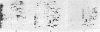Abstract
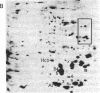
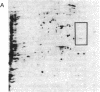
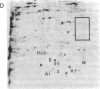
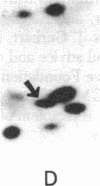
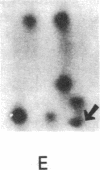
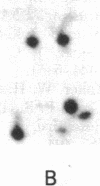
Xenopus laevis oocytes were injected with poly(A)+-mRNA isolated from chorionating follicular epithelium of the domesticated silk moth (Bombyx mori). On two-dimensional gel electrophoresis, the resultant translation products comigrated with authentic, secreted, chorion standards, demonstrating that the frog oocyte system synthesizes and correctly process virtually all major chorion components. A cDNA clone has been shown to contain sequences complementary to those of mRNAs encoding B mori high-cysteine (Hc) chorion proteins Hc6-Hc11. mRNAs were selected by hybridization to plasmid m5000 DNA bound to diazobenzyloxymethyl-cellulose and subsequently translated in X. laevis oocytes into forms that comigrated with authentic chorion standards. The selection of a distinct subset of Hc mRNAs under stringent hybridization conditions (70% formamide/0.2 M NaCl, 60 degrees C) suggests that they are encoded by related genes. This is consistent with the pattern obtained by hybridizing radioactive m5000 DNA to Southern blots prepared from EcoRI-cleaved B. mori chromosomal DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns A. J., van Kraaikamp M., Bloemendal H., Lane C. D. Calf crystallin synthesis in frog cells: the translation of lens-cell 14S RNA in oocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1606–1609. doi: 10.1073/pnas.69.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Kafatos F. C. Secretory kinetics in the follicular cells of silkmoths during eggshell formation. J Cell Biol. 1978 Jul;78(1):131–151. doi: 10.1083/jcb.78.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Kafatos F. C. The control of chorion protein synthesis in silkmoths: mRNA production parallels protein synthesis. Dev Biol. 1977 Jan;55(1):179–190. doi: 10.1016/0012-1606(77)90329-3. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R., Basehoar G. Organization of the chorion genes of Bombyx mori, a multigene family. I. Evidence for linkage to chromosome 2. Genetics. 1978 Oct;90(2):291–310. doi: 10.1093/genetics/90.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. R., Rattner E. C., Koehler M. M., Balikov S. R., Bock S. C. Two-dimensional electrophoresis of small-molecular-weight proteins. Anal Biochem. 1979 Oct 15;99(1):33–40. doi: 10.1016/0003-2697(79)90041-1. [DOI] [PubMed] [Google Scholar]
- Gross P. R. The control of protein synthesis in embryonic development and differentiation. Curr Top Dev Biol. 1967;2:1–46. doi: 10.1016/s0070-2153(08)60282-3. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A. Analysis of mRNA populations by cDNA.mRNA hybrid-mediated inhibition of cell-free protein synthesis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1217–1221. doi: 10.1073/pnas.75.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatrou K., Tsitilou S. G., Goldsmith M. R., Kafatos F. C. Molecular analysis of the GrB mutation in Bombyx mori through the use of chorion cDNA library. Cell. 1980 Jul;20(3):659–669. doi: 10.1016/0092-8674(80)90312-8. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Cavalieri R. L., Yaffe L., Pestka S. Synthesis and glycosylation of the MOPC-46B immunoglobulin in kappa chain in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Dec 7;79(3):625–630. doi: 10.1016/0006-291x(77)91157-3. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Familletti P., Pestka S. Synthesis and processing of the mouse MOPC-321 kappa chain in Xenopus laevis oocytes. Arch Biochem Biophys. 1979 Jan;192(1):290–295. doi: 10.1016/0003-9861(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Structure, organization and evolution of developmentally regulated chorion genes in a silkmoth. Cell. 1980 Dec;22(3):855–867. doi: 10.1016/0092-8674(80)90562-0. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Rosenthal N., Rodakis G. C., Kafatos F. C. Evolution of two major chorion multigene families as inferred from cloned cDNA and protein sequences. Cell. 1979 Dec;18(4):1317–1332. doi: 10.1016/0092-8674(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Regier J. C., Mazur G. D., Nadel M. R., Blau H. M., Petri W. H., Wyman A. R., Gelinas R. E., Moore P. B., Paul M. The eggshell of insects: differentiation-specific proteins and the control of their synthesis and accumulation during development. Results Probl Cell Differ. 1977;8:45–145. doi: 10.1007/978-3-540-37332-2_2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B., Crawford L. V. Translation of encephalomyocarditis viral RNA in oocytes of Xenopus laevis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3665–3669. doi: 10.1073/pnas.69.12.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Nadel M. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. IV. The chorion proteins of Bombyx mori and their program of synthesis. Dev Biol. 1980 Mar;75(1):26–40. doi: 10.1016/0012-1606(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Nadel M. R., Thireos G., Kafatos F. C. Effect of the pleiotropic GrB mutation of Bombyx mori on chorion protein synthesis. Cell. 1980 Jul;20(3):649–658. doi: 10.1016/0092-8674(80)90311-6. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C., Goodfliesh R., Hood L. Silkmoth chorion proteins: sequence analysis of the products of a multigene family. Proc Natl Acad Sci U S A. 1978 Jan;75(1):390–394. doi: 10.1073/pnas.75.1.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C. In vivo kinetics of pyrrolidonecarboxylic acid formation in selected silkmoth chorion proteins. J Biol Chem. 1981 Jun 25;256(12):6444–6451. [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C., Kramer K. J., Heinrikson R. L., Keim P. S. Silkmoth chorion proteins. Their diversity, amino acid composition, and the NH-terminal sequence of one component. J Biol Chem. 1978 Feb 25;253(4):1305–1314. [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Kafatos F. C., Jones C. W., Koehler M. D., Efstratiadis A., Maniatis T. Use of a cDNA library for studies on evolution and developmental expression of the chorion multigene families. Cell. 1979 Dec;18(4):1303–1316. doi: 10.1016/0092-8674(79)90241-1. [DOI] [PubMed] [Google Scholar]
- Thireos G., Kafatos F. C. Cell-free translation of silkmoth chorion mRNAs: identification of protein precursors and characterization of cloned DNAs by hybrid-selected translation. Dev Biol. 1980 Jul;78(1):36–46. doi: 10.1016/0012-1606(80)90316-4. [DOI] [PubMed] [Google Scholar]
- Tsitilou S. G., Regier J. C., Kafatos F. C. Selection and sequence analysis of a cDNA clone encoding a known chorion protein of the A family. Nucleic Acids Res. 1980 May 10;8(9):1987–1997. doi: 10.1093/nar/8.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]