Abstract
Objectives
We characterized two new CTX-M-type extended-spectrum β-lactamase (ESBL) variants in Escherichia coli isolates from stool samples of two elderly patients admitted at the Tel Aviv Sourasky Medical Center, Israel. Both patients underwent treatment with cephalosporins prior to isolation of the E. coli strains.
Methods
ESBLs were detected by the double-disk synergy test and PCR-sequencing of β-lactamase genes. The bla CTX-M genes were cloned into the pCR-BluntII-TOPO vector in E. coli TOP10. The role of amino-acid substitutions V77A and D240G was analyzed by site-directed mutagenesis of the bla CTX-M-94 and bla CTX-M-100 genes and comparative characterization of the resulting E. coli recombinants. MICs of β-lactams were determined by Etest. Plasmid profiling, mating experiments, replicon typing and sequencing of bla CTX-M flanking regions were performed to identify the genetic background of the new CTX-M variants.
Results
The novel CTX-M β-lactamases, CTX-M-94 and -100, belonged to the CTX-M-25-group. Both variants differed from CTX-M-25 by the substitution V77A, and from CTX-M-39 by D240G. CTX-M-94 differed from all CTX-M-25-group enzymes by the substitution F119L. Glycine-240 was associated with reduced susceptibility to ceftazidime and leucine-119 with increased resistance to ceftriaxone. bla CTX-M-94 and bla CTX-M-100 were located within ISEcp1 transposition units inserted into ∼93 kb non-conjugative IncFI and ∼130 kb conjugative IncA/C plasmids, respectively. The plasmids carried also different class 1 integrons.
Conclusions
This is the first report on CTX-M-94 and -100 ESBLs, novel members of the CTX-M-25-group.
Introduction
CTX-Ms are the most prevalent extended-spectrum β-lactamases (ESBLs) in Enterobacteriaceae causing hospital- and community-acquired infections [1]–[3]. The bla CTX-M genes are usually located on plasmids, and are derivatives of chromosomal β-lactamase genes of the Kluyvera genus due to multiple mobilization events [1], [4]. Usually, these plasmids easily spread in microbial populations, also carrying other resistance genes such as those coding for aminoglycoside acetyltransferases, dihydropteroate synthases or other β-lactamases [3]. New CTX-M variants arise rapidly and so far 131 different enzymes of this family have been identified (http://www.lahey.org/studies). They can all be assigned to five different subfamilies, based on amino acid identity: the CTX-M-1, -2, -8, -9, and -25 groups [1]. New CTX-M variants within these groups emerge by the gradual accumulation of mutations, some of which affect enzyme activity and resistance phenotype, and are being selected by antibiotic pressure [5], [6]. Unlike the CTX-M-1, -2 and -9 groups, the CTX-M-25-like β-lactamases have been rarely observed worldwide [7]–[9]. However, the situation in Israel is in stark contrast as a remarkable number of CTX-M-25-group enzymes, namely CTX-M-25, -26, 39 and -41, have been previously reported in there [10], [11]. We recently identified two new CTX-M-25-group variants, CTX-M-94 and -100, in Escherichia coli isolates from patients admitted at the Tel Aviv Sourasky Medical Center, Israel. The aim of this study was to characterize CTX-M-94 and -100 and to understand the phylogenetic relationships between the novel and the known CTX-M-25-group variants.
Methods
Bacterial Strains
Two E. coli clinical strains were recovered in 2008–2009 during an epidemiological study from screening stool samples of two elderly patients hospitalized at the Tel Aviv Sourasky Medical Center, Israel. These isolates were identified using mass spectrometry (MALDI-TOF, Bruker Daltonics, Bremen, Germany). E. coli TOP10 electrocompetent cells (Invitrogen, Carlsbad, CA, USA) were utilized as hosts for cloning and site-directed mutagenesis experiments, and rifampicin-resistant E. coli A15 was used as a recipient in mating tests [12].
Phenotypic ESBL Detection and Susceptibility Testing
The ESBL phenotype of the two clinical isolates was confirmed by the double-disk synergy test using cefotaxime, ceftazidime, cefepime and amoxicillin/clavulanate disks [13]. Susceptibility to β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, ampicillin, aztreonam, cefepime, cefotaxime, cefoxitin, ceftazidime, ceftazidime/clavulanate, ceftriaxone, imipenem, piperacillin, and piperacillin/tazobactam) was tested by disk diffusion (disks from Oxoid, Basingstoke, UK) and by Etest (bioMérieux, Marcy l'Etoile, France) according to the CLSI guidelines [14].
Genotypic ESBL Detection and blaCTX-M Characterization
Total DNA was extracted from the isolates by alkaline lysis as described previously [15]. Plasmid DNA was obtained from the isolates with the PureLink HiPure Plasmid Miniprep Kit according to the manufacturer’s protocol (Invitrogen). β-lactamase genes bla CTX-M, bla SHV, and bla TEM were detected by PCR using universal primers with modified cycling conditions: 10 min at 95°C, 30 cycles of 30s at 95°C, 30s at 59°C, and 1 min at 72°C, and 10 min at 72°C [16]–[18]. The entire bla CTX-M-94 and bla CTX-M-100 genes were amplified using previously described primers CTX-M-25-F (specific for the ISEcp1 element) and CTX-M-25-R primers with modified cycling conditions: 10 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 55°C, and 1.5 min at 72°C, and 10 min at 72°C [10]. The amplicons were sequenced directly on both strands, compared to known bla CTX-M sequences (GenBank and Lahey Clinic), and analyzed using BLASTn (NCBI, http://www.ncbi.nlm.nih.gov) and the Lasergene software (DNASTAR, Madison, WI, USA). The extrachromosomal location of bla CTX-M-94 and bla CTX-M-100 was confirmed by amplification from plasmid DNA in triplicate, as well as by plasmid electroporation into E. coli TOP10.
AmpC Detection
Production of AmpC-like β-lactamases was assessed by the combination disk test with cefotaxime and ceftazidime disks, with and without phenylboronic acid (20 µl of 20 mg/mL solution per disk). A≥5 mm increase in zone diameter after addition of boronate indicated the higher-level expression of AmpC. Genes coding for acquired AmpC types were identified by PCR-sequencing analysis on plasmid DNA with target-specific primer pairs [19].
Strain Typing and Phylogrouping
Sequence types (STs) of the E. coli strains were determined by multilocus sequence typing (MLST) [20], using the MLST database at the ERI, University College Cork (http://mlst.ucc.ie/mlst/dbs/Ecoli). Phylogenetic grouping was performed by PCR [21].
Detection of Enzymatic Activity and Isoelectric Focusing of β-lactamases
The presence of functional β-lactamases was evidenced by a qualitative method utilizing the chromogenic cephalosporin nitrocefin (Calbiochem, Merck Chemicals, Nottingham, UK). Hydrolysis of nitrocefin by β-lactamases results in a distinct colour shift from yellow (λmax = 390 nm at pH 7.0) to red (λmax = 486 nm at pH 7.0). A nitrocefin solution (500 mg/L) was added to crude bacterial sonicates to show presence of functional β-lactamases. Sonicates positive for β-lactamase activity were subjected to isoelectric focusing (IEF) as described previously [12], in a Model 111 Mini IEF Cell (Bio-Rad, Hercules, CA, USA) The IEF gel was homogeneously covered with the nitrocefin solution to mark all bands corresponding to the different β-lactamases present in the bacterial cell extracts.
Cloning of blaCTX-M genes
Blunt-ended amplicons consisting of bla CTX-M genes and ∼30 bp upstream regions were produced using Platinum Pfx DNA Polymerase (Invitrogen) and the primer pair CTX-M-25-F/R with modified cycling conditions: 10 min at 94°C, 30 cycles of 15 s at 94°C, 30 s at 55°C, and 1.5 min at 68°C, and 10 min at 68°C. PCR products were cloned into the pCR-BluntII-TOPO vector (Invitrogen), electrotransformed into E. coli TOP10, and selected on LB agar supplemented with kanamycin (50 mg/L) and cefotaxime (2 mg/L). Transformants (pCR-CTX-M-94 and -100) were screened for presence of the bla CTX-M genes by PCR and sequencing using M13 primers according to the manufacturer’s protocol.
Site-directed Mutagenesis
Single point mutations were introduced into pCR-CTX-M-94 and -100 with the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to the manufacturer’s protocol. Mutagenic primer pairs (available on request) were designed to replace alanine at position 77 with valine (A77V) and glycine at position 240 with aspartic acid (G240D). The mutagenized pCR-CTX-M-94 and -100 plasmids were electrotransformed into E. coli TOP10 and their susceptibility profiles to β-lactams were screened by Etest.
Plasmid Typing
Total plasmid DNA obtained from the clinical isolates was electroporated into E. coli TOP10 to separate coexisting multiple plasmids. Unique plasmids were identified by screening the transformants for presence of bla genes, including bla CTX-M, bla TEM and bla CMY-2, by PCR. Plasmid profiles of the transformants carrying unique plasmids were determined by restriction digestion of their plasmid DNA with HpaI (New England Biolabs, Ipswich, MA, USA). Restriction products were separated along with a 1 Kb DNA Extension Ladder (Invitrogen) on a 0.7% agarose gel for 10 h at 2.5 V/cm with 0.5x Tris-borate-EDTA buffer. Plasmid sizes were estimated by relative mobility calculations. Presence of bla genes was confirmed by hybridization with bla CTX-M-25-group-, bla TEM- and bla CMY-2-specific DIG-labeled probes and a DIG Luminescent Detection Kit (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s protocols. Replicon types of all unique plasmids were identified by PCR-based replicon typing as described previously [22]. Conjugation was carried out as described previously [12], utilizing the clinical strains producing CTX-M-94 and -100 as donors and rifampicin-resistant E. coli A15 strain as a recipient. Transconjugants were selected on LB agar with cefotaxime (2 mg/L) and rifampicin (128 mg/L), and confirmed by the phenotypic disk tests, PCR of bla genes, and MLST against the donors and recipient. Transfer efficiencies were calculated based on the ratio of CFUs/mL of transconjugants per donor cell.
Nucleotide Sequence Accession Numbers
bla CTX-M-94 and bla CTX-M-100 nucleotide sequences were assigned GenBank accession numbers HM167760 and FR682582, respectively.
Results and Discussion
E. coli Isolates Harbouring Novel CTX-M Variants
We recovered the CTX-M-94 and -100-producing E. coli strains from screening stool samples of two elderly hospitalized patients. The patients received various antibiotics, including cephalosporins (ceftriaxone), β-lactam/inhibitor combinations (piperacillin/tazobactam), cephamycins and carbapenems (imipenem). The CTX-M-94-producing E. coli belonged to ST131 and phylogroup B2. ST131 is a uropathogenic clone that has spread worldwide, usually associated with ESBLs, in particular with CTX-M-15 of the CTX-M-1 group [3], [23], [24]. Along with CTX-M-94, the E. coli isolate also harboured two other acquired β-lactamases, TEM-1 and the AmpC-like cephalosporinase CMY-2. The CTX-M-100-producing E. coli belonged to ST88 (clonal complex ST23) and phylogroup A. Strains belonging to phylogroup A are generally more common in the intestinal flora, and both the complex ST23 and the clone ST88 belong to the globally spread members of the phylogroup [25]. The CTX-M-100-harbouring isolate did not express any other acquired β-lactamase.
MICs of β-lactams for the CTX-M-94- and -100-producing isolates are shown in Table 1. The CTX-M-94 harbouring isolate exhibited four- to approximately 200-fold higher MICs of amoxicillin/clavulanate, cefoxitin, cefotaxime, ceftriaxone, ceftazidime (alone and with clavulanate), cefepime and aztreonam, when compared to the CTX-M-100 producer. According to the current CLSI guidelines [14], both isolates were highly resistant to amoxicillin, ampicillin, piperacillin, cefotaxime and ceftriaxone, and susceptible to piperacillin/tazobactam, cefepime and imipenem. The resistance pattern of the CTX-M-100-producing isolate was similar to that of the previously described CTX-M-39-producing E. coli from the same hospital [11], and matched well the typical CTX-M-associated phenotype, with asymmetry in MICs of cefotaxime and ceftazidime and good activity of inhibitor combinations [1], [5], [26]. In contrast, resistance of the CTX-M-94 producer was largely influenced by CMY-2, both in level and type. AmpC β-lactamases like CMY-2 are known to hydrolyze cephamycins and are much less inhibited by class A enzyme inhibitors compared to ESBLs [27], as exemplified by high MICs of cefoxitin and clavulanate combinations, respectively (Table 1). Furthermore, AmpC β-lactamases are also capable of hydrolyzing oxyiminocephalosporins and monobactams, as exemplified by elevated MICs of cefotaxime, ceftazidime, ceftriaxone and aztreonam (Table 1). Finally, the influence of CMY-2 on the phenotype exhibited by the wild-type CTX-M-94-harbouring isolate was confirmed by the decreased resistance pattern of pCR-CTX-M-94, a recombinant bla CTX-M-94-harbouring strain that lacked CMY-2. pCR-CTX-M-94 showed a major decrease in the MICs of cefoxitin (>256 vs. 2 mg/L), ceftazidime/clavulanate (>4 vs. 0.25 mg/L) and cefotaxime (>256 mg/L vs. 16 mg/L), and a more moderate decrease in the MICs of ceftriaxone (>256 vs. 48 mg/L), ceftazidime (12–16 vs. 4 mg/L) and amoxicillin/clavulanate (8 vs. 3 mg/L).
Table 1. MICs of β-lactams of the CTX-M-94- and -100-producing E. coli, E. coli TOP10 transformed with wild-type pCR-CTX-M-94 and -100 and mutagenized pCR-CTX-M-94 and -100.
| Antibiotics | Minimum inhibitory concentrations (mg/L) | |||||||
| E. coli | E. coli | pCR-CTX-M-94 | pCR-CTX-M-94 | pCR-CTX-M-94 | pCR-CTX-M-100 | pCR-CTX-M-100 | pCR-CTX-M-100 | |
| (clinical isolate) | (clinical isolate) | (wild type) | (mutagenized) | (mutagenized) | (wild type) | (mutagenized) | (mutagenized) | |
| (CTX-M-94, TEM-1, CMY-2) | (CTX-M-100) | (A77, L119, G240) | (V77, L119, G240) | (A77, L119, D240) | (A77, F119, G240) | (V77, F119, G240) | (A77, F119, D240) | |
| ( = CTX-M-25) | ( = CTX-M-39) | |||||||
| Amoxicillin | >256* | >256* | >256* | >256* | >256* | >256* | >256* | >256* |
| Amoxicillin/clavulanate | 8** | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Ampicillin | >256* | >256* | >256* | >256* | >256* | >256* | >256* | >256* |
| Aztreonam | 6** | 2 | 4 | 4 | 1.50 | 4 | 4 | 1.50 |
| Cefepime | 8 | 1.50 | 2 | 2 | 1.50 | 2 | 2 | 1 |
| Cefotaxime | >256* | 12* | 16* | 16* | 4* | 16* | 24* | 1.50** |
| Cefoxitin | >256* | 1.50 | 2 | 4 | 1 | 2 | 4 | 1.50 |
| Ceftazidime | 12–16** | 1.50 | 4 | 4 | 0.50 | 3 | 3 | 0.38 |
| Ceftazidime/clavulanate | >4 | 0.06 | 0.25 | 0.25 | 0.19 | 0.25 | 0.25 | 0.19 |
| Ceftriaxone | >256* | 8* | 48* | 24* | 12* | 12* | 32* | 4* |
| Imipenem | 0.75–1.50 | 1.50 | 0.38 | 0.38 | 0.38 | 0.25 | 0.25 | 0.38 |
| Piperacillin | >256* | >256* | >256* | >256* | >256* | >256* | >256* | 64** |
| Piperacillin/tazobactam | 0.75 | 0.50 | 1.50 | 1 | 1 | 1.50 | 2 | 1.50 |
Indicates high-level resistance.
Indicates intermediate resistance.
Other differences in resistance could be attributed to molecular differences between CTX-M-94 and -100 enzymes, which are discussed in the following sections.
Molecular Characterization of Novel CTX-M Variants
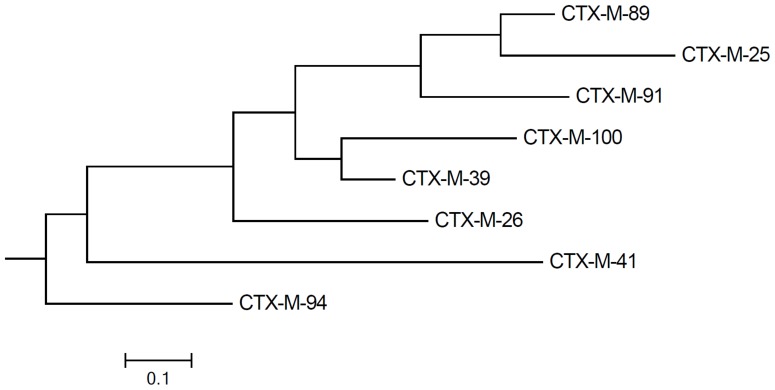
CTX-M-94 and -100 belonged to the CTX-M-25-group, sharing >99% homology with CTX-M-25 and -39 (Figure 1). Both variants differed from CTX-M-25 by the substitution V77A, and from CTX-M-39 by the substitution D240G. Additionally, CTX-M-94 was remarkable in that it had the substitution F119L (the only difference between CTX-M-94 and -100). This is the first report of a CTX-M-25-group member harbouring leucine-119. Leucine is usually present at position 119 in enzymes belonging to CTX-M-1, -2, -8, and -9 groups, with the exception of CTX-M-11 and -85 that have proline here. Its role in resistance towards β-lactams had not been studied before and was therefore investigated in this study by cloning experiments (see next section). Furthermore, silent nucleotide substitutions were absent in either bla CTX-M-94 or bla CTX-M-100 when compared with bla CTX-M-25 and bla CTX-M-39. Genetic relatedness of all known CTX-M-25-group enzymes, calculated using MEGA 5 (Figure 2) [28], suggested earlier branching of the CTX-M-94 branch, possibly due to the F119L substitution. It also indicated that CTX-M-100 is most closely related to CTX-M-39. IEF showed double bands, with pI values of 7.3 and 7.5, for both CTX-M-94 and -100 enzymes. A similar pattern has been shown previously for CTX-M-39, with pIs of 6.8 and 7.0, while CTX-M-25 has a pI of 7.5 [8], [10].
Figure 1. Comparison of the amino acid sequences of the novel CTX-M-25-group variants (CTX-M-94 and -100), with the known CTX-M-25-group enzymes (CTX-M-25 and -39).
Differences are highlighted in bold.
Figure 2. Phylogenetic tree of CTX-M-25-group subtypes. Scale: amino acid substitutions per 100 residues.
Functional Characterization of Wild-type and Mutagenized CTX-Ms
To determine the resistance patterns conferred by the new CTX-M variants and the specific role of the F119L substitution, bla CTX-M-94 and bla CTX-M-100 with upstream regions were cloned in pCR-BluntII-TOPO and expressed in the isogenic background of E. coli TOP10 (Table 1). The analysis revealed high-level resistance towards amoxicillin, ampicillin, piperacillin, cefotaxime and ceftriaxone for both CTX-M-94 and -100 producers without significant differences in MIC values, except for a four-fold lower ceftriaxone MIC in the CTX-M-100 producer (12 mg/L) when compared to that with CTX-M-94 (48 mg/L).
To investigate the role of the V77A and D240G substitutions in the sequence background of CTX-M-25-group β-lactamases, reverse mutations were introduced separately into the bla CTX-M-94 and bla CTX-M-100 genes. In CTX-M-100, the replacement of alanine-77 with valine and glycine-240 with aspartic acid resulted in a reproduction of the protein sequences of CTX-M-25 and -39, respectively. When compared with CTX-M-94 and -100 wild-type strains, the G240D revertants conferred, as expected, a remarkable decrease in the MICs of ceftazidime (4–3 vs. 0.50–0.38 mg/L), and a more moderate decrease in the MICs of aztreonam (4 vs. 1.50 mg/L), cefotaxime (16 vs. 4–1.50 mg/L) and ceftriaxone (48–12 vs. 12–4 mg/L). In addition, the G240D substitution caused a remarkable decrease in the MICs of piperacillin (>256 vs. 64 mg/L) in CTX-M-100, while having no effect in CTX-M-94. These data fit well with previous crystallographic studies showing that glycine-240, located in the B3 β-strand which lines the active site, is a major contributor to ceftazidime resistance. When aspartic acid at position 240 is replaced by glycine, the mobility of the B3 β-strand is increased, resulting in enhanced protein flexibility and facilitation of hydrolysis of β-lactams with bulkier side chains. However, this increased mobility is not only associated with increased activity, but also with decreased stability of the CTX-M enzyme [5], [29]. A recent in vitro-evolution study demonstrated that cefotaxime and ceftazidime probably acted as diversifying agents in the evolution of CTX-M-1 group enzymes [6]. One of the three distinct trajectories analyzed was the D240G route wherein CTX-Ms carrying glycine-240 exhibited moderate increases in ceftazidime MICs, while maintaining high cefotaxime MICs. It was proposed that D240G allows for better adaptation of CTX-M producers to the concurrent exposure to both drugs. Most probably, CTX-M-94 and -100 illustrate this phenomenon in the evolution of the CTX-M-25-group enzymes.
The A77V substitution caused a two-fold increase in cefoxitin MICs (2 vs. 4 mg/L) in CTX-M-94 and-100, and only a minor increase in cefotaxime MICs (16 vs. 24 mg/L) in CTX-M-100. It had no effect on the MICs of ceftazidime. In contrast to our results, Novais et al. observed a minor decrease in cefoxitin MICs and a two-fold increase in MICs of cefotaxime and ceftazidime after introducing A77V into CTX-M-15 [6]. These conflicting outcomes might be explained by differences between CTX-M-1- and -25- group enzymes in amino acid residues that line the highly conserved active site. CTX-M-15, a prototypal CTX-M-1 group enzyme, differs from CTX-M-94 and -100 at positions 103 (valine and isoleucine, respectively) and 133 (valine and threonine, respectively) that lie in the α structural domain of the CTX-M enzyme and in close proximity to the Ω loop (based on the active site structure described in Delmas et al. [30]. Finally, the A77V substitution caused a two-fold decrease (MICs, 48 vs. 12 mg/L) and a three-fold increase (MICs, 12 vs. 32 mg/L) in ceftriaxone MICs in CTX-M-94 and -100, respectively.
Plasmid Typing
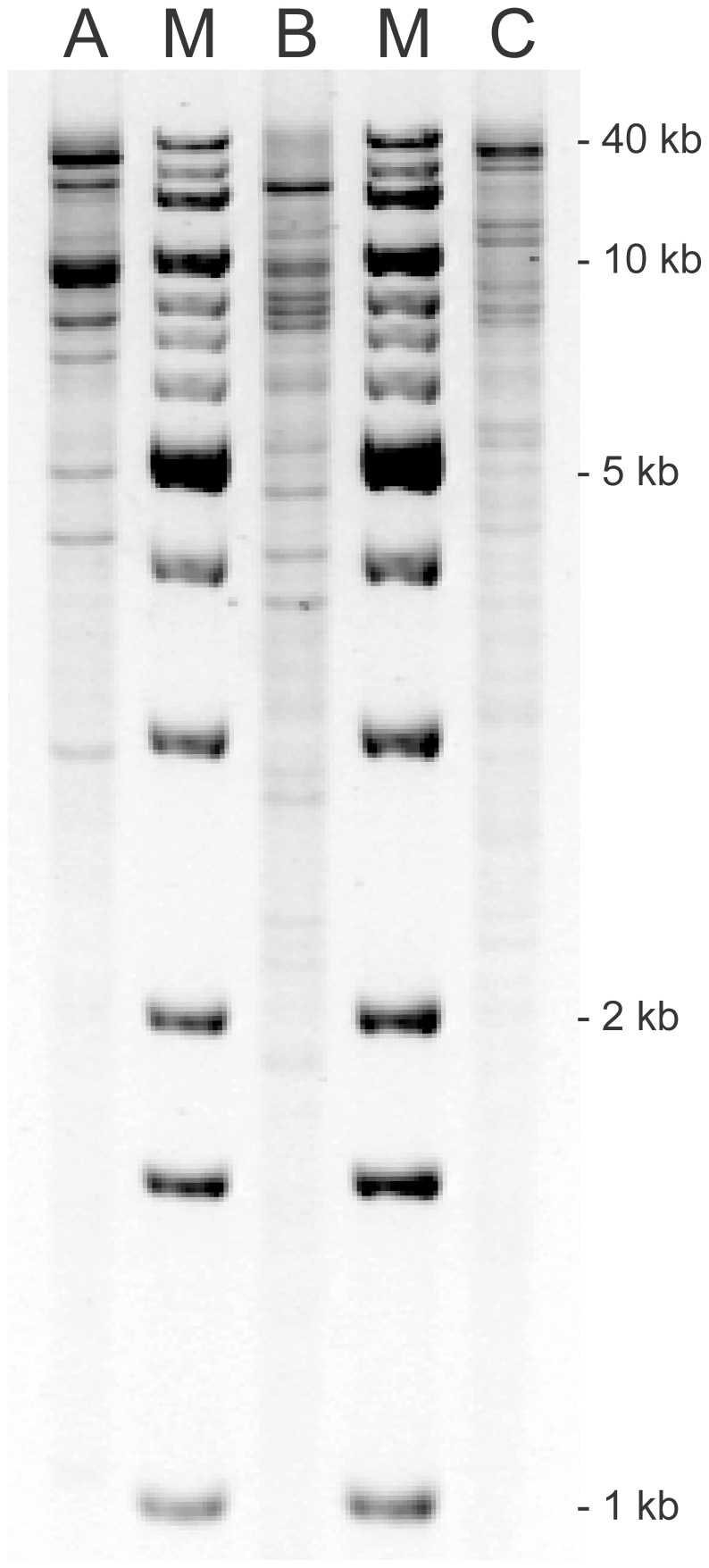
Replicon typing and HpaI plasmid profiling (Figure 3) revealed that bla CTX-M-94 was located on a ∼93 kb IncFI plasmid and bla CTX-M-100 on a ∼130 kb IncA/C plasmid. The bla CMY-2 gene, which was also present in the CTX-M-94-producing isolate, was located on a second ∼98 kb IncI1 plasmid. The bla CTX-M-100-harbouring plasmid was transferred to recipient cells with a high efficiency (5.4×10−2 per donor cell). On the other hand, the bla CTX-M-94-carrying plasmid did not transfer. Instead, the bla CMY-2-harbouring plasmid was transferred with an efficiency of 3.8×10−2 per donor cell.
Figure 3. HpaI plasmid profiles of bla CTX-M-94-, bla CMY-2- and bla CTX-M-100-harbouring plasmids.
A 0.7% agarose gel was loaded with HpaI restricted plasmid DNA purified from three recombinant E. coli TOP10 strains containing the (A) bla CTX-M-94-, (B) bla CMY-2- and (C) bla CTX-M-100-harbouring plasmids, respectively, and stained with GelRed. Relative mobility calculations, based on known band sizes of the 1 Kb DNA Extension Ladder (M), estimated total plasmid sizes to be (A) 93 kb, (B) 98 kb and (C) 130 kb.
Genetic Environment of blaCTX-M Genes
Both bla CTX-M-94 and bla CTX-M-100 were located 36 bp downstream of ISEcp1, the most probable factor of mobilization of these genes from the K. georgiana chromosome [31], and sequences separating the genes from ISEcp1 were identical to each other. Similar distances were previously reported for bla CTX-M-25 and bla CTX-M-26 identified in Canada and the UK, respectively [8]. Interestingly, other bla CTX-M-25-like genes identified in Israel were found to be located 126–128 bp downstream of ISEcp1 [11]. These findings suggest that CTX-M-94 and -100 might not have emerged from other CTX-M-25-group members identified in Israel so far, but from an independent ISEcp1-mediated mobilization of a precursor K. georgiana gene.
This assumption seems to be further supported by the lack of the direct association of bla CTX-M-94 and bla CTX-M-100 with a class 1 integron, demonstrated by a PCR-sequencing based class 1 integron analysis as described previously [11]. Unlike the above mentioned bla CTX-M-25-like genes identified in Israel, which were inserted into a class 1 integron [11], the ISEcp1-bla CTX-M-94 and ISEcp1-bla CTX-M-100 transposition modules were not present in the variable regions of the integrons identified in the bla CTX-M-94- and bla CTX-M-100-containing plasmids. These integrons significantly differed from each other. The one present on the plasmid with bla CTX-M-94 harboured aacC1 (aminoglycoside acetyltransferase; resistance to gentamicin), orfX (hypothetical protein), orfQ (hypothetical protein) and aadA1 (aminoglycoside adenylyltransferase; resistance to spectinomycin and streptomycin) gene cassettes, while the one on the plasmid with bla CTX-M-100 harboured aadB (aminoglycoside adenylyltransferase; resistance to gentamicin/kanamycin/tobramycin), ereA (erythromycin esterase type 1; resistance to erythromycin) and aadA1. For now, it is unclear whether CTX-M-94 and -100 have emerged from each other or from other CTX-M-25-group members by independent mutational events. Because of limited publicly available sequence data, it is not possible to make more global assumptions on the evolutionary paths of the CTX-M-25-group enzymes.
Conclusions
This is the first report describing two novel ESBL variants of the CTX-M-25-group, CTX-M-94 and -100, identified in two E. coli isolates belonging to different clones, ST131 and ST88, respectively. Both bla CTX-M-94 and bla CTX-M-100 were found within ISEcp1 transposition units, located on ∼93 kb non-conjugative IncFI and ∼130 kb conjugative IncA/C plasmids, respectively. CTX-M-94 is the first CTX-M-25-group member carrying leucine-119, which was shown here to be associated with increased resistance to ceftriaxone. We have also confirmed the role of the D240G substitution in the increase of enzyme activity against ceftazidime in the context of the CTX-M-25-type enzymes.
Acknowledgments
We are grateful to the STEC Center at Michigan State University for providing the ECOR-01, -30, -39, and -65 strains, and also to the Health Protection Agency in London for providing the AmpC control strains.
Funding Statement
JV and M. Gazin are supported by funding from the European Community (MOSAR network contract LSHP-CT-2007-037941; SATURN network contract FP7-HEALTH-2009-SINGLE STAGE-N°241796). AB, AG, RI and M. Gniadkowski were also financed by the MOSAR-complementary grant No. 934/6. PR UE/2009/7 from the Polish Ministry of Science and Higher Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bonnet R (2004) Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canton R, Novais A, Valverde A, Machado E, Peixe L, et al. (2008) Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect 14 Suppl 1144–153. [DOI] [PubMed] [Google Scholar]
- 3. Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, et al. (2009) Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother 53: 4472–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlow M, Reik RA, Jacobs SD, Medina M, Meyer MP, et al. (2008) High rate of mobilization for blaCTX-Ms. Emerg Infect Dis 14: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gniadkowski M (2008) Evolution of extended-spectrum beta-lactamases by mutation. Clin Microbiol Infect 14 Suppl 111–32. [DOI] [PubMed] [Google Scholar]
- 6. Novais A, Comas I, Baquero F, Canton R, Coque TM, et al. (2010) Evolutionary trajectories of beta-lactamase CTX-M-1 cluster enzymes: predicting antibiotic resistance. PLoS Pathog 6: e1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGettigan SE, Hu B, Andreacchio K, Nachamkin I, Edelstein PH (2009) Prevalence of CTX-M beta-lactamases in Philadelphia, Pennsylvania. J Clin Microbiol 47: 2970–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munday CJ, Boyd DA, Brenwald N, Miller M, Andrews JM, et al. (2004) Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26. Antimicrob Agents Chemother 48: 4829–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Guo X, Zhang Q (2009) Prevalence characterization of extended-spectrum beta-lactamases among Escherichia coli isolates collected in Zhengzhou. J Clin Lab Anal 23: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chmelnitsky I, Carmeli Y, Leavitt A, Schwaber MJ, Navon-Venezia S (2005) CTX-M-2 and a new CTX-M-39 enzyme are the major extended-spectrum beta-lactamases in multiple Escherichia coli clones isolated in Tel Aviv, Israel. Antimicrob Agents Chemother 49: 4745–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauernfeind A, Grimm H, Schweighart S (1990) A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection 18: 294–298. [DOI] [PubMed] [Google Scholar]
- 13. Drieux L, Brossier F, Sougakoff W, Jarlier V (2008) Phenotypic detection of extended-spectrum beta-lactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect 14 Suppl 190–103. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute (2012) Performance Standards for Antimicrobial Susceptibility Testing: 22nd Informational Supplement M100-S22. Wayne, PA, USA: CLSI.
- 15. Malhotra-Kumar S, Wang S, Lammens C, Chapelle S, Goossens H (2003) Bacitracin-resistant clone of Streptococcus pyogenes isolated from pharyngitis patients in Belgium. J Clin Microbiol 41: 5282–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, et al. (2004) Complete nucleotide sequence of a 9kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother 48: 3758–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, et al. (2007) Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115: 1400–1408. [DOI] [PubMed] [Google Scholar]
- 18. Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, et al. (2003) Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agents Chemother 47: 3554–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perez-Perez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40: 2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wirth T, Falush D, Lan R, Colles F, Mensa P, et al. (2006) Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60: 1136–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clermont O, Bonacorsi S, Bingen E (2000) Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66: 4555–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, et al. (2005) Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63: 219–228. [DOI] [PubMed] [Google Scholar]
- 23. Ewers C, Grobbel M, Stamm I, Kopp PA, Diehl I, et al. (2010) Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-beta-lactamase-producing Escherichia coli among companion animals. J Antimicrob Chemother 65: 651–660. [DOI] [PubMed] [Google Scholar]
- 24. Peirano G, Richardson D, Nigrin J, McGeer A, Loo V, et al. (2010) High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-beta-lactamase-producing Escherichia coli isolates from Canada. Antimicrob Agents Chemother 54: 1327–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mammeri H, Poirel L, Fortineau N, Nordmann P (2006) Naturally occurring extended-spectrum cephalosporinases in Escherichia coli. Antimicrob Agents Chemother 50: 2573–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossolini GM, D'Andrea MM, Mugnaioli C (2008) The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect 14 Suppl 133–41. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby GA (2009) AmpC beta-lactamases. Clin Microbiol Rev 22: 161–182, Table of Contents. [DOI] [PMC free article] [PubMed]
- 28. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Delmas J, Sirot J, Shoichet B, Bonnet R (2005) Atomic resolution structures of CTX-M beta-lactamases: extended spectrum activities from increased mobility and decreased stability. J Mol Biol 348: 349–362. [DOI] [PubMed] [Google Scholar]
- 30. Delmas J, Chen Y, Prati F, Robin F, Shoichet BK, et al. (2008) Structure and dynamics of CTX-M enzymes reveal insights into substrate accommodation by extended-spectrum beta-lactamases. J Mol Biol 375: 192–201. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez MM, Power P, Sader H, Galleni M, Gutkind G (2010) Novel chromosome-encoded CTX-M-78 beta-lactamase from a Kluyvera georgiana clinical isolate as a putative origin of CTX-M-25 subgroup. Antimicrob Agents Chemother 54: 3070–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]