Abstract
The molecular chaperone HSP90, in concert with the co-chaperone CDC37, facilitates the maturation and modulates the activity of a variety of protein kinases. In this article, Gaude and colleagues described the dual activities of the HSP90–CDC37 chaperone machinery in maintaining the stability while inhibiting the activity of LKB1 kinase. LKB1 in complex with HSP90–CDC37 has a longer half-life but is incapable of autophosphorylation, and its kinase activity is increased upon HSP90 inhibition. Dissociation of HSP90 from LKB1 results in its interaction with HSP/HSC70. HSP/HSC70 recruits the ubiquitin ligase CHIP, which ubiquitinates LKB1, leading to its proteasome-mediated degradation. These data emphasize the versatile roles of molecular chaperones associated with LKB1 and warrant future studies to characterize the clinical relevance of these observations.
Keywords: CDC37, CHIP, HSP/HSC70, HSP90, LKB1
Summary of methods & results
It was previously shown that HSP90 associates with and stabilizes LKB1 protein, and that the HSP90-specific inhibitor geldanamycin (GA) induces proteasome-mediated LKB1 degradation [1,2]. It is also known that CDC37 interacts with LKB1, but the implication of this interaction was not clear [1]. To explore the significance of the LKB1/CDC37 association, Gaude et al. treated HeLa cells with the CDC37 inhibitor celastrol [3,4] and found that exogenous LKB1 protein expression was markedly decreased within 2 h. Similar effects were observed with the endogenous LKB1 protein in human kidney epithelial BOSC cells. This stabilizing effect of CDC37 on LKB1 was confirmed by knocking down CDC37 with siRNA. The authors also showed that HSP90 knockdown resulted in similar decreases in LKB1 protein levels, and that the combination of CDC37 and HSP90 knockdown did not lead to a further decrease in LKB1 protein levels compared with individual knockdowns.
It is well recognized that HSP90 maintains the stability of its client kinases by excluding the association of the chaperone HSP/HSC70 and CHIP, a chaperone-associated E3 ubiquitin ligase [5]. Gaude et al. confirmed this model for LKB1. Thus, HSP90 inhibition by GA reduced LKB1 interaction with HSP90, while simultaneously increasing its association with HSP/HSC70 and CHIP. The association of CHIP is most likely mediated by HSP/HSC70, as the K30A CHIP mutant, which has lost interaction with HSP/HSC70, failed to bind to LKB1. Interestingly, the authors found that the interaction sites on LKB1 for HSP90 and HSP/HSC70 are different. While the N-terminal region (1–88 amino acids) and a region in the middle of the kinase domain (146–186 amino acids) are required for HSP90 association, the C-terminal region (318–433 amino acids) of LKB1 is required for HSP/HSC70 association.
Two human LKB1 isoforms encoded by alternative splicing were reported previously [6,7]. The short isoform LKB1-S differs from the longer one (LKB1-L) in the C-terminus, with the last 63 residues replaced by a unique 39-residue sequence. Importantly, both LKB1-S and LKB1-L retain the Hsp70-binding domain. Gaude’s group showed that both LKB1-S and LKB1-L interact with HSP90–CDC37 and are sensitive to celastrol-induced, as well as GA-induced, degradation [1,2]. This is in contrast to LKB1-ΔN, a newly identified isoform whose translation is initiated by using an alternative downstream start codon and that, therefore, lacks the N-terminal 124 residues of LKB1-L. LKB1-ΔN loses association with HSP90–CDC37 and is refractory to celastrol-induced degradation. When cells expressing these three different LKB1 isoforms were treated with celastrol, LKB1-S and LKB1-L showed a time-dependent decrease in protein expression, while the level of LKB-ΔN expression stayed largely unchanged. Another piece of evidence supporting the notion that interaction of HSP90–CDC37 maintains the stability of LKB1 in the cell is that the half-life of LKB1-ΔN is less than half that of LKB1-S and LKB-L.
After characterizing the role of the HSP90–CDC37 chaperone machinery in maintaining the stability of LKB1, Gaude’s group demonstrated how this complex regulates LKB1 kinase activity. The intrinsic kinase activity of LKB1 is rather low. Association with the pseudokinase STRAD dramatically enhances LKB1 kinase activity and the association is stabilized by a third protein, MO25 [8]. Low intrinsic kinase activity probably results from the fact that LKB1 lacks a phosphorylation site in its kinase domain activation loop, which is generally required for inducing and stabilizing the active conformation of a kinase [9]. Interaction with STRAD allosterically modifies LKB1, enabling its activation loop to adopt the active conformation, which is stabilized by MO25 [10]. Indeed, Gaude’s group showed that LKB1 in complex with STRAD was capable of autophosphorylation, and this autophosphorylation was enhanced in the presence of MO25α. By contrast, LKB1 in complex with HSP90–CDC37 was incapable of autophosphorylation, suggesting that, while the HSP90–CDC37 interaction may stabilize LKB1, this complex also inhibits LKB1 kinase activity. Another line of evidence supporting an inhibitory impact of the HSP90–CDC37 chaperone machinery on LKB1 kinase activity is that LKB1 proteins immunoprecipitated from GA-treated cells showed higher autophosphorylation than those immunoprecipitated from untreated cells.
Discussion
The widely expressed serine/threonine kinase LKB1 is a master kinase in the cell, regulating at least 14 downstream proteins that, in turn, modulate the cell cycle, apoptosis, autophagy, energy metabolism, and cell migration and polarity [8]. LKB1 mutation is causally linked to Peutz–Jeghers syndrome, a rare cancer-susceptibility disorder characterized by mucocutaneous melanin pigmentation and early development of intestinal hamartomatous polyposis [11]. Thus, a detailed understanding of the molecular regulation of LKB1 stability and activity is of potential clinical significance. In this study, Gaude’s group showed that both the protein stability and kinase activity of LKB1 are regulated by the HSP90–CDC37 chaperone complex. LKB1 stability requires normal function of both HSP90 and CDC37. Drug-mediated functional inhibition or siRNA-mediated knockdown of either HSP90 or CDC37 resulted in decreased LKB1 expression. It is worth noting that knockdown of HSP90 in addition to CDC37 did not further reduce LKB1 protein expression, suggesting that the two chaperones function together to regulate LKB1 stability.
An inhibitory role for HSP90 has been reported previously for other kinases, including PKR, c-SRC and ERBB2; the current data add support to the concept that HSP90 binding serves to suppress client kinase activity, and suggest an inverse relationship between kinase activity and protein stability [12–14]. Interestingly, Gaude’s group showed that the degree of LKB1 activation caused by GA-mediated HSP90 inhibition depends on the duration of GA treatment. Time-course experiments indicated that LKB1 activity continuously increased with longer GA treatment, reaching a peak at 24 h and subsequently decreasing to basal levels thereafter. These data raise the question of whether the activating effect of GA on LKB1 is a direct consequence of promoting its dissociation from HSP90–CDC37, or whether it is due to as-yet unknown indirect effects on LKB1 post-translational modifications or on interaction with/stabilization of the STRAD–MO25 complex.
It is known that nascent LKB1 protein requires the HSP90–CDC37 chaperone complex for its stability [1], and Gaude’s group show in their paper that the mature LKB1 protein has a similar chaperone requirement. They also demonstrate that LKB1, STRAD and HSP90 can coexist in the same complex. How do the LKB1 interaction partners cooperate to regulate LKB1 stability and kinase activity in a productive manner? The results of Gaude et al.’s study and previous data suggest a scenario in which the HSP90–CDC37 chaperone complex assists the maturation and ensures the stability (and inactivity) of LKB1. When necessary, and perhaps as a result of environmental cues, the molecular complex recruits STRAD in order to activate LKB1 kinase activity. Full activation of LKB1 requires HSP90 dissociation, which conveniently allows for subsequent association of the HSP/HSC70–CHIP complex. CHIP-mediated degradation provides a mechanism to turn off LKB1 signaling after the kinase has fulfilled its function (Figure 1). Along these lines, it would be interesting to determine the impact of LKB1 binding to STRAD–MO25 on the ability of LKB1 to interact with HSP/HSC70–CHIP.
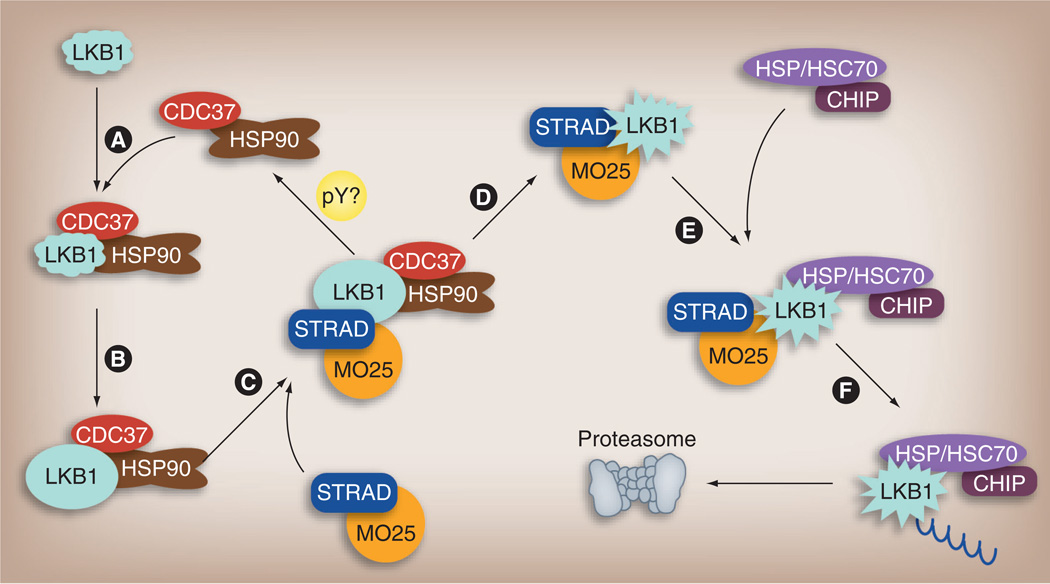
Figure 1. Activity and protein stability of the LKB1 kinase are regulated by molecular chaperones.
The HSP90–CDC37 chaperone complex binds to nascent LKB1 protein (A) and assists its correct folding (B). Folded LKB1 remains bound to this chaperone complex in a stable but inactive state. Association with the STRAD–MO25 heterodimer leads to LKB1 activation (C), but also to dissociation from the HSP90–CDC37 complex (possibly promoted by tyrosine phosphorylation) (D). LKB1 that is not in complex with HSP90–CDC37 is available to bind to the chaperone HSP/HSC70, which recruits the ubiquitin ligase CHIP (E). CHIP ubiquitinates LKB1 (F), leading to its proteasome-mediated degradation. Thus, LKB1 activation and degradation are finely balanced events regulated by dynamic chaperone interactions.
Conclusion
In summary, Gaude et al. have described a very interesting model that highlights the opposing effects of the HSP90–CDC37 chaperone machinery on LKB1 stability and activity. Their discovery furthers our understanding of how this kinase is regulated and is also clinically relevant given the intense ongoing clinical evaluation of HSP90 inhibitors as cancer therapeutics.
Future perspective
While Gaude et al.’s study elucidated the regulatory roles of molecular chaperones on LKB1, their work also raises several interesting questions. Would an LKB1 mutant lacking the HSP/HSC70-association region be resistant to GA-induced degradation? Would an LKB1 mutant lacking either of the HSP90-association regions be inherently unstable (as shown by the authors for LKB1-ΔN)? If so, could the instability be remedied by knockdown of CHIP?
Furthermore, how do the various chaperone components dissociate from LKB1? Tyrosine phosphorylation of HSP90 and CDC37 may be involved in this process. We recently reported that both HSP90 and CDC37 undergo regulated and dynamic tyrosine phosphorylation events that productively drive the HSP90 chaperone cycle and promote the orderly association/dissociation of HSP90, CDC37 and client kinases [15]. The involvement of HSP90 and CDC37 tyrosine phosphorylation in LKB1 activation is worthy of further investigation.
Executive summary.
-
▪
This study characterized the roles of molecular chaperones in regulating the stability and activity of the LKB1 kinase in in vitro experiments.
-
▪
HSP90, as well as its co-chaperone CDC37, stabilize the LKB1 protein in the cell.
-
▪
The chaperone inhibitors geldanamycin and celastrol induce LKB1 degradation; siRNA-mediated knockdown of HSP90 or CDC37 results in decreased LKB1 protein expression.
-
▪
The half-life of LKB1-ΔN, which lacks HSP90–CDC37 association, is less than that of LKB1 proteins that are able to associate with the chaperone complex.
-
▪
The HSP90–CDC37 complex represses LKB1 kinase activity. LKB1 in complex with HSP90–CDC37 is incapable of autophosphorylation, while LKB1 isolated from cells treated with geldanamycin showed higher kinase activity.
-
▪
LKB1 degradation is linked to a remodeling of the associated chaperone complex that is characterized by decreased association of HSP90 and increased association of HSP/HSC70.
-
▪
HSP90 and HSP/HSC70 bind to different regions of LKB1 protein; while the former binds to LKB1 N-terminal and kinase domains, the latter binds to the C-terminal region of LKB1.
-
▪
HSP/HSC70 mediates LKB1 association with CHIP, which ubiquitinates LKB1 protein, leading to its degradation in the proteasome.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR. Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem. J. 2003;370(Pt 3):849–857. doi: 10.1042/BJ20021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nony P, Gaude H, Rossel M, Fournier L, Rouault JP, Billaud M. Stability of the Peutz–Jeghers syndrome kinase LKB1 requires its binding to the molecular chaperones Hsp90/Cdc37. Oncogene. 2003;22(57):9165–9175. doi: 10.1038/sj.onc.1207179. [DOI] [PubMed] [Google Scholar]
- 3.Gaude H, Aznar N, Delay A, et al. Molecular chaperone complexes with antagonizing activities regulate stability and activity of the tumor suppressor LKB1. Oncogene. 2012;31(12):1582–1591. doi: 10.1038/onc.2011.342. [DOI] [PubMed] [Google Scholar]
- 4.Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of Thunder God Vine. Biochem. Biophys. Res. Commun. 2010;394(3):439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl Acad. Sci. USA. 2002;99(20):12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towler MC, Fogarty S, Hawley SA, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem. J. 2008;416(1):1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 7.Denison FC, Hiscock NJ, Carling D, Woods A. Characterization of an alternative splice variant of LKB1. J. Biol. Chem. 2009;284(1):67–76. doi: 10.1074/jbc.M806153200. [DOI] [PubMed] [Google Scholar]
- 8.Sebbagh M, Olschwang S, Santoni MJ, Borg JP. The LKB1 complex–AMPK pathway: the tree that hides the forest. Fam. Cancer. 2011;10(3):415–424. doi: 10.1007/s10689-011-9457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell. 2004;15(5):661–675. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Zeqiraj E, Filippi BM, Deak M, Alessi DR, Van Aalten DM. Structure of the LKB1–STRAD–MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemminki A. The molecular basis and clinical aspects of Peutz–Jeghers syndrome. Cell. Mol. Life Sci. 1999;55(5):735–750. doi: 10.1007/s000180050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donze O, Abbas-Terki T, Picard D. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 2001;20(14):3771–3780. doi: 10.1093/emboj/20.14.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc. Natl Acad. Sci. USA. 2006;103(30):11318–11322. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol. Cell. Biol. 2007;27(1):220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Mollapour M, Prodromou C, et al. Dynamic tyrosine phosphorylation modulates cycling of the Hsp90–p50(Cdc37)–AHA1 chaperone machine. Mol. Cell. 2012 doi: 10.1016/j.molcel.2012.05.015. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]