Abstract
Macrophage stimulating protein (MSP), also known as hepatocyte growth factor-like, is a soluble cytokine that belongs to the family of the plasminogen-related growth factors (PRGFs). PRGFs are α/β heterodimers that bind to transmembrane tyrosine kinase receptors. MSP was originally isolated as a chemotactic factor for peritoneal macrophages. Through binding to its receptor, encoded by the RON gene, it stimulates dissociation of epithelia and works as an inflammatory mediator by repressing the production of nitric oxide (NO). Here, we identify a novel role for MSP in the central nervous system. As a paradigm to analyze this function we chose the hypoglossal system of adult mice. We demonstrate in vivo that either administration of exogenous MSP or transplantation of MSP-producing cells at the proximal stump of the resected nerve is sufficient to prevent motoneuron atrophy upon axotomy. We also show that the MSP gene is expressed in the tongue, the target of the hypoglossal nerve, and that MSP induces biosynthesis of Ron receptor in the motoneuron somata. Finally, we show that MSP suppresses NO production in the injured hypoglossal nuclei. Together, these data suggest that MSP is a novel neurotrophic factor for cranial motoneurons and, by regulating the production of NO, may have a role in brain plasticity and regeneration.
INTRODUCTION
Macrophage stimulating protein (MSP; Skeel et al., 1991; Yoshimura et al., 1993), also known as hepatocyte growth factor-like (Degen et al., 1991), belongs to the family of plasminogen-related growth factors (PRGF; Donate et al., 1994), of which hepatocyte growth factor (HGF) is the prototype. These soluble cytokines share a common structure: they are heterodimeric polypeptides comprising two subunits joined by disulfide bonds, respectively, characterized by the presence of four kringle domains and a nonfunctional serine-protease–like domain (Naldini et al., 1992; Waltz et al., 1997). The PRGF receptors of MSP and HGF identified so far are Ron for MSP (Gaudino et al., 1994) and Met for HGF (Bottaro et al., 1991; Naldini et al., 1991).
MSP/hepatocyte growth factor-like was originally isolated as a chemotactic factor for peritoneal macrophages (Leonard and Skeel, 1978, 1979), although it may also act as a mitogen or morphogen in a variety of other cell types such as osteoclasts (Kurihara et al., 1996, 1998), epithelial cells (Medico et al., 1996), hematopoietic precursors (Broxmeyer et al., 1996), and carcinoma cells (Maggiora et al., 1998; Willett et al., 1998). It has been shown that in exudate macrophages MSP inhibits the production of nitric oxide (NO), an inflammatory mediator produced in response to treatment with bacterial lipopolysaccharide or interferon-gamma (Wang et al., 1994; Chen et al., 1998).
MSP transcripts are present in the liver and, at a lower amount, in kidney and pancreas (Bezerra et al., 1993; Yoshimura et al., 1993). Transcripts of RON (also known as STK in mouse) are detectable in many different organs during murine development. Relatively late in development, high levels of this receptor are found in the trigeminal ganglion and in the hypoglossal nucleus (Gaudino et al., 1995; Quantin et al., 1995). In adult mice, RON transcripts are almost ubiquitous, except in spleen and heart (Gaudino et al., 1995).
MSP knockout animals develop normally, are fertile, and grow to adulthood in spite of liver abnormalities due to lipid-containing cytoplasmic vacuoles in hepatocytes (Bezerra et al., 1998). Two different Ron mutants, generated with different targeting strategies, are available. They display two different phenotypes: the first mutant develops normally to adulthood (Correl et al., 1997), whereas the second shows an embryonic lethal phenotype (Murakoa et al., 1999). Remarkably, both Ron mutant mice are highly prone to septic shock.
Several growth factors such as fibroblast growth factors, insulin-like growth factors (I and II), and HGF itself are essential in brain development (Ebens et al., 1996; Ortega et al., 1998; Gao et al., 1999) and promote motoneuron survival during adulthood (Unsicker et al., 1987; Cuevas et al., 1995; Ebens et al., 1996; Teng et al., 1998; Pu et al., 1999). Many of them, delivered at the proximal nerve stump after axotomy, are taken up and transported to the motoneuron somata (Funakoshi et al., 1993). It has been shown that these paracrine circuits between muscle targets and brainstem motoneuron nuclei are essential in many regenerative and remodeling processes of the central nervous system (CNS) (DiStefano et al., 1992; Li et al., 1994; Blottner et al., 1997). Here, we identify a novel role for MSP, showing that this molecule is a neurotrophic factor that prevents atrophy of motoneurons upon axotomy.
MATERIALS AND METHODS
Reagents
MSP and control solution for the in vivo experiments were prepared from baculovirus-infected Sf9 cells (Maggiora et al., 1998). Both supernatants underwent to two serial precipitations with ammonium sulfate-saturated solution (40%, to remove most of the high molecular weight proteins, and 50% to precipitate MSP). The precipitate was solubilized and dialyzed in phosphate-buffered saline (PBS) to a final concentration of 1 μg/ml. Before treatment on mice, MSP activity was tested performing a Ron phosphorylation assay on T47D cells.
MSP antibodies were obtained by immunizing rabbits with human MSP. MSP cDNA (Gaudino et al., 1994) was treated with the restriction nucleases EcoRI and XhoI. The resulting fragment was cloned in the eukaryotic vector pRK7His previously digested with EcoRI and HindIII. The recombinant construct was transfected in BOSC cells and their conditioned medium was collected after 3 d and after 1 wk. MSP protein was purified onto a nickel column and eluted using imidazol. The factor was dialyzed and rabbits were immunized (500 μg/animal). After 4 wk and two subsequent reimmunizations, polyclonal antibodies were obtained. These antibodies cross-react with the murine homologue, both in blot and in immunohistochemistry, and were used for all the analyses.
Cell-mediated MSP Production
The cDNA encoding the full-length MSP (Gaudino et al., 1994) was treated with the restriction nuclease EcoRI, blunt-ended and retreated with BamHI, and then it was ligated in the eukaryotic expression vector pBat, previously treated with SalI, and then blunted and retreated with BamHI. Neuro2A cells were maintained in DMEM plus 10% fetal calf serum. They were grown to 60% confluence and transfected by calcium-phosphate coprecipitation (CellPhect Transfection kit; Amersham Pharmacia Biotech, Uppsala, Sweden) either with MSP cDNA or with an empty vector (10 μg of DNA/p-100 culture dish). Cells were glycerol-shocked 12 h after transfection and collected after further 48 h. The efficiency of transfection was tested by checking the presence of MSP in the conditioned medium and in the cells by Western blot techniques.
Surgery
Forty 4-mo-old FVB albino mice from our breeding colony were used for this study. Animals had free access to food and water. All experimental procedures on living animals were performed under the supervision of a veterinarian, according to guidelines for care and use of laboratory animals as published by the Italian Ministry of Health (DDL 116/92).
Mice were anesthetized with Avertin (240 mg/kg tribromoethanol, diluted 1.2% in PBS). To perform axotomy, the left hypoglossal nerve was exposed close to the posterior border of the mylohyoid muscle and cut. Upon nerve resection two routes of MSP administration were used.
First, an osmotic minipump (mean pumping rate 0.51 μl/h, Alzet 1007D; Alza, Mountain View, CA) filled with 1 μg/ml MSP was placed in the pectoral region, with the catheter (0.04 cm O.D.) positioned close to the proximal stump of the resected nerve and sutured to the masseter muscle. Conditioned medium from mock-infected Sf9 cells was used as a control.
Second, 300,000 Neuro2A cell pellets transfected with MSP were mixed to a foamy gel and placed close to the proximal stump of the axotomized hypoglossal nerve. Neuro2A cells transfected with an empty vector supplied the control.
All mice were killed with an overdose of anesthetic 48 h or 1 wk after surgery. The ones used for immunohistochemistry and in situ hybridization were perfused through the left ventricle with a washing solution of 0.1 M phosphate buffer pH 7.4 (PB) followed by fixative (4% paraformaldehyde in PB). The brainstem and the tongue were dissected, postfixed 4 h in the same fixative, and immersed in PB plus 30% sucrose overnight for cryoprotection. The brainstems used for polymerase chain reactions (PCRs) and Western blots were dissected and then immediately frozen in liquid nitrogen and transferred at −80°C.
Immunohistochemistry
After fixation, 50-μm-thick sections from mice killed 1 wk after axotomy were reacted free-floating with a polyclonal antibody against choline acetyl transferase (ChAT) (1:500 in PBS; Chemicon International, Temecula, CA). Binding of primary antibody was visualized by incubating in biotinylated goat anti-rabbit IgG antibody (1:200 in PBS; Vector Laboratories, Burlingame, CA) for 1 h at room temperature, followed by avidin-biotin-peroxidase complex (Elite ABC kit; Vector Laboratories); the peroxidase activity was detected using 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich Chemie, Steinheim, Germany) as a chromogen. Sections were transferred on gelatin-coated slides, dehydrated, and mounted in Entellan (Sigma-Aldrich Chemie). In these experiments six axotomized animals were infused with MSP and seven with conditioned medium from mock-infected Sf9 cells; four mice were transplanted with Neuro2A cells expressing MSP and four with Neuro2A transfected with an empty vector. Two animals were infused, after axotomy, with saline solution. Slides were observed and photographed with a Leitz Dialux light microscope (Leitz Gmbh, Oberkochen, Germany) and the number of ChAT-positive profiles in the hypoglossal nuclei of both sides counted. Intergroup differences were statistically evaluated by the two-tailed paired Student's t test and were considered significant when p < 0.05.
After fixation, 50-μm-thick cryostat sections from the brainstem were stained with polyclonal antibodies against Ron (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 in PBS plus 2% bovine serum albumin [BSA]) or MSP (1:100 in PBS plus 2% BSA) for 12 h at room temperature; the signals were visualized using anti-rabbit monoclonal antibodies conjugated either to fluorescein isothiocyanate or to biotin (Amersham Pharmacia Biotech; 1:50 in PBS containing 2% BSA, 1 h of incubation). Fluorescent slides were mounted in Mowiol and observed using a confocal microscope (Molecular Dynamics, Sunnyvale, CA); the others were processed with Elite ABC kit (Vector Laboratories) and the chromogen 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich Chemie) and then observed with a Leitz Dialux light microscope (Leitz, Oberkochen, Germany). Four mice were analyzed for Ron (2 in immunofluorescence and 2 in immunohistochemistry) and six for MSP (4 in immunofluorescence and 2 in immunohistochemistry).
NADPH-Diaphorase Histochemistry
Different groups of mice were analyzed, sacrificing animals either after 2 d (10 mice: 4 infused with MSP, 4 with conditioned medium of Sf9 mock-infected cells and 2 with saline solution) or after 1 wk (10 mice, grouped as described above). Cryostat sections (50-μm-thick) were reacted free-floating for 1 h in a solution of 1 mg/ml NADPH (Sigma-Aldrich Chemie) and 0.2 mg/ml nitroblue tetrazolium (Sigma-Aldrich Chemie, in PB containing 0.5–1% Triton X-100. Sections were thoroughly rinsed in PB and mounted on gelatin-chrome alum-coated slides. Sections were air dried overnight, dehydrated in ascending alcohols, cleared in xylene, mounted in Eukitt mounting medium, and observed with a Leitz Dialux light microscope (Leitz).
For NADPH-diaphorase (NADPH-d) staining the neuronal cell profile was used as unit of counting. Three to seven sections were counted for each animal. NADPH-d-positive motoneurons were counted separately in the nuclei contralateral and ipsilateral to axotomy. These numbers were expressed as percentage of the total number of motoneurons of the corresponding side. The percentage value of the side ipsilateral to axotomy was normalized subtracting the percentage value of the contralateral intact side. Average ±SD was calculated for each group; these data were evaluated performing two-tailed Student's t tests; a p value <0.05 was considered significant.
PCR Analysis
Cerebellum, superior colliculus, and somatosensory cortex were dissected from untreated animals. Hypoglossal nuclei were dissected either from untreated animals or from axotomized mice. In this last case, 48 h after axotomy the hypoglossal nuclei of both sides were separated under dissecting microscope. The following groups of animals were analyzed: two untreated mice, five infused with MSP, four with conditioned medium from mock-infected Sf9 cells, and two infused with saline solution. Extracts were prepared as previously described (di Renzo et al., 1994).
Nucleic acids were treated with DNase and the remaining RNAs were retro-transcribed using an oligo dT primer (Sambrook et al., 1989). The obtained cDNA was split in two parts; each was the template for two or three different PCR reactions, by using primers specific for RON (sense: 5′ TCTTTAGCTTTCTGGGGCC 3′; antisense: 5′ TATTATTTTACACTGTAGTATCTC 3′), for γ-enolase (sense: 5′ AGAATGGGGCTGTGGACCTGGG 3′; antisense: 5′ GCGCTGTGATTCAGACTTTAATGG 3′), or for MSP (sense: 5′ TTGCCTGCTATACCCATGACTGCTGGG 3′; antisense: 5′ ATGTTTGAGAAAGCTTGACATCTC 3′). The same PCR mix was split in the different test tubes and one sample, supplied with all the reagents used for the reverse transcription reaction but without a template DNA, was considered as mock. The reactions were run onto a 4% agarose gel. Because the bands corresponding to RON amplifications were not always visible by staining with ethidium bromide, Southern blot analyses were performed. The gels were blotted and the filters (Hybond-N+; Amersham Pharmacia Biotech) were hybridized with a RON probe, labeled with digoxigenin-dUTP, and detected by chemiluminescence. Probe labeling, hybridization, and chemiluminescent detection were done according to Dig High Prime Labeling and Detection Starter kit II (Roche Molecular Biochemicals, Mannheim, Germany) instructions. Ethidium bromide and chemioluminescent signals were quantified by densitometry with the software ImageQaNT (Molecular Dynamics). The ratio between RON and γ-enolase was calculated for each single reaction. Average ± SD was calculated for each group; data were compared by Student's t test; a p value <0.05 was considered significant.
Northern Blot Analysis
PC12 cells were maintained in Iscove's Modified Dulbecco's Medium (Sigma-Aldrich Chemie) plus 10% fetal calf serum plus 5% horse serum donor herd and plated onto poly-l-lysine-coated dishes. They were grown to 70% confluence and then starved for 24 h in 1% horse serum. Stimulation was performed adding either MSP (150 ng/ml) or conditioned medium of mock-infected Sf9 cells. PC12 cells were lysed before stimulation (time 0) and after 3, 6, 12, 24, and 48 h. RNAs were prepared using RNAwiz (Ambion, Austin, TX) according to the manufacturer's instructions and Northern blot analysis was performed. Total RNAs (10 μg) were loaded in each lane. After running, the gel was blotted onto a nylon membrane (Hybond-N+; Amersham Pharmacia Biotech). A probe for glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) gene was obtained by PCR with cDNA from PC12 cells prepared as described in the previous paragraph (primers: sense 5′ GAAGGTGAAGGTCGGAGTC 3′; antisense 5′ GAAGATGGTGATGGGATTTC 3′) as a template; the full human cDNA was the probe for Ron. Both DNAs were 32P-labeled using the Megaprime DNA labeling system (Amersham Pharmacia Biotech). After hybridization the membrane was washed in 0.5× SSC, 0.1% SDS at 50°C. The signals were visualized with a Storm apparatus (Molecular Dynamics) and quantified by densitometry with the software ImageQaNT (Molecular Dynamics). The ratio of Ron versus GAPDH signals was calculated for each single lane. This value was considered as zero in unstimulated cells. The others were calculated as increments of this reference value.
Western Blot Analysis
Tissues were homogenized and sonicated. The supernatants were clarified by centrifugation for 40 min at 4°C, 13,000 rpm. MSP was immunoprecipitated using 5 μl of polyclonal antibody cross-linked to Sepharose-protein A resin (Amersham Pharmacia Biotech). Ron and γ-enolase were analyzed in the remaining extracts (40 μg/lane). SDS-PAGE gels were blotted onto a nylon membrane (PVDF transfer membrane; Millipore, Bedford, MA). Ron was detected with polyclonal antibodies against the human receptor (Gaudino et al., 1994) 1:200 in Tris-buffered saline (TBS) plus 5% BSA and 0.15% Tween 20); γ-enolase with polyclonal antibodies against neuronal specific enolase (1:1000 in TBS plus 5% BSA and 0.15% Tween 20; Chemicon International) and MSP with the antibody described above (1:500 in TBS plus 5% BSA and 0.15% Tween 20). Protein-A conjugated to a peroxidase activity was used as secondary antibody. The signals were visualized with ECL chemiluminescence system (Amersham Pharmacia Biotech). Extracts from the same animals that underwent to PCRs were analyzed.
In Situ Hybridization
Cryostat sections (10- or 20-μm-thick) were adhered to poly-l-lysine-coated slides. Full-length murine MSP cDNA was labeled with Dig DNA Labeling kit (Roche Molecular Biochemicals) according to the manufacturer's instructions. After labeling, the size of the cDNA fragments was reduced by digestion with the restriction nuclease MspI. The in situ hybridization was carried on at 37°C, with formamide, 12 h, according to Schaeren-Wiemers and Gerfin-Moser (1993); the digoxigenin signals were developed for 2 d. Prior in situ hybridization the tongue sections were stained for actin with tetramethylrhodamine B isothiocyanate-conjugated phalloidin (Sigma-Aldrich Chemie) as described by Maina et al. (1998). Six mice were analyzed.
RESULTS
MSP and Ron in Adult Murine Brain
During murine embryonic development RON transcripts are strongly expressed in specific regions of the CNS (Gaudino et al., 1995; Quantin et al., 1995); however, the role of MSP and its receptor Ron in the CNS is as yet unknown. Thus, we probed whether the ligand and/or the receptor are still expressed in the adult CNS to use adult mice as an experimental model to investigate the function of MSP and Ron in the brain. We analyzed the hypoglossal nuclei, where RON transcripts are abundant during later stages of embryonic development.
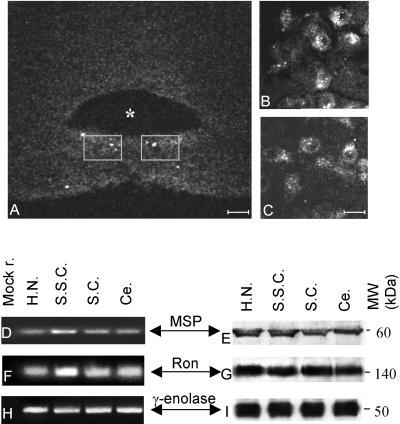
Serial sections of the brainstem from adult mice were stained with antibodies raised against both ligand (6 mice) and receptor (4 mice). Upon treatment with anti-Ron antibodies, the hypoglossal motoneurons showed specific immunoreactivity (Figure 1A, low magnification; B and C, higher magnification). This signal was absent following preabsorption of the antibody with Ron peptides before the staining. The low intensity of the signals with the antibodies against MSP made discerning individual cells impossible; similar results were obtained by in situ hybridization with MSP probes (6 mice). To improve the sensitivity of the assay we thus evaluated the expression of the transcripts by PCR techniques and the proteins by Western blot analyses (see MATERIALS AND METHODS for details) in the brain extracts of two mice pooled together. The left panels of Figure 1 show the results of the PCR amplifications by using specific primers for MSP (Figure 1D) and RON (Figure 1F); the right panels show MSP (Figure 1E) and Ron (Figure 1G) polypeptides in the same extracts. Together with the hypoglossal nuclei three brain regions functionally unrelated were analyzed: cerebellum, somatosensory cortex, and superior colliculus. The neuronal marker γ-enolase was used to compare nucleic acid and proteins contents (Figure 1, H and I, respectively). These brain districts expressed both MSP and Ron, transcripts and proteins. It should be noted that the molecular weight of the bands corresponding to MSP and Ron polypeptides revealed that both proteins are present in their active, processed form. Thus, we can conclude that during adulthood this ligand and its receptor are expressed in the brainstem and Ron still maintains its localization in the hypoglossal nuclei.
Figure 1.
Ron localization in adult brain. (A–C) Cryostat sections reacted with anti-Ron antibodies and observed with a confocal scanning microscope. Asterisk labels the fourth ventricle. The gray squares in A (bar, 80 μm) encompass the region showed at high magnification in B and C (bar, 33 μm). The antibody stains the hypoglossal motoneurons. Transcripts (D, F, and H) and proteins (E, G, and I) from hypoglossal nucleus (H.N.), somatosensory cortex (S.S.C.), superior colliculus (S.C.), and cerebellum (Ce.). cDNAs were amplified with primers specific for MSP (D), RON (F), and γ-enolase (H) and then loaded onto a 4% agarose gel. Mock r. (first lane) is the result of a PCR performed without template. E, G, and I are Western blots showing the expression, respectively, of MSP, Ron, and γ-enolase proteins in the same animals examined by PCRs. The protein extracts were loaded onto 8% SDS-PAGE gels. The gels were blotted and the filters were decorated with specific antibodies. The molecular weights are indicated (MW kDa). Ron and MSP are expressed in all the extracts examined.
MSP Is Expressed in Tongue
The existence of paracrine circuits involving an active transport of many neuronal trophic factors from the target cells backwards to the motoneuron somata has been extensively documented (DiStefano et al., 1992). To assess whether MSP has these properties, we tested by in situ hybridization its expression in the tongue, where the muscles innervated by hypoglossus are located. In this analysis six mice were sacrificed.
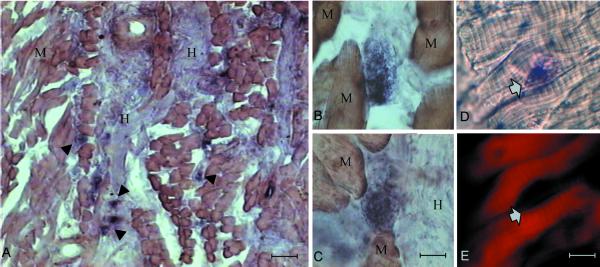
Figure 2 shows cells displaying a very strong signal (arrowheads), which are abundant in proximity to the nerve (Figure 2A) and are mixed to unstained muscle fibers (Figure 2, B and C). To investigate whether MSP was produced by muscle tissue, we took advantage of the typical striped actin distribution in muscle fibers. Therefore, before hybridization, the sections were stained with fluorescent phalloidin to visualize actin. Figure 2, D and E, show, respectively, MSP transcripts and actin localiza-tion within the same section. The structures that express MSP weakly reacted with phalloidin (arrows) and they do not show the actin organization typical of the muscular fibers.
Figure 2.
MSP transcripts in the tongue. In situ hybridization of cryostat sections from adult murine tongue by using digoxigenin-labeled cDNA probes. M, muscle; H, hypoglossal nerves. The arrowheads indicate the structures expressing MSP. Bar, 150 μm (A); 15 μm (B and C); and 30 μm (D and E). D and E show the same section, respectively, reacted with MSP probes or stained with tetramethylrhodamine B isothiocyanate-labeled phalloidin; the arrows indicate the same structure. See RESULTS for details.
These data show that indeed MSP transcripts are produced in the tongue, the target organ of the hypoglossal nerve, although this growth factor is expressed in cells different from mature myotubes, possibly of mesenchymal or glial origin.
Trophic Effect of MSP on Motoneurons of Hypoglossal Nucleus
In adult mammals, axotomy of peripheral nerves results in loss of ChAT immunoreactivity in the motoneuron somata, a phenomenon that reflects a decline in motoneuron functionality expressed as a reduced production of the neurotransmitter acetylcholine (Armstrong et al., 1991; Li et al., 1994). This functional decline may be prevented or reduced by the delivery of a number of growth factors (Chiu et al., 1994; Cuevas et al., 1995; Tuszynski et al., 1996; Teng et al., 1998). To test in vivo whether MSP has a trophic function for hypoglossal motoneurons, we studied whether in adult animals the exogenous delivery of this factor prevented decrease of ChAT immunoreactivity induced by axotomy. Each hypoglossal nerve originates from one of the two hypoglossal nuclei and its axons project to the ipsilateral side of the tongue. The multipolar hypoglossal motoneurons show medium-to-large size somata, which are located ventrally to the dorsal motor nucleus of the X cranial nerve and dorsally to the Roller nucleus (Franklin and Paxinos, 1996). The motoneurons are grouped ventromedially and are more dispersed dorsolaterally. We transected the right hypoglossal nerve and monitored the expression of ChAT by counting the number of immunoreacting cells in both hypoglossal nuclei, one ipsilateral and the other contralateral (control), to the axotomized side. MSP was delivered either by infusion of recombinant factor produced by Sf9-infected cells to the extremity of the proximal hypoglossal nerve stump with an osmotic minipump (1 μg/ml, 6 animals) or by transplanting in the same location Neuro2A cells engineered to produce MSP (4 animals). Control mice were infused with conditioned medium of mock-infected Sf9 cells (7 animals) or transplanted with Neuro2A cells transfected with empty vector (4 animals). Two mice were infused with saline solution.
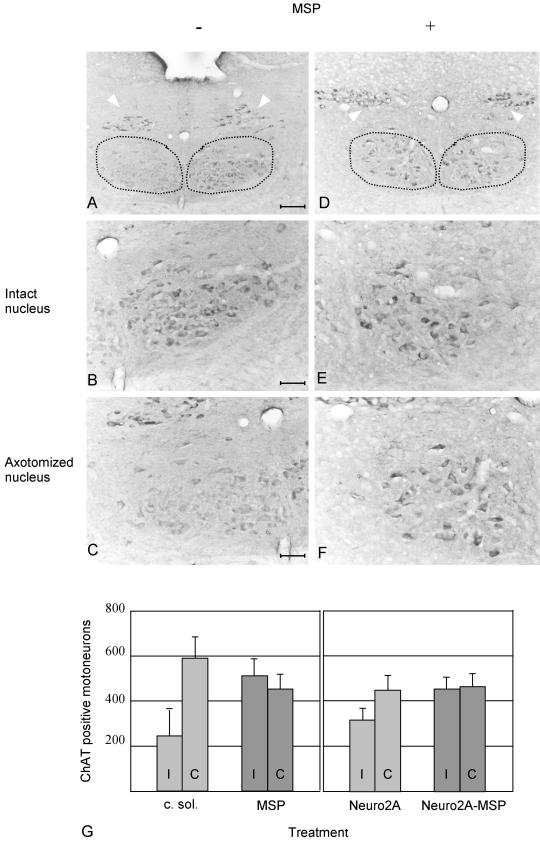
As described in literature (Armstrong et al., 1991), 7 d following hypoglossal nerve section, mice infused with saline solution showed a strong decrease in ChAT immunoreactivity in the nucleus ipsilateral to axotomy compared with the contralateral one. This phenomenon took place in axotomized animals infused with conditioned medium of mock-infected Sf9 cells as well (Figure 3, A, on left, and C). When MSP was infused with an osmotic minipump, this decline of ChAT immunoreactivity was abolished (Figure 3, D, on left, and F). In all the operated mice the contralateral nuclei were unaffected (compare the right side of Figure 3, A and D, at high magnification in B and E). Similar results were achieved when Neuro2A cells expressing MSP were placed at the proximal stump of the axotomized hypoglossal nerve. As described above, in these animals the decrease in ChAT immunoreactivity was prevented, whereas mice transplanted with mock-transfected Neuro2A cells behaved as those infused with saline solution. Notably, in all animals examined, ChAT immunoreactivity in the vagal nucleus was similar in the two sides (white arrowheads in Figure 3, A and D), thus indicating that the differences observed in the hypoglossal nuclei were specifically due to MSP treatment.
Figure 3.
ChAT immunohistochemistry on cryostat sections prepared from axotomized mice treated with conditioned medium of mock-infected Sf9 cells or with MSP. The dotted lines encompass the hypoglossal nuclei; the white arrows point to the dorsal nuclei of the vagal nerve. Mice were sacrificed 1 wk after nerve resection. The hypoglossal nuclei are shown at low (bar, 84 μm, A and D) and high (bar, 33 μm, B, C, E, and F) magnification. In A and D, the nuclei corresponding to the contralateral intact nerves are on the right; on the left are the nuclei of the ipsilateral resected nerves. At high magnification the contralateral nuclei are on the top (B and E) and the ipsilateral on the bottom (C and F). In A–C, mice were treated with conditioned medium of mock-infected Sf9 cells. In D–F, animals were infused with MSP. ChAT activity is lost in axotomized animals treated with conditioned medium of mock-infected Sf9 cells (C) but not in animals treated with MSP (F). The contralateral hypoglossal nuclei (B and E) and the dorsal nuclei of the vagal nerve (arrowheads) are not affected. (G) Diagram showing the number of ChAT-positive hypoglossal motoneurons in treated mice. Both sides ipsilaterally (I) and contralaterally (C) to axotomy were analyzed. On the left side are the data of mice infused with conditioned medium of mock-infected Sf9 cells (c. sol., 7 mice) or with MSP (MSP, 6 mice). On the right are the data of animals transplanted with Neuro2A cells, expressing or not MSP (Neuro2A and Neuro2A-MSP, 4 mice in each group). The differences between ipsilateral and contralateral sides are significant after axotomy and treatment with c. sol. or Neuro2A (p < 0.05), whereas MSP deliver protects the axotomyzed motoneurons from ChAT decline.
The protective effect of MSP was quantified by counting the number of ChAT-positive cells in the hypoglossal nucleus of both contralateral and ipsilateral side in the different groups of animals. In mice treated with conditioned medium of mock-infected Sf9 cells 55% of the ChAT-positive cells were missing in the axotomized side compared with the contralateral one (p < 0.01), whereas in animals treated with MSP both sides had similar ChAT immunoreactivity profiles (Figure 3G). Similar numbers were obtained by supplying MSP through the transplant of Neuro2A cells close to the nerve stump. Here, again, axotomy determined a significant decrease (p < 0.05) in the number of ChAT-positive motoneurons, which was rescued by MSP (Figure 3G). The different efficacy of the two routes of MSP administration may be due to the stickiness of this cytokine, which tends to attach to the proteoglycans ubiquitously present in all tissues. This may happens when MSP diffuses from Neuro2A cells, but not if it is directly taken up from the osmotic minipump. These data prove that MSP has a protective effect on hypoglossal motoneurons, which, upon axotomy, retain their cholinergic phenotype.
MSP Prevents the Induction of Nitric-Oxide Synthase (NOS) in Axotomized Hypoglossal Motoneurons
Together with the loss of cholinergic phenotype, another effect of axon injury in the adult hypoglossal system is an up-regulation of NO synthesis through transcriptional induction of NOS (Yu, 1994). Because MSP represses the production of NO in murine peritoneal macrophages (Wang et al., 1994), we studied whether this function was present in the hypoglossal motoneurons as well. The histochemical reaction for NADPH-d was used as a marker to reveal NOS activity (Bredt et al., 1991) 48 h and 1 wk following axotomy. As described above, we analyzed both ipsilateral and contralateral hypoglossal nuclei from mice in which the hypoglossal nerve of one side was sectioned and infused either with conditioned medium of mock-infected Sf9 cells or with MSP. We sacrificed eight animals at 48 h and eight at 1 wk; always four mice were treated with MSP and four with conditioned medium. Two mice were axotomyzed and treated with saline solution.
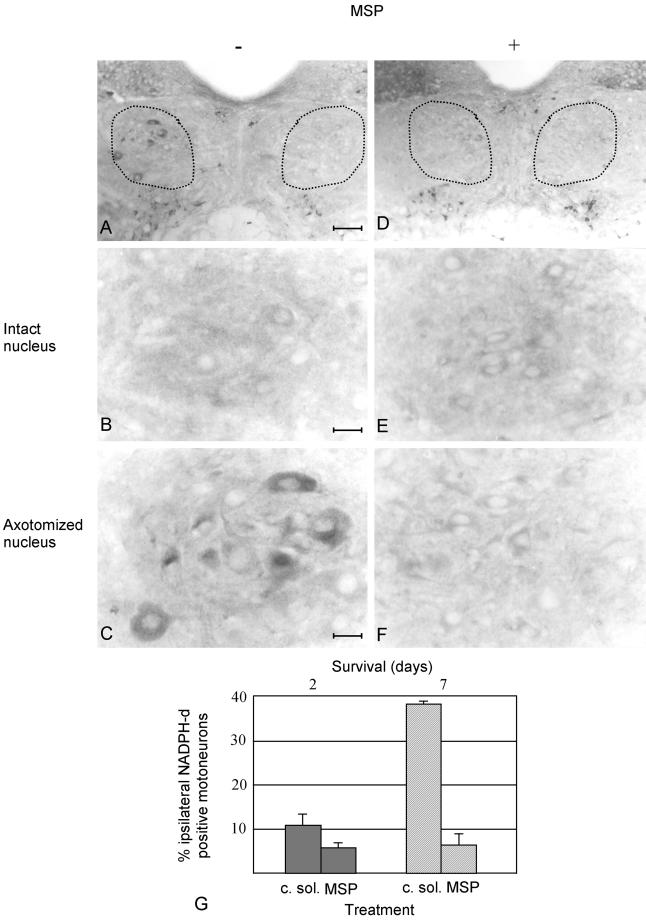
Mice in which one of the two nerves was resected and treated with saline solution, showed increased NADPH-d reactivity in the axotomized side compared with the contralateral intact one, as described in literature (Yu, 1994). Similarly, NADPH-d reactivity increased in the nucleus ipsilateral to axotomy of mice treated with conditioned medium (Figure 4A, on left, and C). When MSP was infused at the proximal stump of the transected nerve, no NADPH-d reactivity was detectable (Figure 4, D, on the left, and F). Contralateral nuclei were unaffected (Figure 4, A and D, on the right, and B and E at higher magnification). Forty-eight hours after axotomy, MSP-treated mice showed a number of NADPH-d-positive hypoglossal motoneurons twofold lower than the animals treated with conditioned medium of mock-infected Sf9 cells (Figure 4G). The effect of MSP treatment was even more evident 1 wk after axotomy, when the difference was fivefold (Figure 4G). These differences are statistically significant (p < 0.05 and < 0.01 after, respectively, 48 h and 1 wk). These data prove unambiguously that MSP prevents NOS up-regulation in axotomized hypoglossal motoneurons.
Figure 4.
NADPH-d activity in sections of axotomized hypoglossal nuclei from animals treated with conditioned medium of mock-infected Sf9 cells or with MSP. Mice were sacrificed 2 d or 1 wk after nerve resection. Four mice were analyzed in each group. The dotted lines encompass the hypoglossal nuclei. Panels are presented as in Figure 3. The hypoglossal nuclei are shown at low (bar, 74 μm, A and D) and high (bar, 16 μm, B, C, E, and F) magnification. In A and D, the nuclei corresponding to the contralateral intact nerves are on the right; on the left are the nuclei of the ipsilateral resected nerves. At high magnification the contralateral nuclei are on the top (B and E) and the ipsilateral on the bottom (C and F). In A–C, the animals were treated with conditioned medium; in D–F with MSP. These representative sections are obtained from animals sacrificed 1 wk after axotomy. The contralateral intact sides do not show NADPH-d activity (compare B and E), whereas it is visible in the nucleus ipsilateral to axotomy in animals treated with conditioned medium. This NADPH-d activity is absent when MSP is supplied (F). (G) Diagram of NADPH-d activity 48 h (filled shapes) and 1 wk (dashed shapes) after axotomy, in animals treated either with conditioned medium of mock-infected Sf9 cells (c. sol.) or with MSP. In each animal three to seven brain sections were analyzed. The neuronal cell profile was used as counting unit. NADPH-d-positive motoneurons were counted separately in the nuclei contra- and ipsi-lateral to axotomy and were expressed as percentage of the total number of hypoglossal motoneurons of the corresponding side. The values of the axotomized sides were obtained by subtracting the percentage of the positive motoneurons in the contralateral intact sides to the number of positive motoneurons in the ipsilateral side. The differences in DADPH-d reactivity between axotomyzed nuclei treated with c. sol. and MSP are significant (p < 0.05).
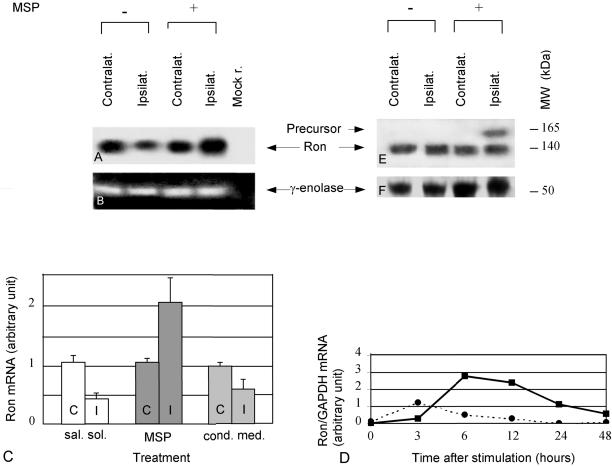
MSP Induces Transcription of RON in Hypoglossal Motoneurons
We then analyzed the expression of RON transcripts and proteins in the nuclei of axotomized animals treated with saline solution, with conditioned medium, or with MSP, all infused as previously described. We evaluated Ron contents 48 h after nerve resection, either by semiquantitative PCR or by Western blot analysis. The neuronal marker γ-enolase, which is unaffected by axotomy (Angelov et al., 1994), was used as a standard to normalize Ron content in each experiment. Five mice were treated with MSP, four with conditioned medium and four with saline solution. Both hypoglossal nuclei were always processed separately and each of them was the template for two series of different PCR reactions.
Interestingly, we observed that the expression of Ron receptor decreased in the nucleus ipsilateral to axotomy compared with the contralateral one (Figure 5A). When MSP was applied to the proximal nerve stump, RON transcripts increased and were more abundant in the nucleus of the resected nerve with respect to the nucleus of the intact nerve (Figure 5A). On the contrary, infusion of conditioned medium of mock-infected Sf9 cells was ineffective in preventing Ron mRNA's decrease. The PCR signals resulting from amplification of γ-enolase transcripts are shown for comparison (Figure 5B). We then quantified by densitometric analysis the amount of RON transcripts in the three groups of animals (Figure 5C). All of them displayed significant differences (p < 0.05) between ipsilateral and contralateral hypoglossal nuclei. It should be noted that all the nuclei contralateral to axotomy expressed similar quantity of RON transcripts (Figure 5C, C). This proves that the differences of RON expression in the ipsilateral nuclei were due to the treatments and not to random variability among the animals.
Figure 5.
MSP and Ron in axotomized hypoglossal nuclei. Mice were sacrificed 48 h after nerve resection. (A) Autoradiogram of a Southern blot showing the expression of RON in the hypoglossal nuclei of axotomized animals untreated (−) or treated (+) with MSP. Transcripts prepared from the hypoglossal nuclei contralateral (1st and 3rd lanes) and ipsilateral (2nd and 4th lanes) to the lesion were amplified with RON primers, loaded onto a 4% agarose gel, transferred to a solid support, and hybridized with RON specific probes. Mock r. (5th lane) is the result of a PCR performed without template. (B) Ethidium bromide staining of an agarose gel showing the same extracts as in A amplified with γ-enolase primers. (C) Diagram showing the quantification of RON expression in animals axotomyzed and treated with saline solution (sal. sol., white), MSP (MSP, dark gray), and supernatant of mock-infected Sf9 cells (cond. med., light gray). C, contralateral sides; I, ipsilateral sides. The signals obtained in the PCR reactions were quantified by densitometric measurements. Each Ron value was normalized to the corresponding γ-enolase value. Axotomy determines a decrease in RON transcripts, which is prevented by MSP treatment; the differences between contralateral and ipsilateral side are always significant (p < 0.05). Note that the contralateral nuclei of each group of animals express the same amount of RON. (D) Diagram showing the quantification by densitometric analysis of a Northern blot obtained by PC12 cells treated with MSP (continuous line) or with conditioned medium (dotted line). After stimulation, RNAs were extracted at the times indicated. The samples were loaded onto a 0.8 denaturing gel and blotted onto a nylon membrane. This membrane was hybridized with probes specific for Ron and GAPDH, a housekeeping gene used as a control. The amount of Ron transcripts loaded in each lane was then normalized to the corresponding value of GAPDH. At time 0 (unstimulated cells), the basal level of Ron mRNA was considered as zero. All the other values are expressed as increments of this basal level. AT 6 and 12 h MSP determines a threefold induction in the amount of Ron transcriptions. E and F are Western blots showing the expression, respectively, of Ron and γ-enolase in the hypoglossal nuclei of the animals described above. Both sides ipsilateral and contralateral to the lesion are shown. The protein extracts were loaded onto an 8% SDS-PAGE gel. This gel was blotted and the filter was divided in two parts, one was stained with anti-Ron antibodies and the other with antibodies against γ-enolase. The molecular weight of Ron is 140 (processed form) and 165 (unprocessed form) kDa; the one of γ-enolase is 50 kDa. MSP treatment determines the appearance of unprocessed Ron.
We then tested whether MSP was able to induce Ron transcription in other cell types. As model we chose PC12 cells, which are known to express this receptor (Gaudino et al., 1995). We performed Northern blot analysis followed by densitometry to compare the amount of Ron transcripts in cells treated either with MSP or with conditioned medium of mock-infected Sf9 cells at 3, 6, 12, 24, and 48 h after stimulation. The transcript of the housekeeping gene GAPDH was used to normalize the amount of RNAs loaded in each lane. Figure 5D shows that at 6 and 12 h there is a threefold induction of Ron mRNA in cells treated with MSP (continuous line), which is absent when the cells are treated with conditioned medium (dotted line). This supports the data previously obtained in the hypoglossal system that MSP induces Ron transcription.
We then compared by Western Blot analysis the content of Ron protein in the extracts obtained from the same animals analyzed above. Forty-eight hours after axotomy, in addition to the band of 140 kDa corresponding the mature form of Ron, which was present in all extracts, a signal with the molecular weight of Ron precursor (165 kDa) appeared in extracts prepared from hypoglossal nuclei of axotomized animals treated with MSP (Figure 5E). This band was absent in the contralateral nuclei of the same group of mice and in both nuclei of animals axotomized and treated with saline solution or with conditioned medium of mock-infected Sf9 cells. The staining of the same blot with antibodies raised against γ-enolase demonstrated that similar amount of proteins were loaded in each lane (Figure 5F).
We thus conclude that axotomy down-regulates RON transcription, whereas administration of MSP prevents this effect. Moreover, because 48 h after axotomy the amount of processed Ron protein in axotomized mice is the same in both the contralateral and ipsilateral side, whereas the amount of RON transcripts decreases, this polypeptide seems to be more stable than its transcripts.
DISCUSSION
In this article we show that MSP behaves as a neurotrophic factor for cranial motoneurons. We demonstrate that in adult mice the MSP receptor (Ron) is expressed in the motoneurons of the hypoglossal nucleus and MSP synthesis is detectable in the tongue, the target of hypoglossal nerve. The absence of this polypeptide in mature myotubes suggests that this factor is produced by neighboring mesenchymal cells, in agreement with its homologue HGF, which is synthesized by forelimb mesenchyme (Maina et al., 1998). Moreover, we demonstrated that exogenous administration of MSP induces novel transcription and biosynthesis of Ron in the hypoglossal motoneuron cell body. This suits with the existence of a paracrine relationship between this ligand, produced in the tongue, and its receptor, located in the brainstem, and supports a neurotrophic function of MSP. Because MSP expression can also be observed in extracts of the hypoglossal nuclei, this factor is likely to be produced in the CNS as well. Glial cells, which are known to synthesize many other neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT-3 and NT-4/5 (Friedman et al., 1998; Wu et al., 1998), are good candidates for being MSP producers. The hypoglossal motoneurons could be a source of MSP as well. If this is the case, an autocrine loop should be hypothesized, similarly to HGF in developing sympathetic neuroblasts (Maina et al., 1998). Interestingly, we show that MSP increases Ron mRNA in PC12 cells, suggesting that, together with the well-documented activation of Ron by phosphorylation (Wang et al., 1994), MSP may regulate its receptor also at the transcriptional level.
In our analyses we detect the processed form of MSP. This growth factor, which is known to be secreted as a single-chain precursor (Wang et al., 1993), is cleaved to a mature α/β polypeptide by specific convertases (Wang et al., 1996; Nanney et al., 1998). Some of them are proteins of the blood coagulation cascade, such as kallikrein, factor XIIa, and factor XIa (Wang et al., 1994a), but the most effective are NGF-γ and epidermal growth factor-binding protein, serine proteases that belong to the subfamily of the glandular kallikrein (Wang et al., 1994b). It is thus intriguing to hypothesize a cleavage of the MSP precursor by NGF-γ and epidermal growth factor-binding protein. These could be transported from the submandibular glands, where they are highly expressed (Drinkwater et al., 1987; Isackson et al., 1987), to the brainstem through the extensive connections that link together masticatory, facial, and lingual neuromuscular systems (Fay and Norgren, 1997).
HGF, the homologue of MSP, promotes survival of motoneurons during development, prevents apoptosis of sympathetic neuroblasts, and is required for the development of sensory neurons of the dorsal root ganglia (Ebens et al., 1996; Maina et al., 1998). Until now, indirect evidence supporting the neurotrophic role of MSP was provided by the findings that rat tongue myoblasts sustain survival and differentiation of dissociated hypoglossal neuroblasts in vitro (Ternaux and Portalier, 1993) and that MSP stimulates mitosis of neuroendocrine PC12 cells in culture (Gaudino et al., 1995). Upon nerve transection, hypoglossal motoneurons cease to express acetylcholine and become atrophic. This represents a change in protein synthesis from neurotransmitter types of molecules, characteristic of fully differentiated motoneurons, toward proteins that are more essential for survival and regeneration (Armstrong et al., 1991). Here, we show that exogenous MSP preserves the cholinergic phenotype of transected hypoglossal motoneurons. This validates the hypothesis of a neurotrophic function of MSP and allows the speculation that, similarly to BDNF or NT-4/5 (but unlike NGF or NT-3; Yan et al., 1993; Koliatsos et al., 1994; Tuszynski et al., 1996), this growth factor supports the differentiated state of adult hypoglossal motoneurons.
A consequence of hypoglossal nerve resection is a dramatic increase in NO. The significance of this induction is not yet fully understood. NO may be protective, functioning as a free radical scavenger (Wink et al., 1993) and facilitating local blood flow (Iadecola et al., 1996; Iadecola et al., 1997). On the other hand, NO may be noxious, promoting motoneuron loss (Ruan et al., 1995). We show that MSP prevents NO up-regulation after axotomy. The ability of MSP to down-regulate NO expression is already documented in macrophages (Wang et al., 1994), where it acts in a phosphatidylinositol 3-kinase-dependent manner (Chen et al., 1998). Thus, we can speculate that the mechanism underlying the neuroprotective role of MSP involves the repression of NO, through activation of the phosphatidylinositol transduction pathway.
During nerve regeneration NO is gradually down-regulated with a time course that depends on the type of neuron analyzed (Gonzalez-Hernandez and Rustioni, 1999). In the hypoglossal motoneurons NO reduction takes place after the proper connections between lingual muscles and hypoglossal axons are restored (Yu, 1997). It is intriguing to propose that, together with its neurotrophic function, MSP may also have a role in regeneration, by participating to NO down-regulation being retrogradedly transported to the motoneuron somata after tongue reinnervation.
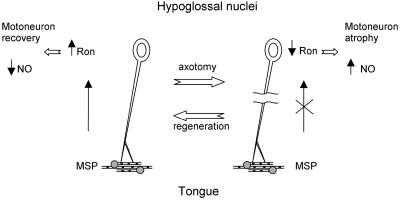
A putative model describing MSP function in the adult nervous system is schematized in Figure 6. MSP, produced in the tongue, would be retrogradedly transported to the hypoglossal motoneuron somata, being sufficient to support a basal level of Ron transcription. The signaling cascade thus activated would be sufficient to block NO production and to sustain motoneuron survival. Upon axotomy, MSP supply is interrupted, determining a decrease in RON synthesis, resulting in motoneuron atrophy and increased NO production. Following regeneration, when MSP can reach once more the CNS, this process would be reversed.
Figure 6.
Schematic representation illustrating the role of MSP in the CNS. MSP, produced in the tongue by specific cells (dashed circles), is retrogradely transported to the motoneuron somata where, through its receptor Ron, acts as a trophic factor and represses NO synthesis. Axotomy interrupts this circuit, which is restored after nerve regeneration. See DISCUSSION for details.
Based on the data shown in this article one would expect that MSP or Ron deprivation determines phenotypic changes in the CNS. Surprisingly, MSP null mice do not present any obvious neuronal alterations, either during embryonic life or in adulthood. This could be due to the presence of MSP homologues, such as the amphibian Livertine (Ruiz i Altaba and Thery, 1996), which may compensate the need of this growth factor. Alterations in the CNS of mice in which the function of Ron has been inactivated have not been described as well. In this animals the existence of other neurotrophic molecules, such as BDNF or NT-4/5, which are known to turn on the same downstream effectors activated by MSP/Ron (Danilkovitch and Leonard, 1999; Yuen and Mobley, 1999), seems to be sufficient to rescue the lack of Ron. Alteration in the CNS of these mutants would then become evident when more than one neurotrophic factor is missing, as in axotomy.
ACKNOWLEDGMENTS
We thank L. Ailles, S. Giordano, P. Gual, P. Longati, and L. Tamagnone for helpful comments. We are grateful to E. Wright for editing the manuscripts and to L. Trusolino and P.G.H. Clarke for critical reading of the manuscript. The excellent technical assistance of L. Palmas is gratefully acknowledged. The experimental work reviewed in this article was supported by Associazione Italiana per la Ricerca sul Cancro and Harvard-Armenise Foundation (to P.M.C.).
REFERENCES
- Angelov DN, Neiss WF, Gunkel A, Guntinas-Lichius O, Stennert E. Axotomy induces intranuclear immunolocalization of neuron-specific enolase in facial and hypoglossal neurons of the rat. J Neurocyt. 1994;23:218–233. doi: 10.1007/BF01275526. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Brady R, Hersh LB, Hayes RB, Wiley R. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J Comp Neurol. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Bezerra JA, Carrick TL, Degen JL, Witte D, Degen SJF. Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J Clin Invest. 1998;101:1175–1183. doi: 10.1172/JCI1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JA, Witte DP, Aronow BJ, Degen SJ. Hepatocyte-specific expression of the mouse hepatocyte growth factor-like protein. Hepatology. 1993;18:394–399. [PubMed] [Google Scholar]
- Blottner D, Stapf C, Meisinger C, Grothe C. Localization, differential expression and retrograde axonal transport suggest physiological role of FGF-2 in spinal autonomic neurons of the rat. Eur J Neurosci. 1997;9:368–377. doi: 10.1111/j.1460-9568.1997.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;15:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi J, Davvsib RM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Cooper S, Li ZH, Lu L, Sarris A, Wang MH, Chang MS, Donner DB, Leonard EJ. Macrophage-stimulating protein, a ligand for the RON receptor protein tyrosine kinase, suppresses myeloid progenitor cell proliferation and synergizes with vascular endothelial cell growth factor and members of the chemokine family. Ann Hematol. 1996;73:1–9. doi: 10.1007/s002770050192. [DOI] [PubMed] [Google Scholar]
- Chen YQ, Fischer JH, Wang MH. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phophatidylinositol-3 kinase is required for RON-mediated inhibition of iNOS expression. J Immunol. 1998;161:4950–4959. [PubMed] [Google Scholar]
- Chiu AY, Chen EW, Loera S. Distinct neurotrophic responses of axotomized motor neurons to BDNF and CNTF in adult rats. Neuroreport. 1994;5:693–696. doi: 10.1097/00001756-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Correl PH, Iwama H, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct. 1997;1:69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Carceller F, Gimenez-Gallego G. Acidic fibroblast growth factor prevents post-axotomy neuronal death of the newborn rat facial nerve. Neurosci Lett. 1995;197:163–186. doi: 10.1016/0304-3940(95)11926-n. [DOI] [PubMed] [Google Scholar]
- Degen SJ, Stuart LA, Han S, Jamison CS. Characterization of the mouse cDNA and gene coding for a hepatocyte growth factor-like protein: expression during development. Biochemistry. 1991;30:9781–9791. doi: 10.1021/bi00104a030. [DOI] [PubMed] [Google Scholar]
- Danilkovitch A, Leonard EJ. Kinases involved in MSP/RON signaling. J Leukoc Biol. 1999;65:345–348. doi: 10.1002/jlb.65.3.345. [DOI] [PubMed] [Google Scholar]
- di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, Sismondi P, Comoglio PM. Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer. 1994;1:658–662. doi: 10.1002/ijc.2910580507. [DOI] [PubMed] [Google Scholar]
- DiStefano P, Friedman B, Radziejewski C, Alexander C, Bolan P, Shick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Donate LE, Gherardi E, Srinivasan N, Sowdhamini R, Aparicio S, Blundell TL. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP) Protein Sci. 1994;3:2378–2394. doi: 10.1002/pro.5560031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater CC, Evans BA, Richards RI. Mouse glandular kallikrein genes: identification and characterization of the genes encoding the epidermal growth factor binding proteins. Biochemistry. 1987;26:6750–6756. doi: 10.1021/bi00395a026. [DOI] [PubMed] [Google Scholar]
- Ebens A, Brose K, Leonardo ED, Gratz H, Anson M, Jr, Bladt F, Birchmeier C, Barres BA, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III Lingual muscle motor systems. Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Black IB, Kaplan DR. Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: an immunocytochemical study. Neuroscience. 1998;84:101–114. doi: 10.1016/s0306-4522(97)00526-5. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1996. [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson A. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WQ, Shinsky N, Ingle G, Beck K, Elias KA, Powell-Braxton L. IGF-I deficient mice show reduced peripheral nerve conduction velocities and decreased axonal diameters and respond to exogenous IGF-I treatment. J Neurobiol. 1999;39:142–152. doi: 10.1002/(sici)1097-4695(199904)39:1<142::aid-neu11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gaudino G, Avantaggiato V, Follenzi A, Acampora D, Simeone A, Comoglio PM. The proto-oncogene RON is involved in development of epithelial, bone and neuro-endocrine tissues. Oncogene. 1995;11:2627–2637. [PubMed] [Google Scholar]
- Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, Godowski PJ, Comoglio PM. Ron is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Rustioni A. Expression of three forms of nitric oxide synthase in peripheral nerve regeneration. J Neurosci Res. 1999;55:198–207. doi: 10.1002/(SICI)1097-4547(19990115)55:2<198::AID-JNR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Clark HB, Ross ME. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke. 1996;27:1373–1380. doi: 10.1161/01.str.27.8.1373. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:157–164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Cayouette MH, Berkemeier LR, Clatterbuck RE, Price DL, Rosenthal A. Neurotrophin 4/5 is a trophic factor for mammalian facial motor neurons. Proc Natl Acad Sci USA. 1994;91:3304–3308. doi: 10.1073/pnas.91.8.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Iwama A, Tatsumi J, Ikeda K, Suda T. Macrophage-stimulating protein activates STK receptor tyrosine kinase on osteoclasts and facilitates bone reabsorption by osteoclast-like cells. Blood. 1996;87:3704–3710. [PubMed] [Google Scholar]
- Kurihara N, Tatsumi J, Arai F, Iwama A, Suda T. Macrophage-stimulating protein (MSP) and its receptor Ron stimulate human osteoclast activity but not proliferation: effect of MSP distinct from that of hepatocyte growth factor. Exp Hemathol. 1998;26:1080–1085. [PubMed] [Google Scholar]
- Isackson PJ, Nisco SJ, Bradshaw RA. Expression of the alpha subunit of 7S nerve growth factor in the mouse submandibular gland. Neurochem Res. 1987;12:959–966. doi: 10.1007/BF00966319. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Skeel AH. Isolation of macrophage stimulating protein (MSP) from human serum. Exp Cell Res. 1978;114:117–126. doi: 10.1016/0014-4827(78)90043-5. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Skeel AH. Enhancement of spreading, phagocytosis and chemotaxis by macrophage stimulating protein (MSP) Adv Exp Med Biol. 1979;121B:181–194. doi: 10.1007/978-1-4684-8914-9_16. [DOI] [PubMed] [Google Scholar]
- Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Maggiora P, Marchiò S, Stella MC, Giai M, Belfiore A, De Bortoli M, di Renzo MF, Costantino A, Sismondi P, Comoglio PM. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- Maina F, Hilton MC, Andres R, Wyatt S, Klein R, Davies AM. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20:835–846. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- Medico E, Mongiovi A, Huff J, Jelinek MA, Follenzi A, Gaudino G, Parsons JT, Comoglio PM. The tyrosine kinase receptors Ron and Sea control “scattering”and morphogenesis of liver progenitors cells in vitro. Mol Biol Cell. 1996;7:495–504. doi: 10.1091/mbc.7.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoa RS, Sun WY, Colbert MC, Waltz SE, Witte DW, Degen JL, Degen SJ. The Ron/STK receptor tyrosine kinase is essential for peri-implantation development in the mouse. J Clin Invest. 1999;103:1277–1285. doi: 10.1172/JCI6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, Birchmeier W, Comoglio PM. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney LB, Skeel A, Luan J, Polis S, Richmond A, Wang MH, Leonard EJ. Proteolytic cleavage and activation of pro-macrophage-stimulating protein and upregulation of its receptor in tissue injury. J Dermatol Invest. 1998;111:578–581. doi: 10.1046/j.1523-1747.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu SF, Zhuang HX, Marsh DJ, Ishii DN. Insulin-like growth factor-II increases and IGF is required for postnatal rat spinal motoneuron survival following sciatic nerve axotomy. J Neurosci Res. 1999;55:9–16. doi: 10.1002/(SICI)1097-4547(19990101)55:1<9::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Quantin B, Schuhbaur B, Gesnel MC, Dollè P, Breathnach R. Restricted expression of the RON gene encoding the macrophage stimulating protein receptor during mouse development. Dev Dyn. 1995;204:383–390. doi: 10.1002/aja.1002040405. [DOI] [PubMed] [Google Scholar]
- Ruan RS, Leong SK, Yeoh KH. The role of nitric oxide in facial motoneuronal death. Brain Res. 1995;698:163–168. doi: 10.1016/0006-8993(95)00887-v. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Thery C. Involvement of Livertine, a hepatocyte growth factor family member, in neural morphogenesis. Mech Dev. 1996;60:207–220. doi: 10.1016/s0925-4773(96)00618-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry. 1993;100:431–40. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Skeel B, Yoshimura T, Showalter SD, Tanaka S, Appella E, Leonard E. Macrophage stimulating protein: purification, partial amino acid sequence, and cellular activity. J Exp Med. 1991;173:1227–1234. doi: 10.1084/jem.173.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibroblast growth factors protect spinal motor neurones in vivo after experimental spinal cord injury. Eur J Neurosci. 1998;10:798–802. doi: 10.1046/j.1460-9568.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- Ternaux JP, Portalier P. Influence of tongue myoblasts on rat dissociated hypoglossal motoneurons in culture. Int J Dev Neurosci. 1993;11:33–48. doi: 10.1016/0736-5748(93)90033-a. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Mafong E, Meyer S. Central infusion of brain-derived neurotrophic factor and neurotrophin-4/5, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neuroscience. 1996;71:761–771. doi: 10.1016/0306-4522(95)00440-8. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Reichert-Preibsch H, Schmidt R, Pettmann B, Labordette G, Sensenbrenner M. Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc Natl Acad Sci USA. 1987;84:5459–5463. doi: 10.1073/pnas.84.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz SE, McDowell SA, Muraoka RS, Air EL, Flick LM, Chen YQ, Wang MH, Degen SJF. Functional characterization of domains contained in hepatocyte growth factor-like protein. J Biol Chem. 1997;272:30526–30537. doi: 10.1074/jbc.272.48.30526. [DOI] [PubMed] [Google Scholar]
- Wang MH, Cox GW, Yoshmura T, Sheffler LA, Skeel A, Leonard EJ. Macrophage-stimulating protein inhibits induction of nitric oxide production by endotoxin- or cytokine-stimulated mouse macrophages. J Biol Chem. 1994;269:14027–14030. [PubMed] [Google Scholar]
- Wang MH, Goniass SL, Skeel A, Wolf BB, Yoshimura T, Leonard EJ. Proteolytic activation of single-chain precursor macrophage-stimulating protein by nerve growth factor-γ and epidermal growth factor-binding protein, members of the kallikrein family. J Biol Chem. 1994b;269:13806–13810. [PubMed] [Google Scholar]
- Wang MH, Skeel A, Leonard EJ. Proteolytic cleavage and activation of pro-macrophage-stimulating protein by resident peritoneal macrophage membrane proteases. J Clin Invest. 1996;97:720–727. doi: 10.1172/JCI118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Skeel A, Yoshimura T, Copeland TD, Sakaguchi K, Leonard EJ. Antibodies to macrophage stimulating protein (MSP): specificity, epitope interactions, and immunoassay of MSP in human serum. J Leukoc Biol. 1993;54:284–295. doi: 10.1002/jlb.54.4.289. [DOI] [PubMed] [Google Scholar]
- Wang MH, Yoshimura T, Skeel A, Leonard EJ. Proteolytic conversion of single chain precursor macrophage-stimulating protein to a biologically active heterodimer by contact enzymes of the coagulation cascade. J Biol Chem. 1994a;269:3436–3440. [PubMed] [Google Scholar]
- Willett CG, Wang MH, Emanuel RL, Graham SA, Smith DI, Shridhar V, Sugarbaker DJ, Sunday ME. Macrophage-stimulating protein and its receptor in non-small-cell lung tumors: induction of receptor tyrosine phosphorylation and cell migration. Am J Respir Cell Mol Biol. 1998;18:489–496. doi: 10.1165/ajrcmb.18.4.2978. [DOI] [PubMed] [Google Scholar]
- Wink DA, Hanbauer I, Krishna MC, DeGraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VW, Nishiyama N, Schwartz JP. A culture model of reactive astrocytes: increased nerve growth factor synthesis and reexpression of cytokine responsiveness. J Neurochem. 1998;71:749–756. doi: 10.1046/j.1471-4159.1998.71020749.x. [DOI] [PubMed] [Google Scholar]
- Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yuhki N, Wang MH, Skeel A, Leonard EJ. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993;268:15461–15468. [PubMed] [Google Scholar]
- Yuen EC, Mobley WC. Early BDNF, NT-3, and NT-4 signaling events. Exp Neurol. 1999;159:297–308. doi: 10.1006/exnr.1999.7148. [DOI] [PubMed] [Google Scholar]
- Yu WH. Nitric oxide synthase in motor neurons after axotomy. J Histochem Cytochem. 1994;42:451–457. doi: 10.1177/42.4.7510317. [DOI] [PubMed] [Google Scholar]
- Yu WH. Regulation of nitric oxide synthase expression in motoneurons following nerve injury. Dev Neurosci. 1997;19:247–254. doi: 10.1159/000111213. [DOI] [PubMed] [Google Scholar]