Abstract
Background
Neuroprotective and neurotrophic properties of leukemia inhibitory factor (LIF) have been widely reported. In the central nervous system (CNS), astrocytes are the major source for LIF, expression of which is enhanced following disturbances leading to neuronal damage. How astrocytic LIF expression is regulated, however, has remained an unanswered question. Since neuronal stress is associated with production of extracellular adenosine, we investigated whether LIF expression in astrocytes was mediated through adenosine receptor signaling.
Methods
Mouse cortical neuronal and astrocyte cultures from wild-type and adenosine A2B receptor knock-out animals, as well as adenosine receptor agonists/antagonists and various enzymatic inhibitors, were used to study LIF expression and release in astrocytes. When needed, a one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test was used for statistical analysis.
Results
We show here that glutamate-stressed cortical neurons induce LIF expression through activation of adenosine A2B receptor subtype in cultured astrocytes and require signaling of protein kinase C (PKC), mitogen-activated protein kinases (MAPKs: p38 and ERK1/2), and the nuclear transcription factor (NF)-κB. Moreover, LIF concentration in the supernatant in response to 5′-N-ethylcarboxamide (NECA) stimulation was directly correlated to de novo protein synthesis, suggesting that LIF release did not occur through a regulated release pathway. Immunocytochemistry experiments show that LIF-containing vesicles co-localize with clathrin and Rab11, but not with pHogrin, Chromogranin (Cg)A and CgB, suggesting that LIF might be secreted through recycling endosomes. We further show that pre-treatment with supernatants from NECA-treated astrocytes increased survival of cultured cortical neurons against glutamate, which was absent when the supernatants were pre-treated with an anti-LIF neutralizing antibody.
Conclusions
Adenosine from glutamate-stressed neurons induces rapid LIF release in astrocytes. This rapid release of LIF promotes the survival of cortical neurons against excitotoxicity.
Keywords: 5′-N-Ethylcarboxamide (NECA), Leukemia inhibitory factor, Neuroprotection, Glutamate
Background
Leukemia inhibitory factor (LIF) is a soluble glycoprotein that belongs to the family of interleukin (IL)-6-type cytokines. Other members of this family include IL-6, IL-11, ciliary neurotrophic factor (CNTF), oncostatin M (OSM), cardiotrophin-1 (CT-1) and novel neurotrophin-1 (NNT-1) [1], which display pronounced trophic as well as protective properties during pathophysiology of the central nervous system (CNS) and are hence referred to as neuropoietic cytokines or neurokines [2]. Specific functions of LIF in the nervous system include induction of cholinergic differentiation of sympathetic neurons, induction of neuropeptide and choline acetyltransferase (ChAT) gene expression [3], regulation of polyneuronal innervation of neuromuscular junction [4,5] and regulation of the HPA axis [6,7]. Furthermore, LIF signaling is crucial for development of the nervous system, including development of sensory and motor neurons [8,9] and glial cells [10]. Consistently, reduced numbers of astrocytes and oligodendrocytes are found in LIF knock-out mice [11]. During inflammation, LIF has been suggested to be both pro- and anti-inflammatory and appears to play a key role in neural injury and regeneration. We and others have previously demonstrated the neuroprotective properties of LIF against damages caused by excitotoxicity, light, et cetera[12-14]. Moreover, promotion of axonal regeneration and oligodendrocyte growth and survival by LIF suggests its potential for reducing damage associated with central inflammatory demyelinating diseases such as multiple sclerosis [15-17].
In the CNS, astrocytes are considered to be the major source for LIF [18,19], and its expression in the brain is significant during pathological conditions including ischemia [20,21], multiple sclerosis [22], Alzheimer’s and Parkinson’s diseases [23] and brain injury [24]. The factors responsible for elevated LIF induction during CNS pathology are largely unknown. One of the candidates identified recently to induce LIF expression in astrocytes is ATP [18,25], levels of which also rise during conditions like high-frequency neuronal activity, seizure, ischemia and hypoxia [26,27]. However, extracellular ATP is rapidly hydrolyzed by a cascade of ectonucleotidases resulting in an enhanced level of adenosine [26,28]. Correspondingly, excitotoxic conditions such as ischemia, hypoxia, seizure and head injury are known to induce a rapid increase in extracellular adenosine concentrations, up to 100 times that of the resting concentration [29-33]. There is abundant evidence for immune regulation by adenosine [34] including expression and release of growth factors and cytokines such as nerve growth factor (NGF), S100beta, IL-6 and CCL2 in glial cells [35-39]. However, it is not known whether adenosine can induce LIF expression in astrocytes.
In the present study, we investigated the potential influence of adenosine receptor activity on LIF release from cultured astrocytes.
Methods
Chemicals and reagents
Neurobasal media, Hank’s balanced salt solution (HBSS), phosphate-buffered saline (PBS), sodium pyruvate, L-glutamine, penicillin-streptomycin, hydroxyethyl piperazineethanesulfonic acid (HEPES), glutaMAX-1 and B27 supplement were obtained from Gibco (Breda, The Netherlands). Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum (FCS) were obtained from PAA Laboratories (Cölbe, Germany). Trypsin was obtained from Life Technologies (Breda, The Netherlands). L-leucine methyl ester (LME) and the remaining cell medium components were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands). Recombinant mouse LIF (rmLIF: LIF2005) was obtained from Millipore (Amsterdam, The Netherlands). Brefeldin A (BFA), caffeine, L-glutamate, adenosine A2B receptor antagonist (MRS 1754), protein kinase A (PKA) inhibitor (KT 5720), protein kinase C (PKC) inhibitor (Ro 31–8220), p38 mitogen-activated protein kinases (MAPK) inhibitor (SB 203580), and adenosine analog (5′-N-Ethylcarboxamide or NECA) were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands). Non-hydrolysable ATP (2MeSATP), adenosine A2A receptor antagonist (ZM 241385), adenosine A2A receptor agonist (CGS 21680) and MEK1/2 inhibitor (U 0126) were obtained from Tocris Bioscience (Bristol, UK). NF-kB inhibitor (BAY 11–7082) and c-Jun N-terminal kinase (JNK) inhibitor (SP 600125) were obtained from Calbiochem (Darmstadt, Germany). Reagents used in immunoblotting experiments were purchased from Bio-Rad Laboratories (Veenendaal, The Netherlands) with the exception of the polyvinylidene fluoride (PVDF) membranes that were obtained from Millipore (Bedford, MA).
Animals
Wild-type C57BL/6 J (1 to 2 days postnatal) mice were obtained from Central Laboratory Animal Facility (University of Groningen, The Netherlands). Adenosine A2B receptor knock-out (A2B KO) mice (1 to 2 days postnatal) with the same genetic background were kindly provided by Professor Marco Idzko (University of Freiburg, Germany). Wild-type C57BL/6 J (14 to 15 days embryonic) mice were obtained from Harlan (Horst, The Netherlands). All procedures were in accordance with the regulation of the Ethical Committee for the use of experimental animals of the University of Groningen, The Netherlands (License number DEC 4623A and DEC 5913A). Animals were housed in standard Makrolon™TM (Bayer AG, Leverkusen, Germany) cages and maintained on a 12 hour light/dark cycle. They received food and water ad libitum.
Primary neuronal culture
Primary culture of cortical neurons from mouse embryo (~E15) was established as described previously [13]. Briefly, cortices from embryonic brains were dissected in ice-cold HBSS supplemented with 30% glucose. Meninges were removed, and the tissues were treated with trypsin before they were gently dissociated by trituration in neuronal culture media (neurobasal medium supplemented with 2% B27, 1 mM sodium pyruvate, 2 mM L-glutamine and 50 U/mL penicillin-streptomycin). The cell suspension was filtered using cell strainer (70 μm) (BD Falcon, Franklin Lakes, NJ, USA) before centrifugation (800 rpm for 10 minutes). Cells were then seeded on poly-D-lysine-coated six-well plates (1.5 x 106 cells/well) and maintained in neuronal culture media in a humidified atmosphere with 5% CO2 at 37°C. The culture medium was refreshed the next day to get rid of debris. The neuronal purity as determined by Microtubule-associated protein 2 (MAP2)-staining was around 98% (data not shown) [13]. Cultures were used after 5 days in vitro.
Induction of excitotoxicity
Cortical neuron cultures were subjected to an excitotoxic challenge with glutamate (50 μM, for 1 hour), after which cultures were refreshed with fresh media and were incubated at 37°C. Supernatants from neuron cultures (untreated and glutamate-treated) were collected 18 hours after glutamate challenge and were applied to the primary astrocyte cultures.
Primary astrocyte cultures
Primary astrocyte cultures were established from cerebral cortices of postnatal (1 to 2 days) C57BL/6 J and A2B KO mice according to a previously described procedure [40], which was modified to reduce microglial contamination [41]. Microglial cells were separated from the astrocytic monolayer by 1-hour shake-off at 150 rpm. This procedure was repeated two times with an interval of 4 days in vitro between each shake off, followed by an overnight shake-off at 240 rpm to remove oligodendrocyte precursor cells. Purified astrocytes were washed with HBSS buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA) and further detached using HBSS with 0.1% trypsin. Cells were reseeded with fresh astrocyte culture medium (DMEM supplemented with 5% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate and 50 U/mL penicillin-streptomycin) in multi-well plates (5 x 104 cells/cm2) and maintained in culture to confluency. To further reduce microglial contamination, confluent astrocyte cultures were treated with 5 mM LME, a lysosomotropic agent [42], for 4 to 5 hours. Astrocytes were ready for experiments after 1 to 2 days. Our cell preparations had a high percentage of astrocytes (≥95%), which was confirmed by immunostaining against GFAP (astrocyte specific marker) and CD11b (microglial specific marker) (data not shown).
Real-time polymerase chain reaction
Total RNA of primary astrocytes was extracted, purified and transcribed into cDNA as described previously [13]. Quality of the cDNA was examined using the following housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer pairs: Fw 5′-CATCCTGCACCACCAACTGCTTAG-3′ and Rev 5′-GCCTGCTTCACCACCTTCTTGATG-3′ [Accession number: NM-008084]. The effect of neuronal supernatants and NECA on LIF mRNA expression in cultured astrocytes was analyzed by real-time PCR (qPCR) using the iCycler and iQ™ SYBR™ Green supermix (Bio-Rad, Veenendaal, The Netherlands ). Mouse Hypoxanthine phosphoribosyltransferase 1 (HPRT1) and GAPDH primers were used for normalization to housekeeping genes (data normalized to HPRT1 are not shown), and these genes showed no variations in response to the experimental treatments. The primer pairs used for qPCR were: LIF (Fw 5′-ATGTGCGCCTAACATGACAG-3′ and Rev 5′-TATGCGACCATCCGATACAG-3′) [Accession number: NM-008501]; GAPDH (Fw 5′-ATGGCCTTCCGTGTTCCTAC-3′ and Rev 5′-GCCTGCTTCACCACCTTCTT-3′) [Accession number: AF106860] and HPRT1 (Fw 5′-GACTTGCTCGAGATGTCA-3′ and Rev 5′-TGTAATCCAGCAGGTCAG-3′) [Accession number: NM-013556]. The comparative Ct method (amount of target amplicon X in Sample S, normalized to a reference R and related to a control sample C), was calculated by:
| (1) |
and was used to determine the relative gene expression levels [43].
Western blot
Western blotting on cultured cortical astrocytes was performed as previously described [13]. Equal amounts of protein (30 μg) were loaded to 12.5 or 15% sodium dodecyl sulfate-polyacrylamide gels and subsequently transferred to PVDF membranes. The membranes were blocked using Odyssey™ Blocking Buffer (OBB; LI-COR Biosciences, Cambridge, UK; diluted 1:1 in PBS) for 1 hour and incubated overnight at 4°C with different combinations of primary antibodies (diluted in 1:1 OBB and PBS + 0.1% Tween 20 (PBS-T)): mouse monoclonal anti-β-actin (1:8000, Abcam, Cambridge, UK); rabbit monoclonal anti-phospho-NF-κB p65 (Ser536) (1:1000, Cell Signaling Technology, Leiden, The Netherlands); and rat monoclonal anti-LIF (MAB449; 1 μg/mL, R&D Systems, Oxford, UK). The next day, membranes were washed in PBS-T (four times for 5 minutes each time) and incubated for 1 hour at room temperature with appropriate fluorescence conjugated secondary antibodies (diluted in PBS-T): donkey anti-mouse IR Dye 680 (1:10000, LI-COR Biosciences, Cambridge, UK); goat anti-rat IR Dye 680 (1:10000, LI-COR); and donkey anti-rabbit IR Dye 800CW (1:10000, LI-COR). Membranes were washed again in PBS-T (four times for 5 minutes each time) and the fluorescent bands were detected using LI-COR’s Odyssey™ infrared imaging system.
Leukemia inhibitory factor ELISA
A total of 1 mL of supernatant was collected from each well of the six-well plates of primary mouse astrocyte cultures, and these samples were stored at −20°C. ELISA plates (96-well, Costar, Corning Life Sciences, Amsterdam, The Netherlands) were coated overnight at room temperature with 100 μl/well of primary antibody goat anti-LIF (AF449; 0.5 μg/mL, R&D Systems, Oxford, UK) diluted in 0.01 M PBS (pH 7.4). The following day, the plates were washed six times with wash buffer (0.25 M Tris–HCl pH 8, 0.15 M NaCl, 0.05% Tween-20) using an automated microplate washer and air dried (this step is repeated after each incubation step). Plates were subsequently incubated for 1 hour at room temperature with 200 μl/well of blocking buffer (0.01 M PBS, 2% BSA). After blocking, the plates were incubated with supernatants from astrocyte cultures (100 μl/well) for 2 hours at room temperature. Two dilutions (1:2 and 1:4) of each sample, diluted in incubation buffer (0.01 M PBS, 0.2% gelatin, 0.05% Tween-20), were made in triplicates. The plates were then incubated for 1 hour at room temperature with 100 μl/well of the detection antibody, biotinylated goat anti-LIF (BAF449; 0.05 μg/mL; R&D Systems, Oxford, UK) diluted in incubation buffer, followed by an incubation for 30 minutes at room temperature with 100 μl/well of Streptavidin-horseradish peroxidase (HRP) conjugate (1:8000, Sanquin Reagents, Amsterdam, The Netherlands). The plates were then incubated for 15 to 20 minutes at room temperature with 100 μl/well of TMB detection buffer (0.1 M acetate buffer, 0.1 M sodium-acetate, pH adjusted with 1 M citric acid (0.21 g/mL; dissolve 2 tablets of 3, 3′, 5, 5′-tetramethyl benzidinedihydrochloride in 11 mL of TMB buffer and add 2 μl of 30% H2O2)). Upon stable color formation the reactions were stopped by adding 100 μl/well of 1 M H2SO4. Absorbance of the samples was measured using VersaMax, a spectrophotometric ELISA plate reader, and SoftMax Pro software (Molecular Devices, CA, USA) at 450 nm, with a background correction at 575 nm. Recombinant mouse LIF (15 to 2000 pg/mL) was used to plot the standard curve.
MTT assay
Survival of cultured embryonic cortical neurons or cultured neonatal astrocytes against various experimental treatments was measured by the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl-) 2,5-diphenyltetrazolium bromide) assay, as described previously [44]. MTT solution (0.5 mg/mL final concentration) was added to cultured cells and incubated for 4 hours, after which, cells were lysed and MTT-formazan solubilized in dimethyl sulfoxide (DMSO) on an orbital shaker for 15 minutes. Optical density measure of each sample was determined using an automated ELISA reader - the Varioskan Flash spectral scanning multimode reader (Thermo Scientific, FL, USA) at 570 nm, with a background correction at 630 nm.
Immunocytochemistry and confocal microscopy
Astrocytes cultured on glass cover slips were fixed for 15 minutes in 4% paraformaldehyde. After several washes in PBS, the cells were blocked for 45 minutes with 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in PBS containing 0.1% TritonX (Sigma, Zwijndrecht, The Netherlands). The cover slips were then incubated overnight at 4°C with rat anti-LIF primary antibody (5 μg/mL, R&D Systems, Oxford, UK) in combination with one of the following primary antibodies: rabbit anti-Rab11 (1:400, Zymed, San Francisco, CA, USA); rabbit anti-chromogranin A & B (1:100, Novus Biologicals, Cambridge, UK); mouse anti-clathrin (1:1000, Abcam, Cambridge, UK); rabbit anti-pHogrin C-terminal (1:100, kind gift of Professor J.C. Hutton (Denver, USA)) and rabbit anti-giantin (1:1000, Covance, Princeton, NJ, USA). The following day, cells were rinsed three times with PBS and incubated for 1 hour with the appropriate secondary antibodies: donkey anti-rat CY3 (1:500, Jackson ImmunoResearch Laboratories, Uden, The Netherlands); donkey anti-rabbit Alexa Fluor 488 (1:500, Molecular Probes, Leiden, The Netherlands)and donkey anti-mouse Alexa Fluor 488 (1:500, Molecular Probes). The cover slips were then rinsed with PBS and mounted on microscopic slide with Mowiol (Sigma, Zwijndrecht, The Netherlands) and analyzed with a Leica SP2 AOBS system (Leica Microsystems, Rijswijk, The Netherlands). Pictures were deconvoluted using the software Huygens Pro (SVI, Hilversum, The Netherlands). Primary antibody omission served as the control.
Statistical data analysis
The absolute data values were normalized to the control in order to allow multiple comparisons. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test, using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA). In all cases, P values < 0.05 were considered statistically significant.
Results
Glutamate-challenged cortical neurons induce LIF expression in cultured astrocytes through adenosine receptor activation
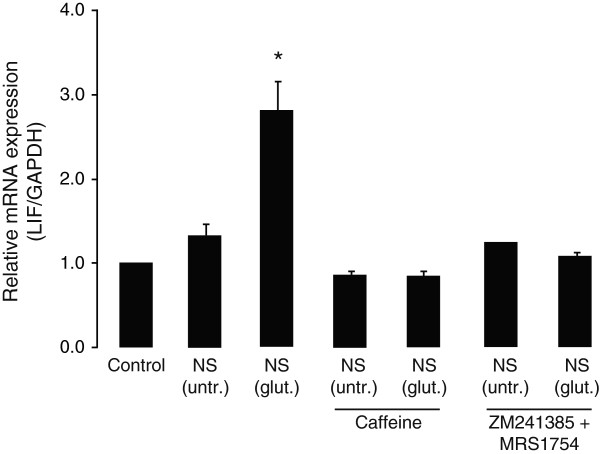
We have previously shown that treatment of cultured cortical neurons with glutamate (50 μM, for 1 hour) reduces cell survival by 60%, when compared to untreated controls [13]. In order to investigate whether neuronal death induces LIF synthesis in astrocytes, supernatants from cultured cortical neurons were collected 18 hours after glutamate treatment (50 μM for 1 hour) and applied to cultured cortical astrocytes. It is shown here that treatment of cultured astrocytes for 2 hours with supernatant from untreated neurons did not change LIF expression (Figure 1). On the other hand, supernatant from glutamate-challenged neurons induced approximately three times greater expression of astrocytic LIF mRNA (Figure 1). The induction of LIF mRNA expression by glutamate-challenged neuronal supernatants was absent in the presence of the non-specific adenosine receptor antagonist (caffeine, 50 μM) (Figure 1) and by cocktail of the specific adenosine A2 receptors antagonists (A2A antagonist: ZM 241385, 250 nM; A2B antagonist: MRS 1754, 250 nM) (Figure 1), suggesting that enhanced LIF expression in astrocytes induced by glutamate-challenged neuronal supernatants is mediated through adenosine A2A and/or A2B receptor subtypes.
Figure 1.
Glutamate-stressed cortical neurons induce leukemia inhibitory factor (LIF) gene expression in primary cortical astrocytes, by a mechanism dependent on astrocytic adenosine receptor activation. Supernatants of primary mouse cortical neurons (NS) collected 18 hours following treatment without (untr.) or with glutamate (glut.; 50 μM, for 1 hour) were applied (1:1 dilution by volume) to the primary cultured cortical astrocytes for 2 hours. Where indicated, astrocytes were pre-treated with caffeine (50 μM) or a cocktail of adenosine A2A and A2B receptor antagonists (A2A antagonist: ZM 241385, 250 nM; A2B antagonist: MRS 1754, 250 nM), for 30 minutes before incubation with neuronal supernatants and were analyzed for LIF mRNA expression (relative to GAPDH) using real-time PCR. Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05.
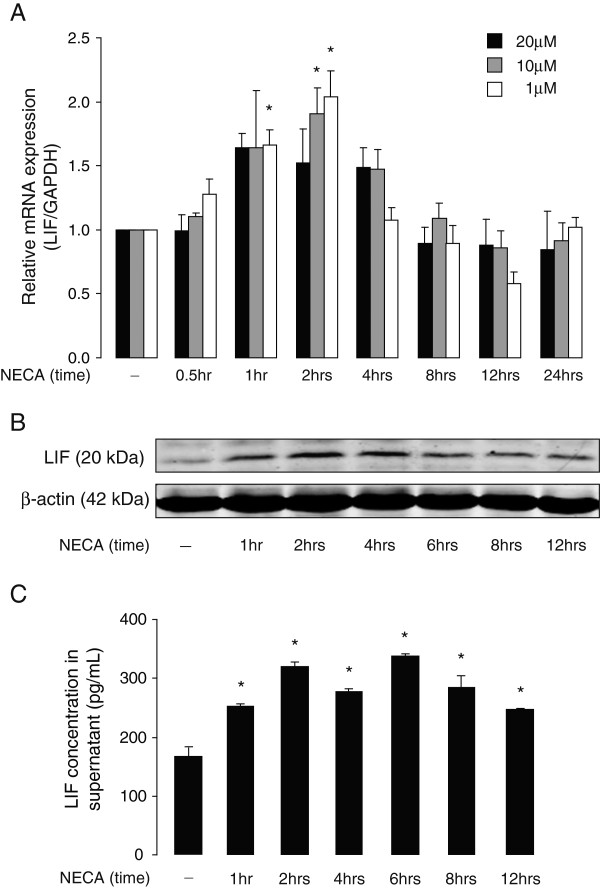
NECA-induced LIF expression and secretion levels in cultured mouse astrocytes is concentration- and time-dependent
In order to further investigate adenosine receptor-mediated LIF expression in astrocytes, we used NECA, a non-selective adenosine receptor agonist. As shown in Figure 2A, NECA-induced LIF mRNA expression in cultured astrocytes was concentration- and time-dependent, with maximum induction after 2 hours of incubation with 1 and 10 μM NECA. Subsequently, the effect of NECA (1 μM) on LIF protein expression was analyzed by Western blot. Elevated LIF protein expression was detected after 1 hour of NECA treatment, with a maximum induction after 2 to 4 hours (Figure 2B). Consistently, ELISA analysis revealed LIF protein content in supernatants from untreated astrocyte cultures, which increased in time upon treatment with NECA (1 μM) (Figure 2C).
Figure 2.
NECA increases leukemia inhibitory factor (LIF) expression and secretion levels in primary mouse astrocytes. (A) Primary cortical astrocytes were treated without or with NECA (1, 10 or 20 μM) for 0.5, 1, 2, 4, 8, 12 and 24 hours. Cells were then analyzed for LIF mRNA expression (relative to GAPDH) by real-time PCR. Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05. (B) shows Western blot analysis of cultured astrocytes treated without or with NECA (1 μM) for 1, 2, 4, 6, 8 and 12 hours to determine LIF protein levels. β-actin served as a loading control. (C) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured astrocytes that were treated without or with NECA (1 μM) for 1, 2, 4, 6, 8 and 12 hours. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. NECA, 5′-N-ethylcarboxamide.
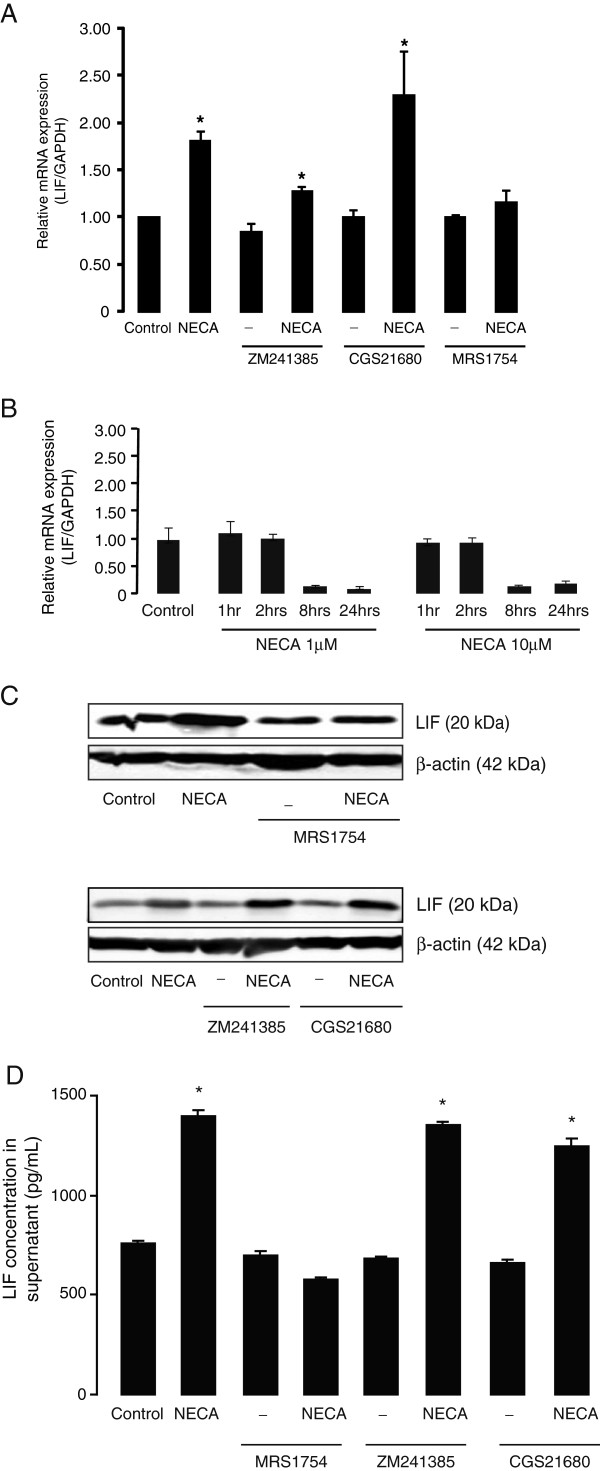
NECA-induced LIF expression and secretion levels is dependent on adenosine A2B receptor activation
In subsequent experiments, specific antagonists of adenosine A2A and A2B receptors (ZM 241385 and MRS 1754, respectively) were used to identify the receptor subtype involved in NECA-induced LIF expression and release in cultured astrocytes. Pre-treatment of astrocytes with ZM 241385 (250 nM, added 30 minutes before NECA) did not abolish NECA-induced LIF mRNA and protein expression (Figure 3A and 3C). In contrast, NECA-induced LIF mRNA (Figure 3A) and protein expression (Figure 3C) and release (Figure 3D) were completely inhibited by MRS 1754 pre-incubation (250 nM, added 30 minutes before NECA). In addition, specific adenosine A2A receptor agonist (CGS 21680; 1 μM) failed to induce LIF mRNA or protein expression and release (Figure 3A, C, D). The involvement of A2B receptors was further confirmed in A2B KO astrocytes where NECA stimulation for up to 24 hours did not induce LIF mRNA expression (Figure 3B). Instead, astrocytes without A2B receptors responded to NECA stimulation with a down-regulation of LIF mRNA at 8 and 24 hours (Figure 3B). Taken together, these results clearly show that NECA-induced LIF expression and release from cultured mouse astrocytes is dependent on the activation of adenosine A2B receptors.
Figure 3.
NECA-induced leukemia inhibitory factor (LIF) expression and secretion levels in primary astrocytes are dependent on adenosine A2Breceptor activation. Primary cortical astrocytes were treated with the adenosine analog, NECA (1 μM) or a selective adenosine A2A receptor agonist (CGS 21680, 1 μM) for 2 hours (for real-time PCR) or 4 hours (for Western blot and ELISA). Where indicated, astrocytes were pre-treated for 30 minutes with selective adenosine A2A receptor antagonist (ZM 241385, 250 nM) or adenosine A2B receptor antagonist (MRS 1754, 250 nM), prior to NECA stimulation. (A and B) show real-time PCR analyses of LIF gene expression (relative to GAPDH) in wild-type and A2B knock-out astrocytes, respectively. Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05. (C) shows Western blot analyses to detect LIF protein levels in wild-type astrocytes. β-actin served as a loading control. (D) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured wild-type astrocytes. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. NECA, 5′-N-ethylcarboxamide.
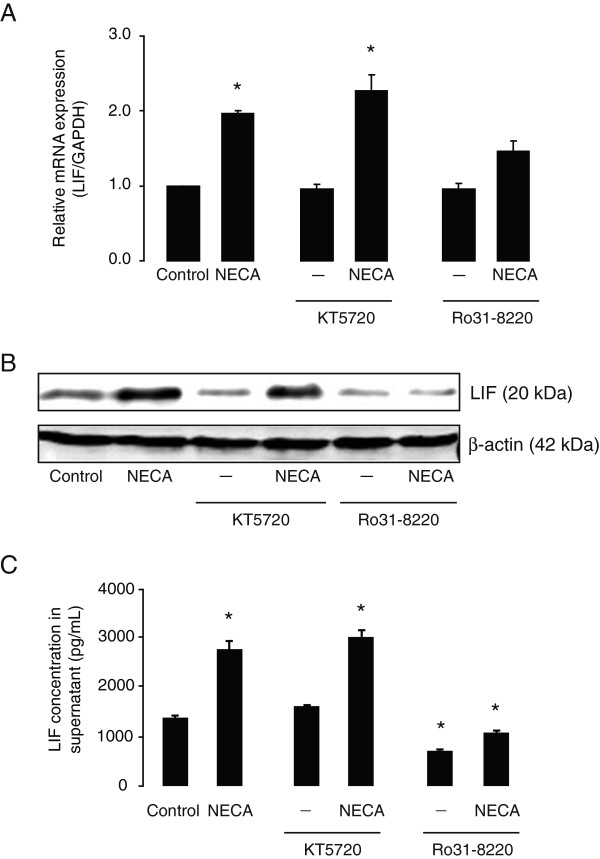
NECA-induced LIF expression and secretion levels in primary astrocytes are mediated through the Gq/11-PLC-PKC and MAPKs, but not through Gs-cAMP-PKA pathway.
In order to analyze the intracellular signaling pathways that couple A2B receptor activity and LIF expression and release in astrocytes, various specific blockers of signaling routes were used. To determine the potential toxicity of these blockers, cultured astrocytes were incubated for 24 hours with the used and the doubled concentration of these blockers, and cellular survival was assessed by MTT assay. None of the used blockers at the appropriate concentration caused significant toxicity; the only blocker that negatively influenced astrocytic survival at the double concentration was the NF-κB inhibitor BAY 11-7082 (Figure 4).
Figure 4.
Effect of signaling pathway inhibitors on survival of cultured astrocytes. Primary cortical astrocytes were treated without or with different signaling pathway inhibitors used in this study (U 0126, 5 to 10 μM; SB 203580, 10 to 20 μM; BAY 11–7082, 10 to 20 μM; Ro 31–8220, 250 to 500nM; KT 5720, 250 to 500nM; SP 600125, 10 to 20 μM) for 24 hours and cell survival assessed by a colorimetric MTT assay. The optical densities were measured at 570 nm, with a 630 nm and blank correction. Data are normalized to percent of control (vehicle) and presented as Mean ± S.E.M of three independent experiments. P < 0.05, when compared to control.
Adenosine A2B receptors are coupled to two types of G-proteins: Gs and Gq/11[45]. Activation of Gs proteins stimulates cyclic AMP (cAMP) leading to PKA activation or Exchange Protein Activated by cAMP (EPAC) signaling pathways [46], whereas Gq/11 proteins stimulate PKC via phospholipase C (PLC). In order to determine which pathway downstream of the A2B receptor is responsible for NECA-induced LIF expression and release, we used specific inhibitors of PKA and PKC (KT 5720 and Ro 31–8220, respectively) [47]. Pre-incubation of astrocytes with KT 5720 (250 nM, added 30 minutes before NECA) did not affect NECA-induced LIF expression (both mRNA and proteins) (Figure 5A and 5B). However, pre-treatment with Ro 31-8220 (250 nM, added 30 minutes before NECA) reduced NECA-induced LIF mRNA (Figure 5A) as well as protein levels (Figure 5B). Consistently, Ro 31-8220, but not KT 5720, suppressed LIF content in the supernatant from NECA-treated astrocyte cultures (Figure 5C), indicating that adenosine A2B receptor-mediated LIF expression and release from astrocytes require PKC, but not PKA, activation.
Figure 5.
NECA-induced leukemia inhibitory factor (LIF) expression and secretion levels in primary astrocytes are blocked by protein kinase C (PKC) inhibitor, but not by protein kinase A (PKA) inhibitor. Primary cortical astrocytes were treated without or with NECA (1 μM) for 2 hours (for real-time PCR) or 4 hours (for Western blot and ELISA). Where indicated, astrocytes were pre-treated for 30 minutes with specific inhibitors of PKA (KT 5720, 250 nM) or PKC (Ro 31–8220, 250 nM), prior to NECA stimulation. (A) shows real-time PCR analysis of LIF gene expression (relative to GAPDH). Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05. (B) shows Western blot analysis to detect LIF protein levels. β-actin served as a loading control. (C) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured astrocytes. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. NECA, 5′-N-ethylcarboxamide.
Basal and NECA-induced LIF expression and secretion levels in primary astrocytes are dependent on ERK1/2- and p38- but not on JNK-MAPK activation
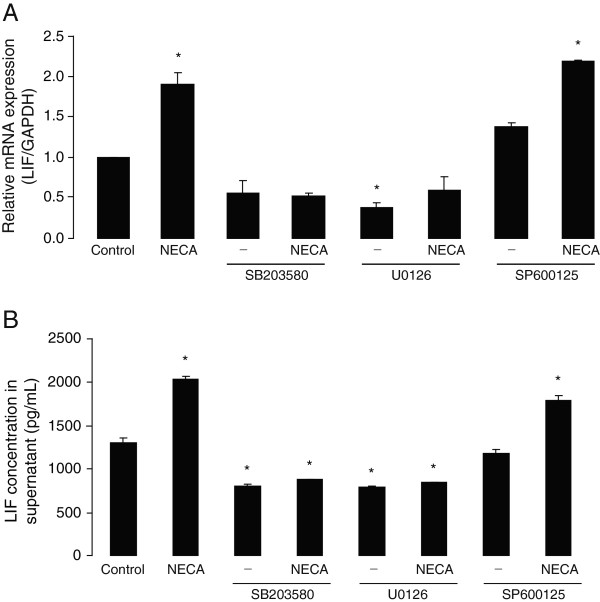
Activation of the PKC pathway has been associated with effects mediated through mitogen-activated protein kinase (MAPK) signaling [48,49]. In order to determine the involvement of MAPKs in NECA-induced LIF expression and release, specific inhibitors of the three MAPK cascades: p38, extracellular signal-regulated kinase (ERK) 1/2, and c-Jun N-terminal kinase (JNK) (SB 203580, U 0126 and SP 600125, respectively), were used. It is shown here that pre-treatment (2 hours before NECA) of cultured astrocytes with SB 203580 (10 μM) and U 0126 (5 μM) significantly reduced basal as well as NECA-induced LIF mRNA expression (Figure 6A) and protein release (Figure 6B). On the other hand, pre-treatment with SP 600125 (10 μM, added 2 hours before NECA) affected neither basal, nor NECA-induced LIF mRNA expression or protein release (Figure 6A and 6B), suggesting that both p38 and ERK1/2, but not JNK, are important for basal, as well as NECA-induced, LIF expression and release in cultured astrocytes.
Figure 6.
Basal and NECA-induced leukemia inhibitory factor (LIF) expression and secretion levels in primary astrocytes are dependent on ERK1/2- and p38- but not on JNK-MAPK activation. Primary cortical astrocytes were treated without or with NECA (1 μM) for 2 hours (for real-time PCR) or 4 hours (for ELISA). Where indicated, astrocytes were pre-treated for 2 hours with specific inhibitors of ERK1/2 (U 0126, 5 μM), p38 (SB 203580, 10 μM) or JNK (SP 600125, 10 μM) pathways, prior to NECA stimulation. (A) shows real-time PCR analysis of LIF gene expression (relative to GAPDH). Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05. (B) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured astrocytes. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NECA, 5′-N-ethylcarboxamide.
Basal and NECA-induced LIF expression and secretion levels in primary astrocytes are dependent on NF-κB activation
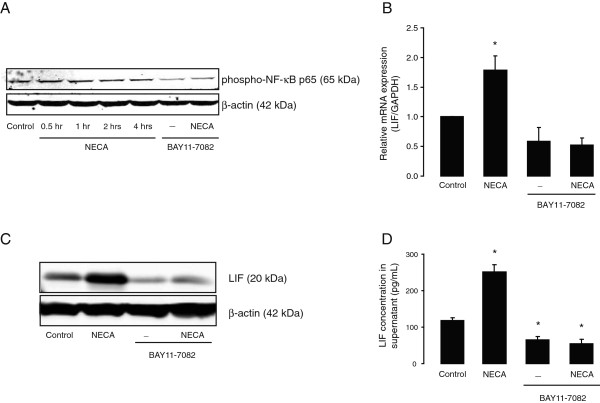
IL-6 gene expression in cultured astrocytes is enhanced by NECA [36,38], similar to our present findings with LIF. Since NF-κB is a key transcription factor that regulates IL-6 gene expression [50,51], we wondered whether NECA-induced LIF gene expression is similarly regulated by NF-κB. Analysis of the mouse LIF promoter region (using Genomatix-MatInspector software (http://www.genomatix.de/)), identified multiple consensus binding sequences for NF-κB at the first 500 bp upstream to the transcription start site (data not shown). Treatment of cultured astrocytes with NECA (1 μM) for 0.5, 1, 2 and 4 hours, induced activation of NF-κB, which was detected by phosphorylation of NF-κB p65 subunit by Western blot (Figure 7A). In addition, the specific inhibitor of NF-κB activation, BAY 11-7082 (10 μM; added 2 hours before NECA) reduced NECA-induced phosphorylation of NF-κB p65 (Figure 7A) and significantly inhibited basal as well as NECA-induced LIF expression (both mRNA and protein) and release (Figure 7B–D), strongly indicating that LIF gene expression is regulated by NF-κB.
Figure 7.
Basal and NECA-induced leukemia inhibitory factor (LIF) expression and secretion levels in primary astrocytes are dependent on NF-κB activation. (A) shows Western blot analysis of primary cortical astrocytes treated without or with NECA (1 μM; for 0.5, 1, 2 and 4 hours) to detect phosphorylation at Ser536 of NF-κB p65 (RelA) proteins. Where indicated, cells were pre-treated with selective inhibitor of NF-κB (BAY 11–7082, 10 μM) for 2 hours prior to NECA stimulation. β-actin served as a loading control. Subsequently, astrocytes were treated without or with NECA (1 μM) for 2 hours (for real-time PCR) and 4 hours (for Western blot and ELISA), in presence or absence of BAY 11–7082 (10 μM, added 2 hours prior to NECA stimulation). (B) shows real-time PCR analysis of LIF gene expression (relative to GAPDH). Data are normalized to the control and presented as Mean ± SEM of three independent experiments. P < 0.05. (C) shows Western blot analysis to detect LIF protein levels. β-actin served as a loading control. (D) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured astrocytes. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. NECA, 5′-N-ethylcarboxamide.
LIF secretion in primary astrocytes is constitutive and independent of NECA stimulation
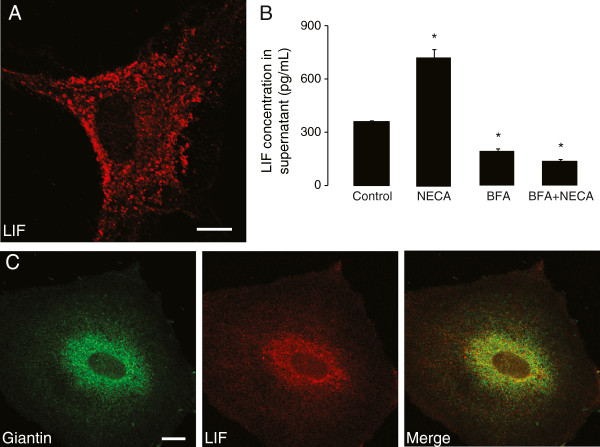
Supernatants from untreated astrocytes contained basal levels of LIF, suggesting that it could be constitutively released from these cells. Since we observed increased LIF concentrations in astrocyte supernatants after NECA stimulation, we investigated whether or not NECA played a direct role in LIF secretion. Thus, we blocked the early secretory pathway with Brefeldin A (BFA), a fungal metabolite that causes Golgi-derived proteins to accumulate in the endoplasmic reticulum [52]. Cultured astrocytes were pre-treated for 1 hour with BFA (5 μg/mL), followed by treatment without or with NECA (1 μM) for 1 or 4 hours (for immunocytochemistry and LIF-ELISA, respectively). In BFA-treated cells, all LIF-immunoreactivity co-localized with the Golgi marker Giantin (Figure 8B), compared to control conditions, where punctate LIF stainings could be observed all over the cytoplasm (Figure 8A). LIF levels in the supernatants from BFA-treated (5 μg/mL, for 5 hours) astrocyte cultures were significantly lower than that of the control (Figure 8C), implying that LIF is indeed constitutively secreted. Moreover, NECA treatment (1 μM, for 4 hours) did not stimulate LIF secretion in BFA-treated astrocytes (Figure 8C), further indicating that increased LIF levels in astrocytic culture supernatants after NECA stimulation require synthesis of new proteins and does not involve a ready-releasable post-Golgi reservoir of LIF.
Figure 8.
Leukemia inhibitory factor (LIF) secretion in primary astrocytes is constitutive and independent of NECA stimulation. (A) illustrates LIF immunocytochemistry in cultured cortical astrocytes, where LIF is found in vesicle-like structures throughout the cytoplasm. Scale bar corresponds to 10 μm. (B) shows complete co-localization of LIF with Giantin, a marker of Golgi apparatus, when cultured astrocytes were treated with Brefeldin A (BFA, 5 μg/mL for 1 hour). Scale bar corresponds to 10 μm. (C) shows a representation of sandwich ELISA experiment performed to detect LIF content in supernatants of cultured astrocytes that were treated without or with NECA (1 μM) for 4 hours. Where indicated, cells were pre-treated with BFA (5 μg/mL) for 1 hour prior to NECA stimulation. Each bar corresponds to the mean concentration of LIF in triplicate samples; error bars indicate SEM. Observations were confirmed by repeating the experiments two additional times. P < 0.05. NECA, 5′-N-ethylcarboxamide.
LIF secretion in primary astrocytes is mediated through recycling endosomes
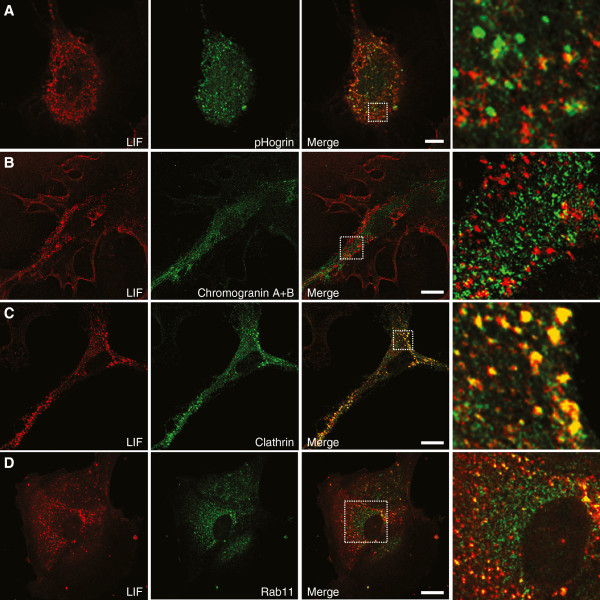
We further investigated the type of organelles responsible for LIF release. Several reports have shown that cytokines such as IL-6 and transforming growth factor β (TGFβ) are secreted through specialized secretory granules called large dense-core vesicles (LDCV) [53,54]. However, when astrocytes were co-stained for LDCV markers such as Chromogranin A & B, or pHogrin and LIF, no co-localization was observed (Figure 9A and 9B). In contrast, co-localization between LIF and clathrin was observed (Figure 9C). Clathrin is a marker for endosomal vesicles and is sometimes used as a marker for constitutive release [55,56]. Furthermore, LIF partially co-localized with Rab11 (Figure 9D), which is a marker for recycling endosomes [57,58], suggesting that recycling endosomes, rather than LDCV, mediate secretion of LIF.
Figure 9.
Leukemia inhibitory factor (LIF) co-localizes with clathrin and Rab11 but not with large dense-core vesicle markers. Immunostaining performed in cultured astrocytes revealed that LIF does not co-localize with pHogrin (A) and Chromogranin A & B (B), which are both markers for large dense-core vesicles. However, we observed a massive co-localization of LIF with clathrin (C) and a partial co-localization with Rab11 (D). Scale bars correspond to 10 μm.
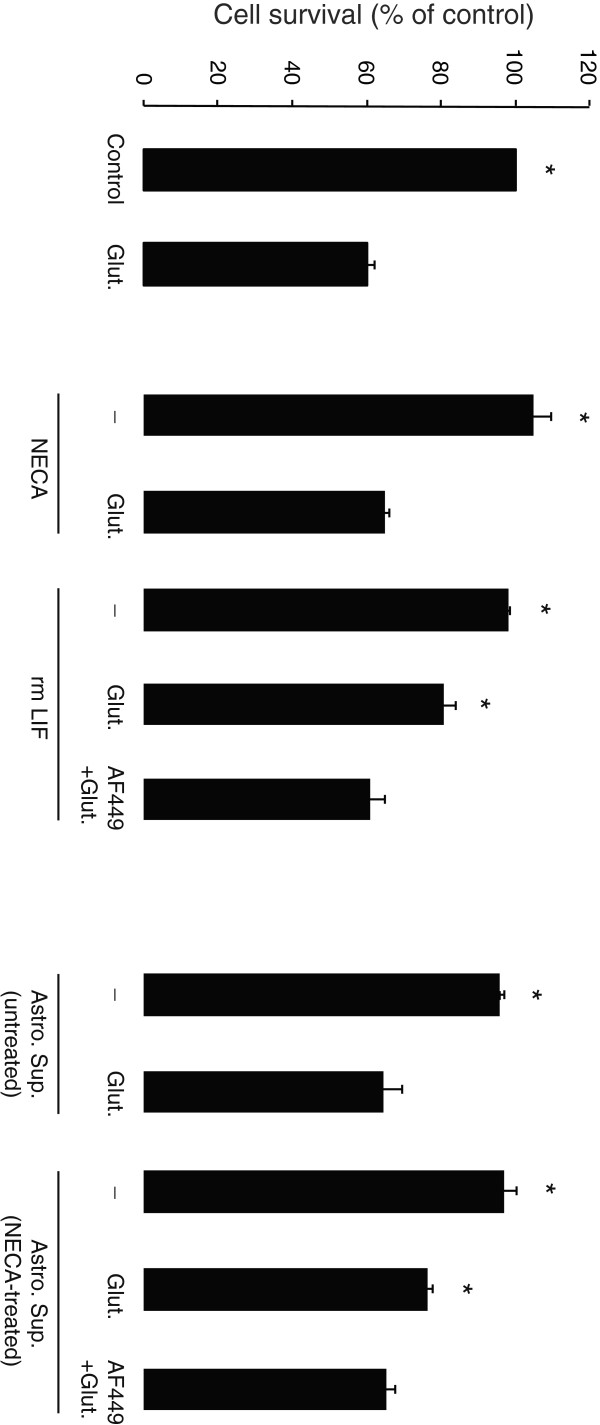
NECA-treated astrocytes induce LIF-mediated protection of cultured cortical neurons against excitotoxicity
We have previously shown that recombinant mouse LIF (rmLIF) protein protects mouse cortical neurons against excitotoxicity [13]. In order to understand whether NECA stimulation of astrocytes specifically would induce accumulation of neuroprotective LIF, astrocyte cultures were refreshed with new media shortly before NECA stimulation (1 μM for 4 hours) and the supernatant was collected. As shown in Figure 10, pre-treatment (1:1 dilution, for 24 hours) with supernatant from NECA-treated astrocytes significantly reduced the glutamate-induced cell death of cultured cortical neurons. A similar protective effect was observed by pre-treatment with rmLIF (0.1 ng/mL, for 24 hours) (Figure 10). Pre-treatment of the neurons (for 24 hours before application of glutamate) with NECA (1 μM) or supernatant from untreated astrocytes did not affect glutamate-induced neuronal cell death (Figure 10). We further investigated whether neuroprotection induced by NECA-treated astrocyte supernatant was mediated by LIF, by incubating the supernatants for 1.5 hours at 37°C with a LIF-neutralizing antibody (goat polyclonal anti-LIF, AF449, 100 ng/mL, R&D Systems, Oxford, UK) before applying to the neuronal cultures. The optimum concentration of LIF-neutralizing antibody was standardized by an efficiency test, performed according to manufacturer’s recommendations (data not shown). In addition, the effect of rmLIF protein treated with LIF-neutralizing antibody, on neuronal survival against glutamate, served as a control (Figure 10). Interestingly, LIF-neutralized supernatant from NECA-treated astrocyte cultures failed to protect neurons against glutamate (Figure 10), suggesting a direct neuroprotective mechanism of the endogenous LIF produced by astrocytes in response to NECA stimulation.
Figure 10.
Effect of untreated and NECA-treated astrocyte supernatants on survival of cultured cortical neurons against excitotoxicity. Primary cortical neurons were treated without or with recombinant mouse Leukemia inhibitory factor (rmLIF, 0.1 ng/mL), NECA (1 μM) or astrocyte supernatants (untreated or treated with 1 μM NECA for 4 hours; diluted 1:1 in neuronal culture medium) for 24 hours. Where indicated, rmLIF and NECA-treated astrocyte supernatants were treated with an anti-LIF neutralizing antibody (AF449; 100 ng/mL for 1.5 hours) before applying them to cultured neurons. Subsequently, the neurons were treated without or with glutamate (Glut.; 50 μM for 1 hour) and cell survival assessed 24 hours after glutamate treatment by a colorimetric MTT assay. The optical densities were measured at 570 nm, with a 630 nm and blank correction. Data are normalized to percent of control and presented as Mean ± S.E.M of four independent experiments. P < 0.05, when compared to glutamate-treated condition. NECA, 5′-N-ethylcarboxamide.
Discussion
We have previously shown that recombinant LIF protects neurons against glutamate-induced excitotoxicity [13]. In this study, we investigated the mechanism by which astrocytes produce and release LIF. Here we show that glutamate-induced neuronal excitotoxicity leads to adenosine receptor-mediated increase in LIF mRNA expression in cultured cortical astrocytes. We demonstrate that the upregulation of LIF mRNA and protein is adenosine A2B receptor-dependent, and is mediated through Gq/11-PLC-PKC-MAPK-NF-κB signaling pathways. We furthermore show that LIF is transiting through the Golgi and is found in recycling endosomes rather than in LDCV. Finally, LIF produced by astrocytes can protect neurons against excitotoxicity.
It has been known for more than a decade that astrocytes are the major source for LIF in the CNS [18,19,59,60]. However, the factors responsible for the regulation of LIF expression in these cells are still largely unknown. It is well known that stressed neurons release nucleotides such as ATP and adenosine [30,61]. Recently, it was demonstrated that astrocytes increase LIF production and release in response to ATP receptor stimulation [18]. In this study, the authors demonstrate that neurons during action potentials can secrete ATP, which triggers LIF production in astrocytes. This ATP-dependent upregulation of LIF by astrocytes is responsible for the promotion of oligodendrocyte-mediated myelination around neuronal axons. ATP is also known to be secreted by neurons during stressful conditions such as seizure, ischemia and hypoxia [26,27]. However, when we blocked adenosine receptors with the non-selective antagonist caffeine, or with specific A2A/A2B receptor antagonists, the effect of glutamate-stressed neuronal supernatants on LIF expression in astrocytes was absent, suggesting that adenosine, but not ATP, is responsible for astrocytic LIF production during glutamate-induced neuronal stress. Thus, it might be hypothesized that depending on the CNS status, astrocytic LIF expression and secretion is differentially regulated; during normal neuronal activity and development ATP is involved whereas during neuronal insults, adenosine might enhance LIF secretion by astrocytes.
Several studies have demonstrated the involvement of adenosine A2B receptors in the regulation of IL-6 expression in various cell types in vitro[38,47,48,62,63] as well as in vivo[64], suggesting that A2B receptors might also be essential in the regulation of other IL-6-type cytokines. Our results show that adenosine-dependent LIF regulation is mediated through the A2B receptor, since no increase in LIF expression was found in cultured astrocytes from A2B receptor deficient mice. Instead NECA caused a down-regulation of LIF mRNA after 8 and 24 hours in these cells, indicating that knocking out A2B receptors may have unmasked an inhibitory effect on LIF mRNA expression of an unidentified adenosine receptor. Whether or not this might explain the very short-lived effect of NECA on LIF mRNA expression in wild-type astrocytes is at the moment unclear and a subject of future investigations. We furthermore demonstrated that A2B-mediated LIF expression is dependent on the PKC, but not the PKA pathway. These data are in line with the study of Aloisi and colleagues, which demonstrated that LIF modulation by pro-inflammatory cytokines in human astrocytes was mediated through PKC activation [59]. Moreover, PKC has also been shown to be essential in IL-6 regulation [47,48,62,65], revealing a prominent role for PKC in the signaling pathway controlling LIF gene expression.
MAPKs have been reported to be involved in adenosine A2B receptor-mediated regulation of IL-6 gene expression in astrocytoma cells [48]. In our experiments, both basal as well as NECA-induced LIF gene expression and release in cultured astrocytes were inhibited by specific inhibitors of p38 and ERK1/2, but not JNK-MAPKs. In line with our findings, it has been shown that LIF expression in Schwann cells is mediated through PKC pathway-induced ERK1/2 activation [49]. Furthermore, we show here that adenosine-dependent LIF expression in astrocytes is regulated through the NF-κB transcription factor. This observation is in line with several studies showing an NF-κB-dependent regulation of IL-6 gene by this transcription factor in several cell types [38,50,51,65,66]. It has been shown that NECA-induced NF-κB activation and the resultant IL-6 gene expression was abolished by inhibitors of MAPK pathways [65]. In our study, preliminary observations indicate that NECA-induced activation of the NF-κB pathway is reduced by selective inhibitors of p38 and ERK1/2 pathways (data not shown), suggesting that these pathways might play as upstream mediators in NF-κB-dependent LIF expression in astrocytes.
Recent evidence indicates that, depending on the cell type, different secretory pathways are employed for cytokine release [67]. For example, T cells use two different release mechanisms: IL-2 and IFN-γ are secreted at the immunological synapse whereas CCL3 and TNF-α are secreted multidirectionally, suggesting different secretory pathways [68]. In neurons or neuron-like cells, secretory granules called LDCVs are the organelles used for the selective secretion of IL-6, TGF-β2 and CCL21 [53,54,69]. The same organelles are also used in immune cells such as mast cells and neutrophils [67]. Here we show that LIF protein is transported through Golgi but its secretion by astrocytes is not mediated by secretory granules. Instead, LIF co-localizes with Rab11, a known marker of recycling endosomes [57,58]. Moreover, we observed a partial co-localization of LIF with clathrin, which also associates with recycling endosomes where it is implicated in protein sorting [56]. Recycling endosomes have now been shown to be responsible for cytokine secretion in several cell types. For example, IFN-γ and TNF-α secretion from natural killer cells require Rab11 [70]. Recycling endosomes are also responsible for the constitutive secretion of IL-6 and TNF-α in macrophages [71]. Further studies will be needed to better understand LIF sorting, trafficking and release by these vesicles.
Interestingly, our data indicate that LIF is constitutively released from astrocytes. Indeed constant levels of LIF were present in the supernatants of untreated astrocytes when measured by ELISA. Similar data were observed in human astrocyte cultures [59]. Whether this observation is representative of the physiological behavior of astrocytes in vivo or is due to the culture conditions remains to be determined. We further show that by blocking the early secretory pathway with BFA, the LIF concentration in the culture supernatant was not increased upon NECA stimulation. The inhibitory effect of BFA indicates that LIF passes through the Golgi prior to its secretion, and thus does not follow non-conventional secretory pathways that by-pass the Golgi and is typically insensitive to BFA, which has recently been reported to be used by other cytokines [72]. Importantly, the inhibitory effect of BFA suggests that NECA-stimulated release of LIF by astrocytes requires de novo LIF synthesis, and does not involve a ready-releasable post-Golgi pool of LIF.
It is now clear that one of the major roles of LIF is directed toward cell protection. Indeed, it has been shown that LIF is up regulated in astrocytes and neurons after cerebral ischemia [21] as well as in astrocytes after cortical brain injury [24], suggesting a role of LIF in neuronal repair or protection. In line with these data, treatment of rat with LIF prevented loss of motoneurons after peripheral nerve injury [73,74] and protection of retinal ganglia cells was compromised in LIF knock-out mice after lens injury [12]. Finally, LIF was shown to limit demyelination in an experimental autoimmune encephalomyelitis mouse model [16] and has become a prominent therapeutic candidate for multiple sclerosis [17]. We have previously shown that LIF can protect cortical as well as hippocampal neurons against glutamate-induced excitotoxicity [13]. Here we show that LIF coming from the supernatant of NECA-treated astrocytes has the same protective effect. Indeed, astrocytes produce several other cytokines and neurotrophic factors including IL-6, NGF, brain-derived neurotrophic factor, neurotrophin-3, S-100β protein and TGFβ [75], that might help neurons to cope with excitotoxic stress. Accordingly, conditioned media from astrocyte cultures protected cortical neurons against glutamate (data not shown). In order to confine the neuroprotective effect of astrocytic factors that are released in response to NECA treatment, we had to refresh astrocyte culture medium prior to NECA treatment and testing supernatant on glutamate-stressed neurons. This, together with LIF neutralization, indicates that LIF produced by astrocytes after adenosine receptor stimulation is necessary to witness neuronal protection. Our results provide further evidence for a role of adenosine in neuronal protection. Indeed, it has been shown that adenosine can protect neurons during hypoxia [76,77], ischemia [78,79] and excessive neuronal activity [80,81]. This adenosine protection is often mediated through the A1 receptor subtype [82-84], but here we show that an indirect protection of adenosine through the stimulation of A2B receptor on astrocytes leading to LIF upregulation exists. This A2B receptor activation might be related to an anti-inflammatory process as observed previously by others [85-87].
Conclusions
We demonstrate a protective role of LIF against glutamate neurotoxicity and we provide clear evidence that adenosine is required for an increased production of LIF by astrocytes. These data further confirm a neuroprotective role of adenosine in the brain.
Abbreviations
A2B KO, A2B receptor knock-out; BFA, Brefeldin A; bp, base pairs; ChAT, choline acetyltransferase; CNS, central nervous system; CNTF, ciliary neurotrophic factor; CT-1, cardiotrophin-1; DMEM, Dulbecco’s modified Eagle’s medium; DMSO, dimethyl sulfoxide; EDTA, ethylenediaminetetraacetic acid; EPAC, Exchange Protein Activated by cAMP; ERK, extracellular signal-regulated kinase; FCS, fetal calf serum; HBSS, Hank’s balanced salt solution; HEPES, hydroxyethyl piperazineethanesulfonic acid; HRP, horseradish peroxidase; IL, interleukin; IFN, interferon; JNK, c-Jun N-terminal kinase; LDCV, large dense-core vesicles; LIF, leukemia inhibitory factor; LME, L-leucine methyl ester; MAP2, Microtubule-associated protein 2; MAPK, mitogen-activated protein kinase; NECA, 5′-N-ethylcarboxamide; NGF, nerve growth factor; NNT-1, novel neurotrophin-1; NT, nuclear transcription factor; OBB, Odyssey™ Blocking Buffer; OSM, oncostatin M; PBS, phosphate-buffered saline; PBS-T, phosphate-buffered saline plus 0.1 % Tween 20; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PVDF, polyvinylidene fluoride; qPCR, real-time PCR; rmLIF, recombinant mouse LIF; TGFβ, transforming growth factor β.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SM performed the majority of experiments including cell culturing, ELISA and Western blotting. JV did the immunofluorescent part of the study as well as all the microscopy and statistical analysis. Both SM and JV were involved in the redaction of the manuscript. EW did the qPCR experiments. JB took part in ELISA experiments. CHS performed the toxicity and the A2B KO experiments. SCDI and PB were both involved in the secretion part of the study. They gave precious antibodies, were essential in the design of the experiments and participated actively in writing the manuscript. HWGMB and KPHB were both involved in the conception and design of the study as well as in the manuscript redaction. All authors read and approved the final manuscript.
Contributor Information
Shamsudheen Moidunny, Email: shaamzee@gmail.com.
Jonathan Vinet, Email: vinetj@gmail.com.
Evelyn Wesseling, Email: e.m.wesseling@umcg.nl.
Johan Bijzet, Email: j.bijzet@umcg.nl.
Chu-Hsin Shieh, Email: chu-hsin.shieh@uniklinik-freiburg.de.
Sven CD van Ijzendoorn, Email: s.c.d.van.ijzendoorn@umcg.nl.
Paola Bezzi, Email: Paola.Bezzi@unil.ch.
Hendrikus WGM Boddeke, Email: h.w.g.m.boddeke@umcg.nl.
Knut Biber, Email: Knut.Biber@uniklinik-freiburg.de.
Acknowledgements
This study was supported financially by the school of Behavioral and Cognitive Neurosciences (S. Moidunny) and by the Deutsche Forschungsgemeinschaft (K. Biber), grant number: FOR1336 and CA 115/5-4.
References
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. The emerging neuropoietic cytokine family: first CDF/LIF, CNTF and IL-6; next ONC, MGF, GCSF? Curr Opin Neurobiol. 1992;2:94–97. doi: 10.1016/0959-4388(92)90169-L. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Leukemia inhibitory factor, a cytokine at the interface between neurobiology and immunology. Proc Natl Acad Sci USA. 1994;91:7833–7835. doi: 10.1073/pnas.91.17.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20:815–822. doi: 10.1002/(SICI)1097-4598(199707)20:7<815::AID-MUS5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kwon YW, Abbondanzo SJ, Stewart CL, Gurney ME. Leukemia inhibitory factor influences the timing of programmed synapses withdrawal from neonatal muscles. J Neurobiol. 1995;28:35–50. doi: 10.1002/neu.480280105. [DOI] [PubMed] [Google Scholar]
- Akita S, Webster J, Ren SG, Takino H, Said J, Zand O, Melmed S. Human and murine pituitary expression of leukemia inhibitory factor. Novel intrapituitary regulation of adrenocorticotropin hormone synthesis and secretion. J Clin Invest. 1995;95:1288–1298. doi: 10.1172/JCI117779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Auernhammer CJ, Melmed S. Murine leukemia inhibitory factor gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology. 1998;139:2209–2216. doi: 10.1210/en.139.5.2209. [DOI] [PubMed] [Google Scholar]
- Li M, Sendtner M, Smith A. Essential function of LIF receptor in motor neurons. Nature. 1995;378:724–727. doi: 10.1038/378724a0. [DOI] [PubMed] [Google Scholar]
- Murphy M, Reid K, Hilton DJ, Bartlett PF. Generation of sensory neurons is stimulated by leukemia inhibitory factor. Proc Natl Acad Sci USA. 1991;88:3498–3501. doi: 10.1073/pnas.88.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol. 1998;36:509–524. doi: 10.1002/(SICI)1097-4695(19980915)36:4<509::AID-NEU5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moidunny S, Dias RB, Wesseling E, Sekino Y, Boddeke HW, Sebastiao AM, Biber K. Interleukin-6-type cytokines in neuroprotection and neuromodulation: oncostatin M, but not leukemia inhibitory factor, requires neuronal adenosine A1 receptor function. J Neurochem. 2010;114:1667–1677. doi: 10.1111/j.1471-4159.2010.06881.x. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–796. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- Slaets H, Hendriks JJ, Stinissen P, Kilpatrick TJ, Hellings N. Therapeutic potential of LIF in multiple sclerosis. Trends Mol Med. 2010;16:493–500. doi: 10.1016/j.molmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GM, Song Y, Ong E, Lee YL, Schmidt KG, Bocchini V, Eng LF. Leukemia inhibitory factor mRNA is expressed in cortical astrocyte cultures but not in an immortalized microglial cell line. Neurosci Lett. 1995;184:48–51. doi: 10.1016/0304-3940(94)11165-F. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Mitsios N, Perikleous C, Cuadrado E, Montaner J, Sanfeliu C, Luque A, Kumar S, Kumar P, Gaffney J. Leukaemia inhibitory factor is over-expressed by ischaemic brain tissue concomitant with reduced plasma expression following acute stroke. Eur J Neurol. 2008;15:29–37. doi: 10.1111/j.1468-1331.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Ito D, Dembo T, Kosakai A, Fukuuchi Y. Immunohistochemical detection of leukemia inhibitory factor after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:661–668. doi: 10.1097/00004647-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Salehi Z. Expression of leukemia inhibitory factor in the cerebrospinal fluid of patients with multiple sclerosis. J Clin Neurosci. 2011;18:951–954. doi: 10.1016/j.jocn.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Broberg E, Roytta M, Mattila P, Rinne J, Hukkanen V. Expression of LIF and LIF receptor beta in Alzheimer’s and Parkinson’s diseases. Acta Neurol Scand. 2010;121:44–50. doi: 10.1111/j.1600-0404.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- Banner LR, Moayeri NN, Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp Neurol. 1997;147:1–9. doi: 10.1006/exnr.1997.6536. [DOI] [PubMed] [Google Scholar]
- Yamakuni H, Kawaguchi N, Ohtani Y, Nakamura J, Katayama T, Nakagawa T, Minami M, Satoh M. ATP induces leukemia inhibitory factor mRNA in cultured rat astrocytes. J Neuroimmunol. 2002;129:43–50. doi: 10.1016/S0165-5728(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Lupica CR, Dunwiddie TV. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Berman RF, Fredholm BB, Aden U, O’Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 2000;872:44–53. doi: 10.1016/S0006-8993(00)02441-0. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, Alexander KM, Jarvis MF, Kowaluk EA. Inhibition of adenosine kinase during oxygen-glucose deprivation in rat cortical neuronal cultures. Neurosci Lett. 1998;252:207–210. doi: 10.1016/S0304-3940(98)00376-0. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J Neurochem. 2004;88:1305–1312. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res. 2005;27:153–160. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- von Lubitz DK. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept? Eur J Pharmacol. 1999;371:85–102. doi: 10.1016/S0014-2999(99)00135-1. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D’Alimonte I, D’Onofrio M, Nicoletti F, Caciagli F. Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia. 1999;27:275–281. doi: 10.1002/(SICI)1098-1136(199909)27:3<275::AID-GLIA9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Biber K, Gyufko K, Berger M, Bauer J, van Calker D. Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J Neurochem. 1996;66:1426–1431. doi: 10.1046/j.1471-4159.1996.66041426.x. [DOI] [PubMed] [Google Scholar]
- Heese K, Fiebich BL, Bauer J, Otten U. Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A2a-receptors. Neurosci Lett. 1997;231:83–86. doi: 10.1016/S0304-3940(97)00545-4. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- Wittendorp MC, Boddeke HW, Biber K. Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia. 2004;46:410–418. doi: 10.1002/glia.20016. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Feng W, Song Y, Chen C, Lu ZZ, Zhang Y. Stimulation of adenosine A(2B) receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-delta-P38 signalling pathway. Br J Pharmacol. 2010;159:1598–1607. doi: 10.1111/j.1476-5381.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Biber K, Hamke M, Schmidt C, Butcher RD, van Calker D, Willmroth F. IL-6 expression induced by adenosine A2b receptor stimulation in U373 MG cells depends on p38 mitogen activated kinase and protein kinase C. Neurochem Int. 2005;46:501–512. doi: 10.1016/j.neuint.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Vaccariello SA, Zigmond RE. The levels of leukemia inhibitory factor mRNA in a Schwann cell line are regulated by multiple second messenger pathways. J Neurochem. 1999;72:1871–1881. doi: 10.1046/j.1471-4159.1999.0721871.x. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren A, Kooijman R, Lintermans B, Van Craenenbroeck K, Vermeulen L, Haegeman G, Gerlo S. Cooperation of NFκB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal. 2010;22:871–881. doi: 10.1016/j.cellsig.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JC, Kruttgen A, Burmester R, Weis J, Oertel WH, Shooter EM. Release of interleukin-6 via the regulated secretory pathway in PC12 cells. Neurosci Lett. 2006;400:75–79. doi: 10.1016/j.neulet.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Specht H, Peterziel H, Bajohrs M, Gerdes HH, Krieglstein K, Unsicker K. Transforming growth factor beta2 is released from PC12 cells via the regulated pathway of secretion. Mol Cell Neurosci. 2003;22:75–86. doi: 10.1016/S1044-7431(02)00023-4. [DOI] [PubMed] [Google Scholar]
- McPherson PS. Proteomic analysis of clathrin-coated vesicles. Proteomics. 2010;10:4025–4039. doi: 10.1002/pmic.201000253. [DOI] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn SC, Mostov KE, Hoekstra D. Role of rab proteins in epithelial membrane traffic. Int Rev Cytol. 2003;232:59–88. doi: 10.1016/s0074-7696(03)32002-9. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn SC. Recycling endosomes. J Cell Sci. 2006;119:1679–1681. doi: 10.1242/jcs.02948. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Rosa S, Testa U, Bonsi P, Russo G, Peschle C, Levi G. Regulation of leukemia inhibitory factor synthesis in cultured human astrocytes. J Immunol. 1994;152:5022–5031. [PubMed] [Google Scholar]
- Dallner C, Woods AG, Deller T, Kirsch M, Hofmann HD. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia. 2002;37:374–378. doi: 10.1002/glia.10048. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DA, Lewis BM, Lewis MD, Francis K, Scanlon MF, Ham J. Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol. 2003;140:764–772. doi: 10.1038/sj.bjp.0705488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- Vazquez JF, Clement HW, Sommer O, Schulz E, van Calker D. Local stimulation of the adenosine A2B receptors induces an increased release of IL-6 in mouse striatum: an in vivo microdialysis study. J Neurochem. 2008;105:904–909. doi: 10.1111/j.1471-4159.2007.05191.x. [DOI] [PubMed] [Google Scholar]
- Kim MO, Kim MH, Lee SH, Suh HN, Lee YJ, Lee MY, Han HJ. 5'-N-ethylcarboxamide induces IL-6 expression via MAPKs and NF-κB activation through Akt, Ca(2+)/PKC, cAMP signaling pathways in mouse embryonic stem cells. J Cell Physiol. 2009;219:752–759. doi: 10.1002/jcp.21721. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 2009;214:601–612. doi: 10.1016/j.imbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- de Jong EK, Vinet J, Stanulovic VS, Meijer M, Wesseling E, Sjollema K, Boddeke HW, Biber K. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. FASEB J. 2008;22:4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- Reefman E, Kay JG, Wood SM, Offenhauser C, Brown DL, Roy S, Stanley AC, Low PC, Manderson AP, Stow JL. Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells. J Immunol. 2010;184:4852–4862. doi: 10.4049/jimmunol.0803954. [DOI] [PubMed] [Google Scholar]
- Manderson AP, Kay JG, Hammond LA, Brown DL, Stow JL. Subcompartments of the macrophage recycling endosome direct the differential secretion of IL-6 and TNFalpha. J Cell Biol. 2007;178:57–69. doi: 10.1083/jcb.200612131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Cheema SS, Richards LJ, Murphy M, Bartlett PF. Leukaemia inhibitory factor rescues motoneurones from axotomy-induced cell death. Neuroreport. 1994;5:989–992. doi: 10.1097/00001756-199404000-00034. [DOI] [PubMed] [Google Scholar]
- Tham S, Dowsing B, Finkelstein D, Donato R, Cheema SS, Bartlett PF, Morrison WA. Leukemia inhibitory factor enhances the regeneration of transected rat sciatic nerve and the function of reinnervated muscle. J Neurosci Res. 1997;47:208–215. doi: 10.1002/(SICI)1097-4547(19970115)47:2<208::AID-JNR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Schwartz JP. Expression of neurotrophic factors and cytokines and their receptors on astrocytesin vivo.In Advances in Molecular and Cell Biology: Non-Neuronal Cells of the Nervous System: Function and Dysfunction Volume 31. Elsevier, Amsterdam; 2003. pp. 561–573. [Google Scholar]
- Gribkoff VK, Bauman LA. Endogenous adenosine contributes to hypoxic synaptic depression in hippocampus from young and aged rats. J Neurophysiol. 1992;68:620–628. doi: 10.1152/jn.1992.68.2.620. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycemia in rat hippocampal slices. Neurosci Lett. 1993;157:83–86. doi: 10.1016/0304-3940(93)90648-5. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindstrom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-M. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;128:1035–1044. doi: 10.1038/sj.bjp.0702888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin B, Neville LF, Pan J, Roberts PJ. 2-chloroadenosine attenuates kainic acid-induced toxicity within the rat straitum: relationship to release of glutamate and Ca2+ influx. Br J Pharmacol. 1989;98:225–235. doi: 10.1111/j.1476-5381.1989.tb16886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd HG, Perkins A, Spence I. Effect of magnesium on depression of the monosynaptic reflex induced by 2-chloroadenosine or hypoxia in the isolated spinal cord of neonatal rats. Neurosci Lett. 1989;101:175–181. doi: 10.1016/0304-3940(89)90526-0. [DOI] [PubMed] [Google Scholar]
- Pingle SC, Jajoo S, Mukherjea D, Sniderhan LF, Jhaveri KA, Marcuzzi A, Rybak LP, Maggirwar SB, Ramkumar V. Activation of the adenosine A1 receptor inhibits HIV-1 tat-induced apoptosis by reducing nuclear factor-kappaB activation and inducible nitric-oxide synthase. Mol Pharmacol. 2007;72:856–867. doi: 10.1124/mol.106.031427. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Hallam DM, Nie Z. Adenosine, oxidative stress and cytoprotection. Jpn J Pharmacol. 2001;86:265–274. doi: 10.1254/jjp.86.265. [DOI] [PubMed] [Google Scholar]
- Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, McGann K, Hauss-Wegrzyniak B, Wenk GL. The influence of brain inflammation upon neuronal adenosine A2B receptors. J Neurochem. 2003;86:220–227. doi: 10.1046/j.1471-4159.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P. et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]