Abstract
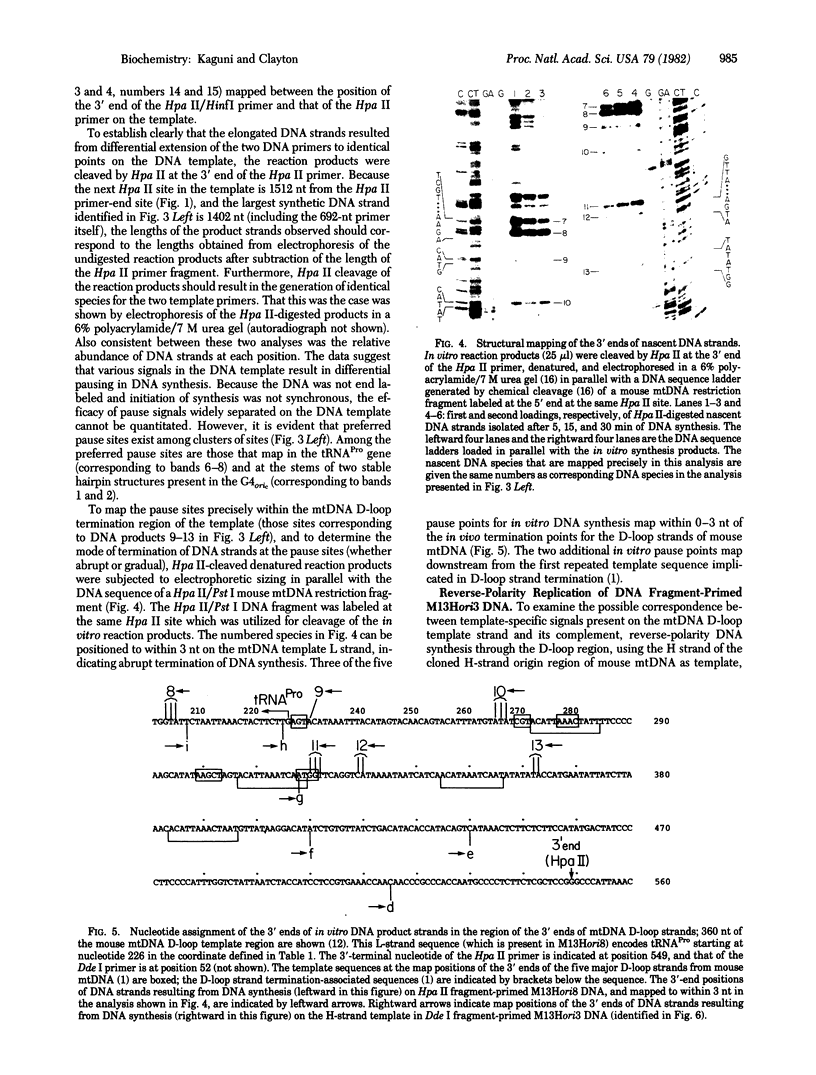
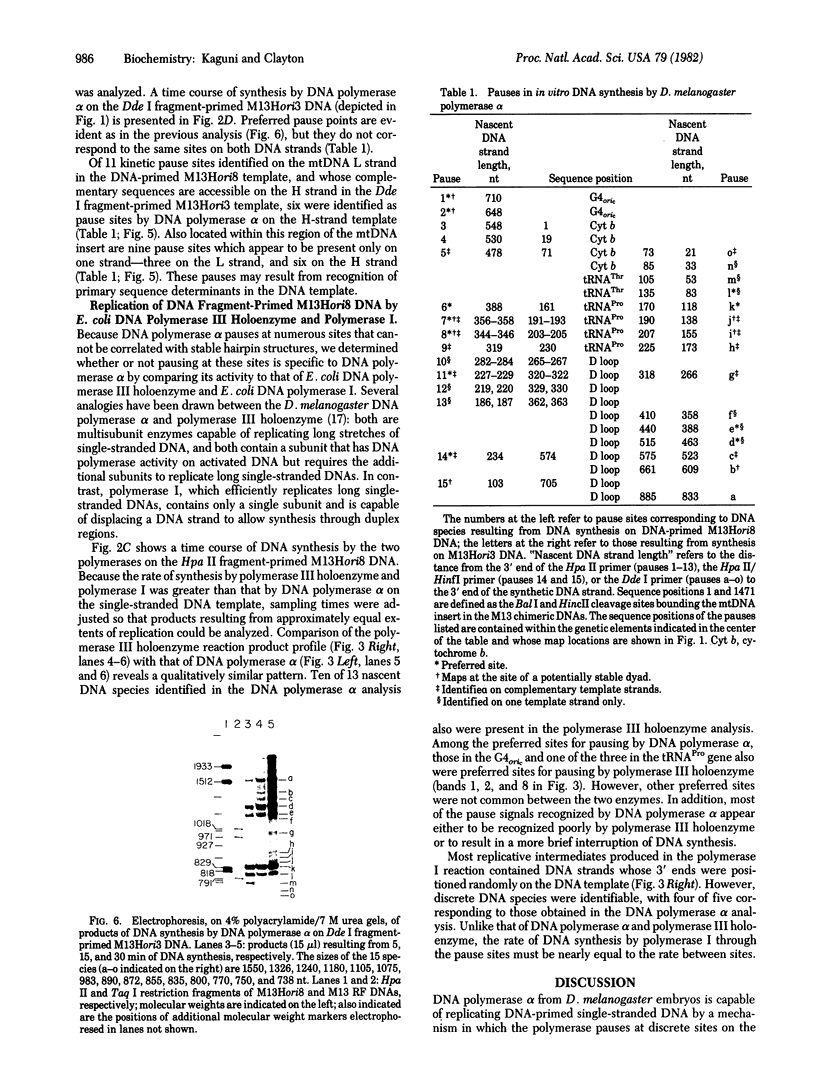
The activity of Drosophila melanogaster DNA polymerase alpha on DNA-primed single-stranded DNA templates has been examined. The DNA templates contain a 1471-nucleotide sequence from the heavy-strand origin region of mouse mtDNA inserted into the single-stranded bacteriophage vector M13Gori1. Preferred sites for pausing of in vitro DNA synthesis have been mapped within the cloned mtDNA insert and in the G4 cDNA strand origin which is contained within the vector DNA. Analysis of nascent DNA strands from replicative intermediates has revealed that pause sites are discrete and lie both at the positions of predicted stable dyads and in regions lacking the potential for formation of such structures. The patterns of kinetic pause sites observed for Escherichia coli DNA polymerase III holoenzyme is qualitatively similar to that found for DNA polymerase alpha. A subset of the observed kinetic pause signals are recognized by E. coli DNA polymerase I under similar conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Doda J. N., Wright C. T., Clayton D. A. Elongation of displacement-loop strands in human and mouse mitochondrial DNA is arrested near specific template sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6116–6120. doi: 10.1073/pnas.78.10.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Chen J. T., Korn D. Enzymological characterization of KB cell DNA polymerase-alpha. Regulation of template binding by nucleic acid base composition. J Biol Chem. 1981 Jan 10;256(1):133–141. [PubMed] [Google Scholar]
- Godson G. N., Barrell B. G., Staden R., Fiddes J. C. Nucleotide sequence of bacteriophage G4 DNA. Nature. 1978 Nov 16;276(5685):236–247. doi: 10.1038/276236a0. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Pauses at positions of secondary structure during in vitro replication of single-stranded fd bacteriophage DNA by T4 DNA polymerase. Anal Biochem. 1980 Mar 15;103(1):127–139. doi: 10.1016/0003-2697(80)90246-8. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sherman L. A., Gefter M. L. Studies on the mechanism of enzymatic DNA elongation by Escherichia coli DNA polymerase II. J Mol Biol. 1976 May 5;103(1):61–76. doi: 10.1016/0022-2836(76)90052-8. [DOI] [PubMed] [Google Scholar]
- Villani G., Fay P. J., Bambara R. A., Lehman I. R. Elongation of RNA-primed DNA templates by DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1981 Aug 10;256(15):8202–8207. [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]