of anemia to assess the relationship between baseline and changes in levels of systemic NGAL with changes in indices of anemia. We also did not have measures of functional iron deficiency (such as serum iron, total iron binding capacity, or ferritin) to confirm the presence of underlying functional iron deficiency, despite the lack of a gold standard for such condition. The cross-sectional nature of our study limits any direct demonstration of a cause-and-effect for different mechanistic pathways that may underlie the link between NGAL and anemia.
Despite these potential limitations, we have demonstrated the association between NGAL and anemia to exist after adjustment for underlying renal function and systemic markers of inflammation and oxidative stress. While additional studies need to performed to determine whether NGAL plays a causal role in the pathogenesis of anemia in heart failure, it is interesting to hypothesize that systemic and local NGAL upregulation may contribute to anemia through iron sequestration and erythrocyte growth regulation. NGAL blockade consequently may offer a novel therapeutic approach. In addition, systemic NGAL levels may help identify patient subpopulations who will benefit most from intravenous iron therapy. Further studies are warranted to better understand the role of NGAL in predisposing to anemia in the setting of disease progression of chronic heart failure.
CONCLUSION
Systemic NGAL levels are associated with indices of anemia independent of underlying renal function and systemic markers of inflammation and oxidant stress in chronic systolic heart failure.
Acknowledgments
SUPPORT
The main ADEPT study was supported in part by grant funding from American Society of Echocardiography, GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc. Dr. Tang is funded in part by NIH grant 1R01HL103931-02.
Footnotes
STATEMENT OF FINANCIAL DISCLOSURE
Dr Tang serves as a consultant for Medtronic, Inc, and St Jude Medical and has received research grant support from Abbott Laboratories. All other authors have no relationship to disclose.
References
- 1.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000 Oct 18;1482(1–2):298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007 Feb;18(2):407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 3.Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J Immunol. 2003 Dec 15;171(12):6630–6639. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 4.Hemdahl AL, Gabrielsen A, Zhu C, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006 Jan;26(1):136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 5.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997 Oct 1;45(1):17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 6.Cowland JB, Muta T, Borregaard N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol. 2006 May 1;176(9):5559–5566. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- 7.Bu DX, Hemdahl AL, Gabrielsen A, et al. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am J Pathol. 2006 Dec;169(6):2245–2253. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu SY, Carlson M, Engstrom A, Garcia R, Peterson CG, Venge P. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand J Clin Lab Invest. 1994 Aug;54(5):365–376. doi: 10.3109/00365519409088436. [DOI] [PubMed] [Google Scholar]
- 9.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993 May 15;268(14):10425–10432. [PubMed] [Google Scholar]
- 10.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002 Nov;10(5):1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 11.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004 Dec 16;432(7019):917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007 May;71(10):967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005 Mar;115(3):610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002 Nov;10(5):1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 15.Barasch J, Mori K. Cell biology: iron thievery. Nature. 2004 Dec 16;432(7019):811–813. doi: 10.1038/432811a. [DOI] [PubMed] [Google Scholar]
- 16.Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. May 11;55(19):2024–2033. doi: 10.1016/j.jacc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Bolignano D, Coppolino G, Lombardi L, Buemi M. NGAL: a new missing link between inflammation and uremic anemia? Ren Fail. 2009;31(7):622–623. doi: 10.1080/08860220903003438. [DOI] [PubMed] [Google Scholar]
- 18.Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001 Aug 3;293(5531):829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 19.Bolignano D, Coppolino G, Donato V, Lacquaniti A, Bono C, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL): a new piece of the anemia puzzle? Med Sci Monit. 2010 Jun;16(6):RA131–135. [PubMed] [Google Scholar]
- 20.Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2008 May;215(2):526–537. doi: 10.1002/jcp.21334. [DOI] [PubMed] [Google Scholar]
- 21.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. Faseb J. 2005 Nov;19(13):1881–1883. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- 22.Sephton RG, Hodgson GS, De Abrew S, Harris AW. Ga-67 and Fe-59 distributions in mice. J Nucl Med. 1978 Aug;19(8):930–935. [PubMed] [Google Scholar]
- 23.Opasich C, Cazzola M, Scelsi L, et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J. 2005 Nov;26(21):2232–2237. doi: 10.1093/eurheartj/ehi388. [DOI] [PubMed] [Google Scholar]
- 24.Nanas JN, Matsouka C, Karageorgopoulos D, et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006 Dec 19;48(12):2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Troughton RW, Prior DL, Pereira JJ, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004 Feb 4;43(3):416–422. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 26.Troughton RW, Prior DL, Frampton CM, et al. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005 Jul 15;96(2):257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009 Feb;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 30.Urrechaga E. The new mature red cell parameter, low haemoglobin density of the Beckman-Coulter LH750: clinical utility in the diagnosis of iron deficiency. Int J Lab Hematol. Feb;32(1 Pt 1):e144–150. doi: 10.1111/j.1751-553X.2008.01127.x. [DOI] [PubMed] [Google Scholar]
- 31.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009 Dec 17;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 32.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010 Oct 1; doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawar RD, Pitashny M, Gindea S, et al. Neutrophil gelatinase associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis. Arthritis Rheum. 2011 Nov 14; doi: 10.1002/art.33485. [DOI] [PMC free article] [PubMed] [Google Scholar]