Abstract
Objective
The role of the endogenous secretory receptor for advanced glycation end products (esRAGE) in depression of diabetes patients and its clinical significance are unclear. This study investigated the role of serum esRAGE in patients with type 2 diabetes mellitus with depression in the Chinese population.
Patients and Methods
One hundred nineteen hospitalized patients with type 2 diabetes were recruited at Fujian Provincial Hospital (Fuzhou, China) from February 2010 to January 2011. All selected subjects were assessed with the Hamilton Rating Scale for Depression (HAMD). Among them, 71 patients with both type 2 diabetes and depression were included. All selected subjects were examined for the following: esRAGE concentration, glycosylated hemoglobin (HbA1c), blood lipids, C-reactive protein, trace of albumin in urine, and carotid artery intima-media thickness (IMT). Association between serum esRAGE levels and risk of type 2 diabetes mellitus with depression was also analyzed.
Results
There were statistically significant differences in gender, age, body mass index, waist circumference, and treatment methods between the group with depression and the group without depression (P<0.05). Multiple linear regression analysis showed that HAMD scores were negatively correlated with esRAGE levels (standard regression coefficient −0.270, P<0.01). HAMD-17 scores were positively correlated with IMT (standard regression coefficient 0.183, P<0.05) and with HbA1c (standard regression coefficient 0.314, P<0.01).
Conclusions
Female gender, younger age, obesity, poor glycemic control, complications, and insulin therapy are all risk factors of type 2 diabetes mellitus with combined depression in the Chinese population. Inflammation and atherosclerosis play an important role in the pathogenesis of depression. esRAGE is a protective factor of depression among patients who have type 2 diabetes.
Introduction
Currently, diabetes is a threat to human health around the world. The prevalence of diabetes has been increasing rapidly.1 In 2010, the global prevalence rate of adult diabetes was 6.4%, and it is projected that it would increase to 7.7% by 2030.1 The incidence of depression in patients with diabetes has been significantly higher than that of the general population.2–4 Depression and depression-related symptoms increase medical costs, reduce quality of life of patients, and increase risk of complications and mortality.5
In recent years, attention has focused on risk factors of depression in patients with diabetes and its associated detrimental effects.6–8 However, its pathogenesis is still not clear. Recent studies show that inflammation and atherosclerosis may be a common pathogenesis of accelerated development of diabetes and depression.9,10 Systemic immune activation,11 vascular endothelial dysfunction,12 and modified platelet function13 may be potential pathophysiological mechanisms of diabetes and depression. Seldenrijk et al.14 found that compared with normal controls, persons with depressive symptoms showed a two- to threefold increased odds of low ankle brachial index, indicating that patients with depression are more likely to have subclinical atherosclerosis compared with healthy controls. Many studies have shown that depression will further worsen glycemic control in diabetes patients and influence occurrence and development of macrovascular and microvascular complications,2,15,16 but little is known about the risk factors of type 2 diabetes mellitus with combined depression in the Chinese population.
There are several types of advanced glycation end product (AGE) receptors; among them, the receptor for AGEs (RAGE) plays the most essential role in pathogenesis of AGEs.17 RAGE, which can be expressed in endothelial cells and mesangial cells, is involved in inflammation, neuronal differentiation, and other physiological and pathological processes. RAGE is closely related to chronic complications of diabetes.18–20 Serum total cleaved RAGE and endogenous secretory RAGE (esRAGE) are called soluble RAGE (sRAGE). The sRAGE can bind to AGEs and block phosphorylation of AGE-induced extracellular signal-regulated kinase, inhibiting its atherosclerosis-promoting effects.18,20 esRAGE can capture the RAGE ligand and can counteract AGE-induced damages to endothelial cells, suggesting its potential protection against vascular injury in diabetes.21,22 Therefore, individual differences of serum esRAGE levels may be determining factors of susceptibility and resistance of vascular complications of diabetes. Currently, the role of esRAGE in depression of diabetes patients and its clinical significance are unclear. In this study, we analyzed risk factors of concomitant depression in patients with type 2 diabetes through a case-control study. Through measuring intima-media thickness (IMT), C-reactive protein (CRP), serum concentration of esRAGE, and the Hamilton Rating Scale for Depression (HAMD) in patients with type 2 diabetes and combined depression, we explored the pathogenesis of concomitant depression in patients with type 2 diabetes.
Subjects and Methods
Patients
One hundred nineteen hospitalized patients with type 2 diabetes were recruited at the Department of Endocrinology, Fujian Provincial Hospital, Fuzhou, China, from February 2010 to January 2011. There were 71 patients with type 2 diabetes and combined depression (26 men and 45 women). Ages ranged from 32 to 75 years (mean, 57.39±9.80 years). There were 48 patients with type 2 diabetes and no depression (30 men and 18 women). Ages ranged from 48 to 75 years (mean, 63.56±7.60 years). The study was approved by the Hospital Internal Review Board of Fujian Provincial Hospital, and written informed consent was obtained from all subjects. All patients had complete medical records.
Diagnosis of type 2 diabetes was based on criteria published by the International Diabetes Federation in 2005 of fasting plasma glucose ≥7.0 mmol/L and/or 2-h plasma glucose level after glucose load ≥11.1 mmol/L or diagnosed diabetes with hypoglycemia agents. Diagnostic criteria for depression followed the Structured Clinical Interview for DSM-III-R23 diagnostic criteria for depression and the HAMD. An HAMD score of ≥17 indicated type 2 diabetes and combined depression. Information of each patient included in this study was recorded, including their admission number, name, gender, age, course of disease, body mass index, abdominal circumference, microvascular complications (diabetic peripheral neuropathy, diabetic nephropathy, and diabetic retinopathy), and treatments.
The HAMD scale used in this study is a main tool to evaluate the severity of depressive symptoms and the most commonly used tool to access the severity of depressive symptoms in the world.24,25 An HAMD score of <7 indicates no depression, a score of 7–17 indicates possible depression, and a score of ≥17 confirms depression.25 Investigators evaluated every patient using a standard questionnaire and standardized questions. According to the HAMD score of each patient, we selected 71 patients with type 2 diabetes whose HAMD score was ≥17 (the depression group) and chose 48 patients with type 2 diabetes whose HAMD score was ≤7 (the control group).
Auxiliary examination included glycosylated hemoglobin (HbA1c), triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and CRP. Traces of albumin in the urine were detected with an automatic biochemical analyzer. To measure the concentration of human circulating esRAGE in serum, fasting blood samples were collected, and specimens were immediately centrifuged and stored at −80°C. esRAGE concentrations were measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN). Carotid artery IMT was detected through high-frequency color Doppler ultrasonography. All data were presented as mean±SD values and analyzed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL). All tests were two-sided. The significance level α was set at 0.05. P<0.01 indicated a highly significant difference. Normal data were analyzed using t test, and nonnormal data were analyzed using a nonparametric test (Mann–Whitney ∪ test). Association was examined using multiple linear stepwise regression analysis.
Results
Comparison of clinical data
The χ2 test showed that gender was significantly different between patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P<0.01). The t test indicated statistically significant differences in age and abdominal circumference between the two groups (P<0.01). The Mann–Whitney ∪ test showed that body mass index (BMI) was significantly different between patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P<0.01). The Mann–Whitney ∪ test showed that duration of diabetes was not significantly different between patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P>0.05). The χ2 test indicated that there were no statistically significant differences in microvascular complications of diabetes, diabetic retinopathy, diabetic peripheral neuropathy, or diabetic nephropathy between patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P>0.05). The χ2 test also showed that treatments were not statistically significant (P<0.05) (Tables 1 and 2).
Table 1.
Characteristics of the Study Group
| Variable | Diabetes, complicating depression (n=71) | Diabetes, unconsolidated depression (n=48) | χ2 | P value |
|---|---|---|---|---|
| Gender | 7.699 | 0.006 | ||
| Male | 26 (36.62%) | 30 (62.50%) | ||
| Female | 45 (63.38%) | 18 (37.50%) | ||
| Microvascular complications of diabetes | 2.519 | 0.112 | ||
| Yes | 50 (70.42%) | 27 (56.25%) | ||
| No | 21 (29.58%) | 21 (43.75%) | ||
| Diabetic retinopathy | 0.002 | 0.965 | ||
| Yes | 18 (25.35%) | 12 (25.00%) | ||
| No | 53 (74.65%) | 36 (75.00%) | ||
| Diabetic peripheral neuropathy | 0.802 | 0.371 | ||
| Yes | 37 (52.11%) | 21 (43.75%) | ||
| No | 34 (47.89%) | 27 (56.25%) | ||
| Diabetic nephropathy | 0.475 | 0.491 | ||
| Yes | 25 (35.21%) | 46 (64.79%) | ||
| No | 14 (29.17%) | 34 (70.83%) | ||
| Treatment type | 4.709 | 0.03 | ||
| Oral therapy | 33 (46.48%) | 32 (66.67%) | ||
| Insulin therapy | 38 (53.52%) | 16 (33.33%) |
Data are number of patients (%).
Table 2.
Characteristics and Biochemical Parameters of the Studied Groups
| Variable | Diabetes, complicating depression (n=71) | Diabetes, unconsolidated depression (n=48) | Z/t | P value |
|---|---|---|---|---|
| Age (years) | 57.47±9.84 | 63.56±7.60 | 3.61 | 0.000 |
| Diabetes course (years) | 9.20±6.35 | 9.74±6.70 | −0.38 | 0.704 |
| BMI (kg/m2) | 23.63±3.13 | 21.16±2.76 | −4.323 | 0.000 |
| Waist circumference (cm) | 9.20±6.35 | 9.74±6.70 | 5.942 | 0.000 |
| esRAGE (μg/L) | 4.23±5.16 | 6.37±5.43 | −3.47 | 0.001 |
| CRP (mg/L) | 23.63±3.13 | 21.16±2.76 | −2.91 | 0.004 |
| HbA1c (%) | 9.75±2.69 | 8.32±1.95 | −2.790 | 0.005 |
| IMT (mm) | 1.21±0.30 | 1.06±0.20 | 3.389 | 0.001 |
| TGs (mmol/L) | 1.87±2.22 | 1.35±0.73 | −1.554 | 0.120 |
| CHOL (mmol/L) | 4.97±1.42 | 4.72±1.17 | 0.982 | 0.328 |
| HDL-C (mmol/L) | 1.25±0.70 | 1.20±0.40 | −0.368 | 0.713 |
| LDL-C (mmol/L) | 2.94±1.22 | 2.81±1.06 | 0.607 | 0.545 |
| Urine tracealbumin (mg/L) | 287.05±856.20 | 130.35±525.58 | −1.466 | 0.143 |
Data are mean±SD values.
BMI, body mass index; CHOL, total cholesterol; CRP, C-reactive protein; esRAGE, endogenous secretory receptor for advanced glycation end products; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol; TGs, triglycerides.
Comparisons of laboratory test results of patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression
The Mann–Whitney ∪ test indicated that esRAGE, CRP, and HbA1c levels were significantly different between patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P<0.01). The t test indicated statistically significant differences in IMT between the two groups (P<0.01). The Mann–Whitney ∪ test showed that triglycerides, high-density lipoprotein cholesterol, and microalbuminuria were not significantly different in patients with type 2 diabetes without depression and patients with type 2 diabetes with combined depression (P>0.05). The t test indicated that there were no statistically significant differences in total cholesterol or low-density lipoprotein cholesterol between the two groups (P>0.05) (Tables 1 and 2).
Multiple linear stepwise regression analysis results of diabetes patients without depression and diabetes patients with combined depression were as follows. HAMD score was the dependent variable, and esRAGE, IMT, CRP, and HbA1c were independent variables.
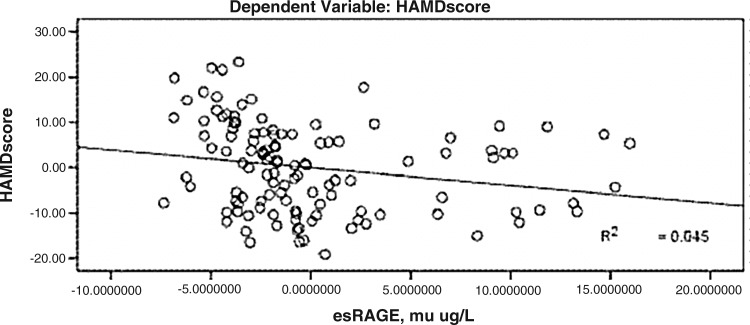
HAMD scores were significantly negatively correlated with esRAGE levels, and the standard regression coefficient was −0.270 (P<0.01) (Fig. 1).
FIG. 1.
Correlation between Hamilton Rating Scale for Depression (HAMD) score and the endogenous secretory receptor for advanced glycation end products (esRAGE).
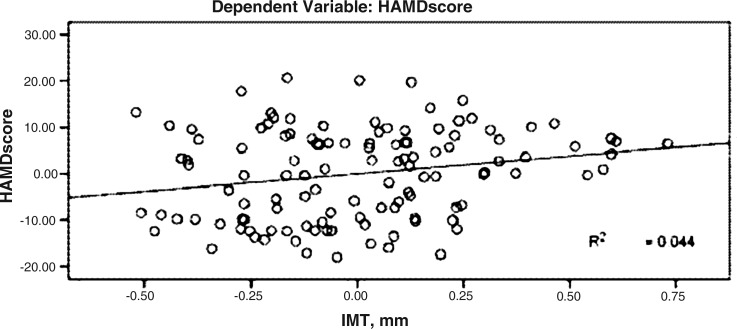
HAMD scores were significantly positively correlatedwith IMT, and the standard regression coefficient was 0.183 (P<0.05) (Fig. 2).
FIG. 2.
Correlation between Hamilton Rating Scale for Depression (HAMD) score and glycosylated hemoglobin (HbA1c).
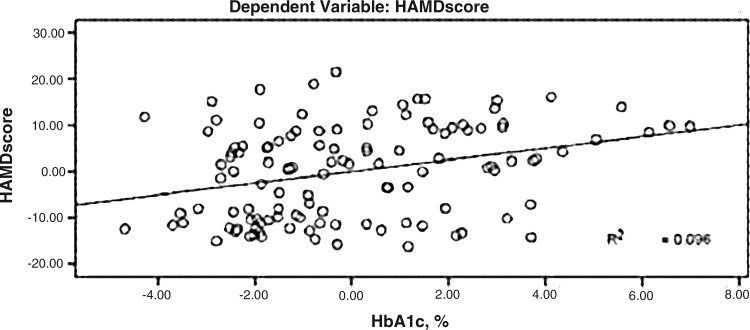
HAMD scores were significantly positively correlated with HbA1c, and the standard regression coefficient was 0.314 (P<0.01) (Fig. 3).
FIG. 3.
Correlation between Hamilton Rating Scale for Depression (HAMD) score and intima-media thickness (IMT).
Discussion
Risk factors of concomitant depression in patients with type 2 diabetes
The prevalence of diabetes has increased steadily in recent years, and diabetes has become the third most serious disease following cancer and cardiovascular and cerebrovascular diseases. It has been shown that patients with type 2 diabetes have significantly higher incidence of depression than the normal population.5,26 Previous studies show that female gender, young age, low education levels, low family income, high BMI, and high HbA1c levels are all risk factors of concomitant depression in patients with type 2 diabetes.27,28 In the current study, risk factors of concomitant depression in patients with type 2 diabetes were analyzed.
Gender
We found that there were significantly more women in the concomitant depression and type 2 diabetes group than in the control group. The χ2 test showed that gender was statistically significant between these two groups. It was found that female patients with type 2 diabetes are more likely to develop depression than male patients with type 2 diabetes.29 This may be closely related to social factors and women's own psychological and physiological characteristics. Women's characteristics show more sensitivity, and women are prone to anxiety and depression.
Age
We found that patients with type 2 diabetes with depression were younger in age compared with the patients in the control group. This may be due to the following: (1) Diabetes requires lifelong treatment. Relatively young diabetes patients may think they are still young and have more fear of prognosis of diabetes and possible death. (2) Relatively young diabetes patients have shorter courses of diabetes compared with older diabetes patients. In particular, those patients who were hospitalized for the first time had incomplete awareness of diabetes, and they might believe that diabetes is a disease that can cause a high morbidity and disability whether the disease is controlled well or not. Therefore, they may have pessimism and depression. It can be seen that younger age may be a potential risk factor of concomitant depression in patients with type 2 diabetes, which is consistent with previous reports.28 BMI and abdominal circumference are predictors of obesity and abdominal obesity. We found that the BMI of patients with concomitant depression and type 2 diabetes was significantly higher than that of controls, indicating that obesity is an important risk factor for depression.
Blood glucose control
The key issue of diabetes treatment is blood glucose control. A high HbA1c level indicates that blood sugar control is not satisfactory.30 Poor glycemic control can result in complications, seriously affecting quality of life of patients, and may cause great psychological and economic burden to patients.2 Our results indicated that the HbA1c level was significantly higher in patients with type 2 diabetes with concomitant depression compared with that of controls. This suggests that poor blood glucose control is another risk factor for concomitant depression in patients with type 2 diabetes.
Complications
Complications may cause inferiority, feeling worthless, feeling despair, and reduced self-care ability or defective self-care ability of patients. This may result in different levels of depressive symptoms. In this study it was found that diabetes complications were significantly associated with depressive symptoms in patients with type 2 diabetes and that depressive symptoms were significantly associated with the number and severity of complications.3,7,31 We also found a higher incidence of complications in patients with concomitant depression compared with that of controls. However, the difference was not statistically significant. We did not observe significant differences in diabetic retinopathy, diabetic peripheral neuropathy, or diabetic nephropathy between the two groups. This may be due to the small number of cases in this study, which caused an insignificant difference in results.
Treatments
We also observed that the percentage of patients with type 2 diabetes using insulin therapy with concomitant depression was higher than that of controls, indicating that treatment is also a risk factor for depression. Chinese patients have a strong fear of insulin treatment and believe that they have serious diabetes when receiving insulin treatment, which may lead to pessimism and depression. In addition, regular insulin therapy requires frequent monitoring of peripheral blood glucose, which increases the economic burden of patients, and inevitably influences quality of learning, living, and work. This will further increase the spiritual burden of patients, and patients may have strong resistance to the therapy and depression.
We also compared duration of diabetes between two groups, but the difference was not statistically significant. Because patients in this study were all hospitalized patients with type 2 diabetes, most patients had a long disease history, which may result in no significant difference in duration between the two groups. The lack of significant difference may be also due to the small number of cases in the current study, which warrants an epidemiological study with a large sample size.
Correlation between concomitant depression in patients with type 2 diabetes and inflammation or atherosclerosis
The increased CRP level is a predictor of depression in patients with type 2 diabetes. Literature shows that clinical depression and depressive symptoms are positively correlated with CRP levels after controlling for age, gender, BMI, high-density lipoprotein cholesterol concentrations, and other traditional risk factors of depression.11,32,33 Our results show that patients with combined depression had significantly higher CRP levels than controls. This result is consistent with recent reports. Maes34 found that depression is an inflammatory disease and that inflammation and cellular immune activation are key factors for depression. It was also found that patients with depressive symptoms have haplotype variation in the CRP gene through genetic polymorphism studies, indicating that elevated levels of inflammatory markers play an important role in the pathogenesis of depression.35
Atherosclerosis and depression are risk factors of each other.10 Depressive symptoms and depression-related diseases have been widely regarded as independent risk factors of cardiovascular abnormalities.36,37 Recent studies show that patients with depression or anxiety disorder have a higher prevalence of subclinical atherosclerosis compared with that of healthy controls, which increases the risk of cardiovascular disease in patients with depression and anxiety.14 AGEs can interact with cell surface receptors to promote cell dysfunction, leading to diabetes, chronic inflammation, cancer, and other diseases.18,20–22 In addition, AGEs development is also closely related to inflammation. Catalase and superoxide dismutase can produce reactive oxygen species during inflammation. Activation of oxidative stress can promote more AGEs production, further inducing inflammation.
There is a positive feedback loop that AGEs and inflammation connect through oxidative stress, which can promote atherosclerosis development.38 The sRAGE can interact with AGEs and block phosphorylation of AGEs-induced extracellular signal-regulating kinase, which can further inhibit atherosclerosis. Ultrasound measurements of carotid IMT can be used as an alternative indicator of subclinical atherosclerosis.39 Katakami et al.40 did a 4-year follow-up study to observe carotid artery plaque progression in patients with type 1 diabetes. It was found that the esRAGE level is an independent risk factor of carotid artery plaque progression in type 1 diabetes patients. The average serum esRAGE change was negatively correlated with mean carotid artery thickness and the annual change in carotid artery IMT. These results indicate that a decrease of serum esRAGE level is a protective factor of atherosclerosis. We also found that patients with type 2 diabetes with depression had significantly lower serum esRAGE than controls and that carotid artery IMT was thicker than that of controls. HAMD depression scores were negatively correlated with serum esRAGE but were positively correlated with IMT. These results indicate that the patients with more severe depressive symptoms had lower serum esRAGE and more severe carotid atherosclerosis, which demonstrates that depression plays an important role in pathogenesis of atherosclerosis and that esRAGE, as a protective factor of atherosclerosis, is a protective factor for depression in patients with type 2 diabetes.
Concomitant depression in patients with type 2 diabetes is significantly correlated with inflammation and atherosclerosis. Development of concomitant depression in patients with type 2 diabetes may be due to AGE–RAGE system-mediated inflammation, immune response, endothelial function disorder, and activation of oxidative stress. esRAGE may inhibit the above-mentioned series of reactions of the AGE–RAGE system to inhibit development of concomitant depression in type 2 diabetes. Recently, Emanuele et al.41 investigated the relationship between mental diseases and serum sRAGE in a cross-sectional study involving 74 patients and found that lower serum sRAGE was directly correlated with mental illness (including schizophrenia, anxiety, and severe depression). Our results are consistent with findings of that study. These results indicate that esRAGE measurement can predict incidence of arteriosclerosis in patients with depression.
Limitations
The sample size was small because of the short study duration. All patients were hospitalized patients at the Department of Endocrinology, Fujian Provincial Hospital. Subramaniam et al.42 found that depressive symptoms were significantly associated with the total number of hospitalizations and the days of hospitalization among individuals with diabetes and depression. Nonetheless, our findings may not apply in the larger population of people with type 2 diabetes who are not hospitalized. Multicenter, larger-scale studies are lacking. We only studied patients with type 2 diabetes mellitus and combined depression and patients with type 2 diabetes mellitus and no depression. Patients with type 1 diabetes mellitus and possible combined depression were not included for comparisons in the current study. Finally, given the cross-sectional nature of this study, no inference regarding direct causality between type 2 diabetes mellitus with combined depression and esRAGE can be made.
Conclusions
We found that female gender, younger age, obesity, poor glycemic control, complications, and insulin therapy are all risk factors for depression in patients with type 2 diabetes. Inflammation and atherosclerosis play an important role in the pathogenesis of depression. esRAGE is a protective factor of depression among patients who have type 2 diabetes.
Acknowledgments
This work was supported by grants C071002 and 2009Y0011 from the Natural Science Foundation, Fujian Province, China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Petrak F. Hautzinger M. Plack K. Kronfeld K. Ruckes C. Herpertz S. Müller MJ. Cognitive behavioural therapy in elderly type 2 diabetes patients with minor depression or mild major depression: study protocol of a randomized controlled trial (MIND-DIA) BMC Geriatr. 2010;10:21–29. doi: 10.1186/1471-2318-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lustman PJ. Anderson RJ. Freedland KE. de Groot M. Carney RM. Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE. Katon WJ. Lin EH. Ludman E. VonKorff M. Ciechanowski P. Young BA. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry. 2005;27:344–351. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Ali S. Stone MA. Peters JL. Davies MJ. Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 5.Wexler DJ. Grant RW. Wittenberg E. Bosch JL. Cagliero E. Delahanty L. Blais MA. Meigs JB. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49:1489–1497. doi: 10.1007/s00125-006-0249-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang CX. Chen YM. Chen WQ. Association of psychosocial factors with anxiety and depressive symptoms in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:523–530. doi: 10.1016/j.diabres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Kokoszka A. Pouwer F. Jodko A. Radzio R. Muko P. Bieńkowska J. Kuligowska E. Smoczyńska O. Skodowska Z. Serious diabetes-specific emotional problems in patients with type 2 diabetes who have different levels of comorbid depression: a Polish study from the European Depression in Diabetes (EDID) Research Consortium. Eur Psychiatry. 2009;24:425–430. doi: 10.1016/j.eurpsy.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RJ. Freedland KE. Clouse RE. Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 9.Pizzi C. Manzoli L. Mancini S. Bedetti G. Fontana F. Costa GM. Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors. Atherosclerosis. 2010;212:292–298. doi: 10.1016/j.atherosclerosis.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Seldenrijk A. van Hout HP. van Marwijk HW. de Groot E. Gort J. Rustemeijer C. Diamant M. Penninx BW. Depression, anxiety, and arterial stiffness. Biol Psychiatry. 2011;69:795–803. doi: 10.1016/j.biopsych.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Ford DE. Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 12.Pizzi C. Mancini S. Angeloni L. Fontana F. Manzoli L. Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–532. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 13.Bruce EC. Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67(Suppl 1):S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 14.Seldenrijk A. Vogelzangs N. van Hout HP. van Marwijk HW. Diamant M. Penninx BW. Depressive, anxiety disorders, risk of subclinical atherosclerosis. Findings from the Netherlands Study of Depression and Anxiety (NESDA) J Psychosom Res. 2010;69:203–210. doi: 10.1016/j.jpsychores.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Black SA. Diabetes, diversity, and disparity: what do we do with the evidence? Am J Public Health. 2002;92:543–548. doi: 10.2105/ajph.92.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makine C. Karida C. Kadiolu P. Ilkova H. Karida K. Skovlund SE. Snoek FJ. Pouwer F. Symptoms of depression, diabetes-specific emotional distress are associated with a negative appraisal of insulin therapy in insulin-naive patients with Type 2 diabetes mellitus. A study from the European Depression in Diabetes [EDID] Research Consortium. Diabet Med. 2009;26:28–33. doi: 10.1111/j.1464-5491.2008.02606.x. [DOI] [PubMed] [Google Scholar]
- 17.Hori O. Brett J. Slattery T. Cao R. Zhang J. Chen JX. Nagashima M. Lundh ER. Vijay S. Nitecki D. Morser J. Stern D. Schmidt AM. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 18.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Grossin N. Wautier MP. Meas T. Guillausseau PJ. Massin P. Wautier JL. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab. 2008;34:392–395. doi: 10.1016/j.diabet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt AM. Yan SD. Yan SF. Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonekura H. Yamamoto Y. Sakurai S. Petrova RG. Abedin MJ. Li H. Yasui K. Takeuchi M. Makita Z. Takasawa S. Okamoto H. Watanabe T. Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson BI. Harja E. Moser B. Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;25:879–882. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]
- 23.Williams JB. Gibbon M. First MB. Spitzer RL. Davies M. Borus J. Howes MJ. Kane J. Pope HG Jr. Rounsaville B. Wittchen HU. The structured clinical interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre RS. Konarski JZ. Mancini DA. Fulton KA. Parikh SV. Grigoriadis S. Grupp LA. Bakish D. Filteau MJ. Gorman C. Nemeroff CB. Kennedy SH. Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. CMAJ. 2005;173:1327–1334. doi: 10.1503/cmaj.050786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballesteros J. Bobes J. Bulbena A. Luque A. Dal-Ré R. Ibarra N. Güemes I. Sensitivity to change, discriminative performance, and cutoff criteria to define remission for embedded short scales of the Hamilton Depression Rating Scale (HAMD) J Affect Disord. 2007;102:93–99. doi: 10.1016/j.jad.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Egede LE. Zheng D. Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- 27.Katon W. von Korff M. Ciechanowski P. Russo J. Lin E. Simon G. Ludman E. Walker E. Bush T. Young B. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 28.Engum A. Mykletun A. Midthjell K. Holen A. Dahl AA. Depression and diabetes: a large population-based study of sociodemographic, lifestyle, and clinical factors associated with depression in type 1 and type 2 diabetes. Diabetes Care. 2005;28:1904–1909. doi: 10.2337/diacare.28.8.1904. [DOI] [PubMed] [Google Scholar]
- 29.Bell RA. Smith SL. Arcury TA. Snively BM. Stafford JM. Quandt SA. Prevalence and correlates of depressive symptoms among rural older African Americans, Native Americans, and whites with diabetes. Diabetes Care. 2005;28:823–829. doi: 10.2337/diacare.28.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair M. Prabhakaran D. Narayan KM. Sinha R. Lakshmy R. Devasenapathy N. Daniel CR. Gupta R. George PS. Mathew A. Tandon N. Reddy KS. HbA1c values for defining diabetes and impaired fasting glucose in Asian Indians. Prim Care Diabetes. 2011;5:95–102. doi: 10.1016/j.pcd.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams LH. Miller DR. Fincke G. Lafrance JP. Etzioni R. Maynard C. Raugi GJ. Reiber GE. Depression and incident lower limb amputations in veterans with diabetes. J Diabetes Complications. 2011;25:175–182. doi: 10.1016/j.jdiacomp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danner M. Kasl SV. Abramson JL. Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 33.Penninx BW. Kritchevsky SB. Yaffe K. Newman AB. Simonsick EM. Rubin S. Ferrucci L. Harris T. Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 34.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Halder I. Marsland AL. Cheong J. Muldoon MF. Ferrell RE. Manuck SB. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C-reactive protein. Brain Behav Immun. 2010;24:160–167. doi: 10.1016/j.bbi.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Kooy K. van Hout H. Marwijk H. Marten H. Stehouwer C. Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson A. Kuper H. Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 38.Basta G. Schmidt AM. De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Poredos P. Intima-media thickness: indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc Med. 2004;9:46–54. doi: 10.1191/1358863x04vm514ra. [DOI] [PubMed] [Google Scholar]
- 40.Katakami N. Matsuhisa M. Kaneto H. Matsuoka TA. Sakamoto K. Yasuda T. Umayahara Y. Kosugi K. Yamasaki Y. Serum endogenous secretory RAGE level is an independent risk factor for the progression of carotid atherosclerosis in type 1 diabetes. Atherosclerosis. 2009;204:288–292. doi: 10.1016/j.atherosclerosis.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Emanuele E. Martinelli V. Carlin MV. Fugazza E. Barale F. Politi P. Serum levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with different psychiatric disorders. Neurosci Lett. 2011;487:99–102. doi: 10.1016/j.neulet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Subramaniam M. Sum CF. Pek E. Stahl D. Verma S. Liow PH. Chua HC. Abdin E. Chong SA. Comorbid depression and increased health care utilisation in individuals with diabetes. Gen Hosp Psychiatry. 2009;3:220–224. doi: 10.1016/j.genhosppsych.2009.01.001. [DOI] [PubMed] [Google Scholar]