Abstract
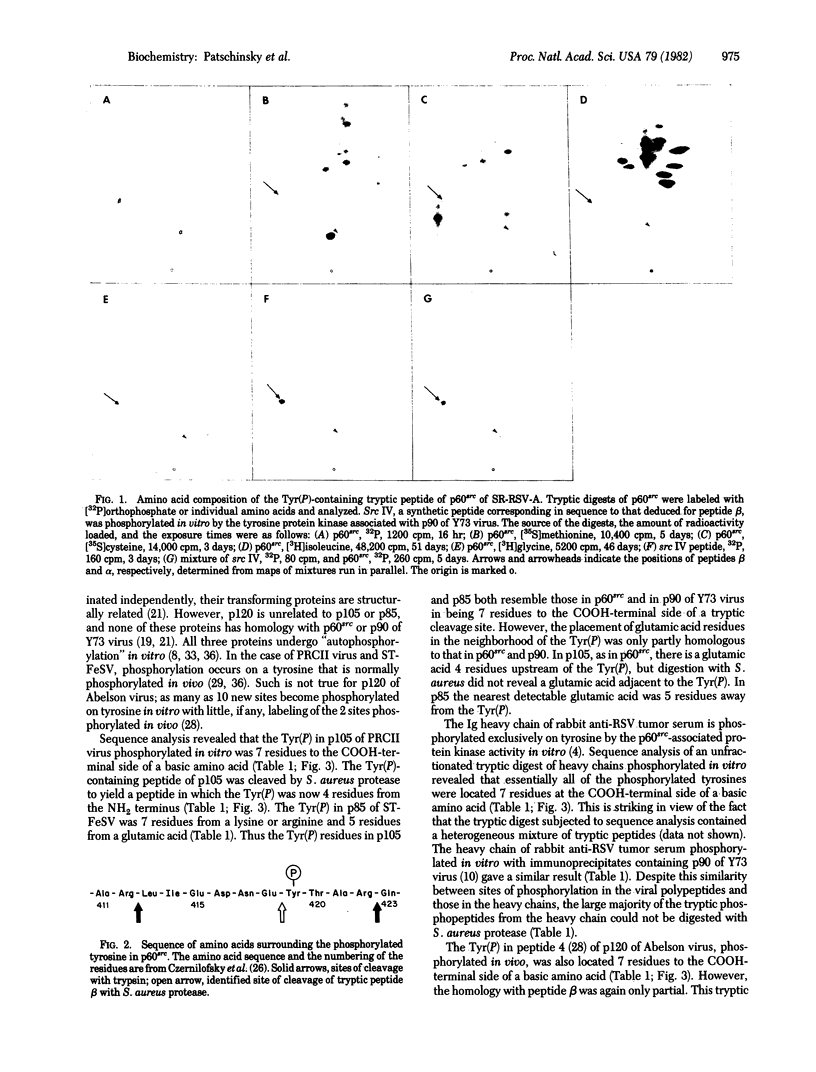
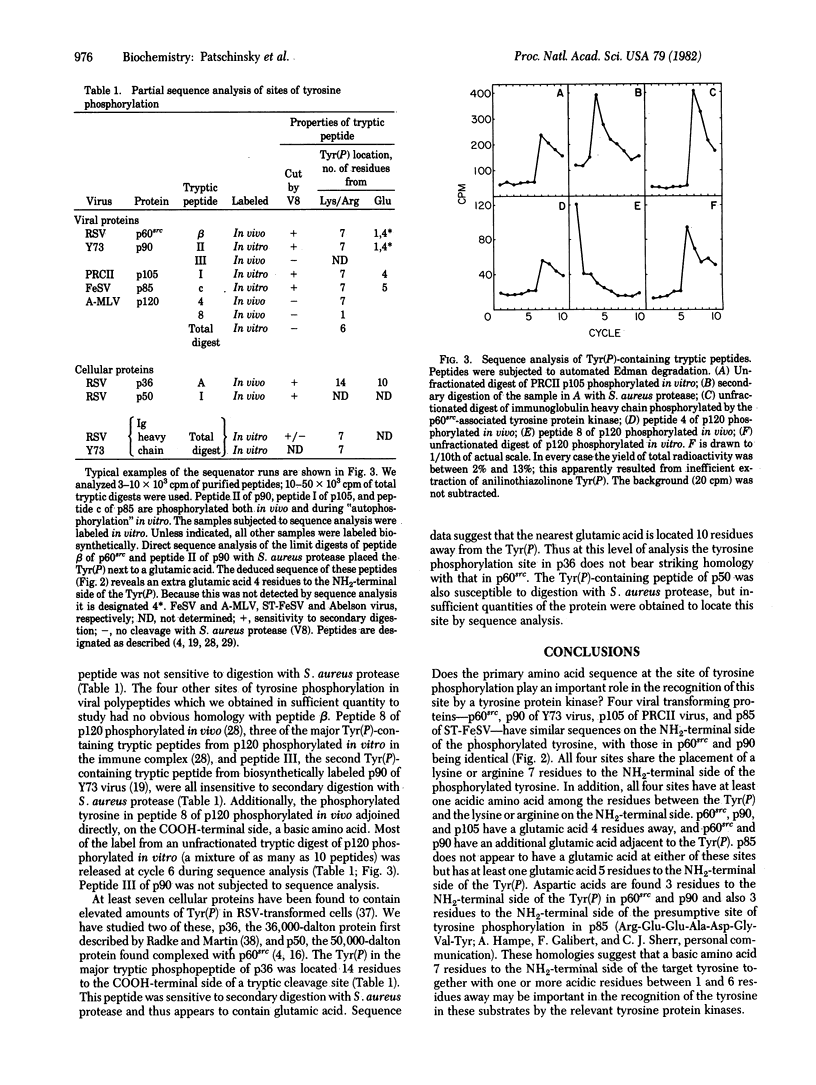
We have identified the single phosphorylated tyrosine in p60src, the transforming protein of Rous sarcoma virus, as part of the sequence. NH2-Arg-Leu-Ile-Glu-Asp-Asn-Glu-Tyr(P)-Thr-Ala-Arg-COOH. Therefore, this is a sequence that is recognized efficiently by a tyrosine protein kinase in vivo. Phosphorylation of tyrosine in cellular proteins appears to play a role in malignant transformation by four classes of genetically distinct RNA tumor viruses. Phosphorylated tyrosines in several other proteins resemble of the tyrosine in p60src in that they are located 7 residues to the COOH-terminal side of a basic amino acid and either 4 residues to the COOH-terminal side of, or in close proximity to, a glutamic acid residue. Therefore it is possible that these features play a role in the selection of sites of phosphorylation by some tyrosine protein kinases. However, several clear exceptions to this rule exist.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Blomberg J., Van de Ven W. J., Reynolds F. H., Jr, Nalewaik R. P., Stephenson J. R. Snyder-Theilen feline sarcoma virus P85 contains a single phosphotyrosine acceptor site recognized by its associated protein kinase. J Virol. 1981 Jun;38(3):886–894. doi: 10.1128/jvi.38.3.886-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Mercier J. C., Grosclaude F., Ribadeau-Dumas B. Structure primaire de la caséine s1 -bovine. Séquence complète. Eur J Biochem. 1971 Nov 11;23(1):41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K., Smart J. E. Homologous tyrosine phosphorylation sites in transformation-specific gene products of distinct avian sarcoma viruses. Nature. 1981 Jun 25;291(5817):675–677. doi: 10.1038/291675a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Sefton B. M. Evidence that there exist four classes of RNA tumor viruses which encode proteins with associated tyrosine protein kinase activities. J Virol. 1981 Jul;39(1):104–114. doi: 10.1128/jvi.39.1.104-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Raschke W. C. Evidence that the Abelson virus protein functions in vivo as a protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1552–1556. doi: 10.1073/pnas.78.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Bingham E. W., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Site-specific phosphorylation of casein variants. Eur J Biochem. 1979 Mar;94(2):497–504. doi: 10.1111/j.1432-1033.1979.tb12918.x. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M., Esch F. S. Amino-terminal sequence analysis of alphavirus polypeptides. J Virol. 1981 Jun;38(3):968–972. doi: 10.1128/jvi.38.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]



