Abstract
A systematic study was conducted on round spermatids (ROS) injection (ROSI) using the goat model. After ROSI, the oocytes were treated or not with ionomycin (ROSI+I and ROSI−I, respectively) and compared with intracytoplasmic sperm injection (ICSI). After ROSI−I, most oocytes were arrested with premature chromatin condensation and few oocytes formed pronuclei. In contrast, most oocytes formed pronuclei after ROSI+I. Some ROS were observed to form asters that organized a dense microtubule network after ROSI+I, but after ROSI−I, no ROS asters were observed. Whereas most of the oocytes showed Ca2+ rises and a significant decline in maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities after ROSI+I, no such changes were observed after ROSI−I. Due to the lack of Ca2+ oscillations after ROSI−I, oocytes were injected with more ROS. Interestingly, different from the results observed in a single ROS injection, injection with four ROS effectively activated oocytes by inducing typical Ca2+ oscillations. Whereas ROSI+I oocytes and ICSI oocytes both showed extensive microtubule networks, no such a network was observed in parthenogenetic oocytes. Together, the results suggest that goat ROS is not able to trigger intracellular Ca2+ rises and thus to inhibit MPF and MAPK activities, but artificial activation improved fertilization and development of ROSI goat oocytes. Goat ROS can organize functional microtubular asters in activated oocytes. A ROS-derived factor(s) may be essential for organization of a functional microtubule network to unite pronuclei. Goat centrosome is of paternal origin because both ROS and sperm asters organized an extensive microtubule network after intra-oocyte injection.
Introduction
Obstructive azoospermia can be treated by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using spermatozoa retrieved from the epididymis (Craft et al., 1995; Temple-Smith et al., 1985) or by testicular biopsy (Craft et al., 1993; Silber et al., 1995). Nonobstructive azoospermia, however, can be treated only by micromanipulation-assisted fertilization using spermatogenic cells including spermatids. Attempts have been made to produce transgenic offspring using sperm and round spermatids (ROS) as nuclear donors (Huang et al., 2000; Lavitrano et al., 2002; Maione et al., 1998). As carriers of exogenous DNA, ROS have been suggested to be more effective than spermatozoa, because ROS chromatin is less compact and easier for exogenous DNA insertion. In addition, intraoocyte ROS injection (ROSI) has been used to rescue male infertility caused by transgene insertion (Hirabayashi et al., 2002; Meng et al., 2002) and natural mutation (Ogura et al., 1996). However, although reports suggested the feasibility of ROSI in humans (Al-Hasani et al., 1999; Gianaroli et al., 1999; Tesarik et al., 2000a), overall fertilization rates were lower than with ICSI using mature sperm or elongating spermatids (Balaban et al., 2000; Levran et al., 2000; Schoysman et al., 1999; Vicdan et al., 2001).
The current problems with ROSI include incomplete nuclear protein maturation of spermatids, cell cycle asynchrony, impaired oocyte activation, and potential adverse effects on the embryonic centrosome (Sousa et al., 1998). Histones of spermatids are progressively replaced with transition proteins and protamines during spermatid development. Protamines are responsible for the compaction of the sperm nucleus, which protects the male chromatin during genital transit and fertilization. Thus, when a ROS nucleus that lacks or is deficient in protamines is introduced into the metaphase II oocyte, the ROS chromatin is driven to an inappropriate metaphase block by the high maturation-promoting factor (MPF) activity. In some mammalian species, the embryonic centrosome normally derives from spindle elements contributed by spermatozoa (Schatten, 1994). Because ROS have not yet developed mature centriolar complexes with spindles, their injection into oocytes has the theoretical potential for unexpected and possibly adverse effects on subsequent embryonic development (Practice Committee, 2003). Although it has been suggested that these problems could be solved if oocytes were activated properly before or during injection to destroy MPF (Edwards et al., 1994; Fishel et al., 1996; Tesarik et al., 1995; Tesarik and Mendoza, 1996), systematic studies have not been reported to test this speculation. Furthermore, the centrosome origin of the goat is unknown.
During normal fertilization, oocyte activation is initiated after sperm delivers a sperm-borne oocyte-activating factor (SOAF) into the ooplasm (Runft et al., 2002). Thus, the impaired oocyte activation after ROSI is most probably due to a lack or insufficiency of SOAF in ROS (Tesarik et al., 2000b; Yanagida et al., 2000; Yazawa et al., 2000). Significant species differences in the ability of ROS to activate oocytes have been reported among different mammals. For example, while ROS from mice (Kimura and Yanagimachi, 1995; Yazawa et al., 2000), Mastomys (Ogonuki et al., 2003), rabbit (Tachibana et al., 2009), and human (Vanderzwalmen et al., 1997) displayed no or a limited oocyte-activating ability, ROS from hamster (Haigo et al., 2004) and cynomolgus monkeys (Ogonuki et al., 2001) induced oocyte activation efficiently after intraoocyte injection. However, it is not known whether ROS from goats have the ability to activate oocytes. Furthermore, although rabbit (Tachibana et al., 2009) and human (Vanderzwalmen et al., 1997) ROS could not activate oocytes of the same species effectively, they activated mouse oocytes efficiently after ROSI (Yanagida et al., 2000; Yazawa et al., 2000). The mechanism by which rabbit and human ROS activate mouse oocytes is unclear.
The objectives of the present study were to: (1) determine whether goat ROS could activate oocytes and support early embryo development following ROSI; (2) see whether the ROSI problems caused by cell cycle asynchrony and ROS incomplete maturation of nuclear proteins and centriolar complexes could be improved by proper oocyte activation; (3) investigate why ROS of some species cannot activate oocytes of the same species while activating mouse oocytes efficiently; (4) correlate dynamics of MPF and mitogen-activated protein kinase (MAPK) activities with intracellular Ca2+ oscillations following ROSI; and (5) explore the parental origin of centrosomes in the goat.
Materials and methods
All chemicals and reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA) unless otherwise specified.
Goat and mouse oocyte recovery
Caprine ovaries were obtained from a local abattoir and transported within 3 h to the laboratory in sterilized saline containing 100 IU/mL penicillin and 0.05 mg/mL streptomycin maintained at 30–35°C. Oocyte aspiration and selection were performed in Dulbecco's phosphate-buffered saline (D-PBS), supplemented with 0.1% polyvinyl alcohol (PVA). The oocytes were aspirated with a syringe from antral follicles of 1.5–4 mm in diameter and were examined under a stereomicroscope. Only oocytes with more than three complete layers of cumulus cells and a finely granulated homogeneous ooplasm were cultured for maturation.
Kunming mice were kept in a room with 14 h/10 h light–dark cycles, and the dark cycle starting from 8 p.m. The animals were handled by the rules stipulated by the Animal Care and Use Committee of Shandong Agricultural University. Female mice, 6–8 weeks after birth, were induced to superovulate with equine chorionic gonadotropin [eCG, 10 IU, intraperitoneally (i.p.)] followed 48 h later by human chorionic gonadotropin (hCG, 10 IU, i.p.). Oocytes were collected from oviductal ampullae in M2 medium 15–16 h after hCG injection. Oocytes recovered were freed of cumulus cells by pipetting in M2 medium containing 0.1% hyaluronidase before use.
Goat oocyte maturation in vitro
The maturation medium used was tissue culture medium-199 (TCM-199; Gibco, Grand Island, New York, USA) supplemented with 10 % (vol/vol) fetal calf serum (FCS; Gibco), 1 μg/mL 17 β-estradiol, 24.2 mg/L sodium pyruvate, 0.05 IU/mL follicle-stimulating hormone (FSH), 0.05 IU/mL luteinizing hormone (LH), and 10 ng/mL epiderminal growth factor (EGF). After being washed in D-PBS and maturation medium, groups of 20–30 oocytes were transferred into 100 μL of culture medium under mineral oil and were cultured in maturation medium at 38.5°C in 5% CO2 in humidified air. At hour 27 of the maturation culture, oocytes were freed of cumulus cells by pipetting in D-PBS containing 0.1% hyaluronidase, and those with first polar bodies were selected for use.
Preparation of ROS and ejaculated spermatozoa
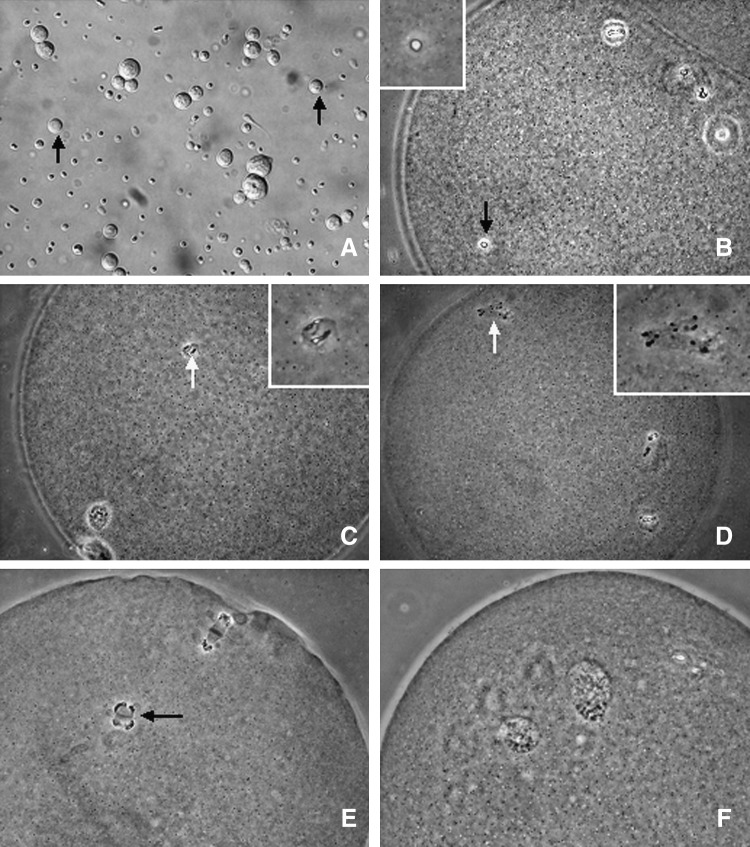
Testes of mature bucks were obtained from the same abattoir and transported in the same way to the laboratory as described above for caprine ovaries. Procedures for isolation of ROS reported by Ogura and Yanagimachi (1993) were used with modifications. Testes were placed one at a time in erythrocyte-lysing buffer (155 mM NH4Cl, 10 mM KHCO3, 2 mM EDTA, pH 7.2). After removal of the tunica albuginea with fine forceps, seminiferous tubules were allowed to spread in the buffer. They were washed thoroughly in D-PBS containing 5.6 mM glucose and 5.4 mM sodium lactate (GL-PBS) and cut into small pieces with a pair of fine scissors. The spermatogenic cells were dispersed throughout the medium by gently pipetting in and out of the pipette. The cell suspension was filtered through a 50-μm nylon mesh and centrifuged at 200×g for 5 min. Sedimented cells were then treated for 1 min with 0.2 mg/mL pronase in GL-PBS and centrifuged at 400×g for 5 min to eliminate elongating spermatids and mature spermatozoa from the cell population. The cells were resuspended in GL-PBS, washed twice (5 min each) by centrifugation at 200×g, and stored at 4°C before use. Most of the round, nucleated cells thus isolated were either spermatocytes or ROS. Using a phase-contrast microscope, the smaller ones with a diameter of about 10 μm were identified as ROS (Fig. 1A).
FIG. 1.
Phase-contrast micrographs of goat oocytes with different nuclear configurations after ROSI or ROSI followed by ionomycin activation (ROSI+I). (A) ROS (arrows) selected for use for injection. (B–D) Oocytes after ROSI alone (ROSI−I) with ROSN (arrows) showing intact nuclear envelope, NEBD, and PCC, respectively. The insets in B, C, and D represent enlarged views of ROSN. (E) A ROSI+I oocyte with PCC chromosomes separated by the anaphase spindle into two groups that move to the opposite poles of the oocyte. (F) A ROSI+I oocyte with two well-developed pronuclei. Magnification, 400×.
Semen was collected from fertile bucks aged between 2 and 5 years using an artificial vagina. The collected semen was diluted, cooled, and stored at 5°C until use, as we reported previously (Zhao et al., 2009). The motile sperm fraction was separated by a swim-up method. Briefly, 100 μL of fresh or stored semen was layered under 1 mL of D-PBS at the bottom of a 5-mL tube and incubated for 30 min in a humidified atmosphere of 5% CO2 in air at 38.5°C. The upper fraction containing highly motile spermatozoa was then recovered for injection.
Intraooplasmic microinjection
Micromanipulation was conducted using an inverted phase-contrast microscope equipped with micromanipulators and a piezo drive unit. Micromanipulation involving goat oocytes was performed in m-D-PBS [D-PBS supplemented with glucose, pyruvate, and bovine serum albumin (BSA)] containing 8% PVA, and micromanipulation involving mouse oocytes was carried out in HCZB medium containing 5 μg/mL cytochalasin B. To perform ICSI, motile spermatozoa were immobilized by rubbing at the midpiece region of the tail or by cutting off the tail using a bevel-tipped injection pipette with a 10 μm outer diameter. A spermatozoon was aspirated and injected into goat oocyte in a microdrop of m-D-PBS containing 8% PVA. To confirm the penetration of the pipette into the ooplasm, a small amount of ooplasm was aspirated. The injection pipette was then inserted more deeply and the spermatozoon was released into the oocyte with small amount of ooplasm and manipulation solution.
The ROS membrane was ruptured by gently aspirating with injection pipette to avoid loss of any ROS cytoplasm during the process. The same procedures described above for ICSI were repeated to conduct ROSI in goat oocytes. Goat ROS were injected into mouse oocytes using an injection pipette of 7–9 μm in inner diameter. Strong piezo pulses were applied to drill through the zona, and mild piezo pulses were used to rupture the oolemma. As the injection pipette was retracted following ROS injection, a small portion of ooplasm along with the medium injected were aspirated and pinched off to seal the oolemma.
Oocyte activation
The ionomycin and 6-dimethylaminopurine (6-DMAP) stocks were prepared in dimethyl sulfoxide (DMSO) and diluted to the desired concentrations in CR1aa (Rosenkrans et al., 1993) supplemented with 3 mg/mL BSA and 5% FCS before use. Four activation protocols were adopted in this study. In the post-ROSI ionomycin treatment (ROSI+I), oocytes were exposed immediately after ROSI to 10 μM ionomycin for 2 min at room temperature before culture in CR1aa at 38.5°C under 5% CO2 in humidified air. In the pre-ROSI ionomycin treatment (I+ROSI), oocytes were treated for 2 min with 10 μM ionomycin 1 h before ROSI. In the pre- and post-ROSI ionomycin treatment (I+ROSI+I), oocytes were treated with 10 μM and 5 μM ionomycin for 2 min, respectively, at 1 h before and immediately after ROSI. In the I+ROSI+I plus 6-DMAP (I+ROSI+I + 6D) treatment, oocytes were treated with 2 mM 6-DMAP for 2 h at 1.5 h following the first ionomycin treatment. During ionomycin- or 6-DMAP-treatment intervals, the oocytes were cultured in regular CR1aa without drugs. The total time of culture following ROSI was 6 h unless otherwise specified.
Embryo culture
Embryos produced by ROSI or ICSI were cocultured on cumulus cell monolayers in CR1aa containing 3 mg/mL BSA and 5% FCS. To prepare cumulus cell monolayer, cumulus cells were collected from in vitro matured oocytes and cultured in DMEM/F12 supplemented with 10% FCS in wells of a 96-well culture plate. The DMEM/F12 in wells with growing cumulus cell monolayers were replaced with CR1aa (100 μL per well) and equilibrated for 3 h prior to coculture. After ICSI or ROSI and activation treatment, embryos were transferred to the wells (20 per well) with cumulus cell monolayers and incubated at 38.5°C under 5% CO2 in humidified air. Culture medium was renewed every 48 h. Cleavage and blastulation were examined on days 1 and 9 of culture, respectively.
Assessment of nuclear morphology
To observe oocyte meiotic progression and sperm or spermatid nuclear remodeling following ICSI, ROSI, and activation treatment, oocytes were assessed for nuclear morphology at different times. For that, oocytes were mounted on glass slides. Vaseline and paraffin wax were used to keep the coverslip in contact with the oocytes without extensive pressure. For fixation, the slides were immersed in ethanol:acetic acid (3:1 vol/vol) for at least 24 h. Oocytes were stained in 1% aceto-orcein and examined under a phase-contrast microscope.
Immunofluorescence microscopy
Procedures were conducted at room temperature unless otherwise specified. Oocytes were washed in D-PBS between treatments. Oocytes were: (1) fixed with 4% paraformaldehyde in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 4 mM MgSO4, pH 7.0) for 20 min; (2) treated for 10 min in 1% Triton-X 100 in PHEM; and (3) blocked in PHEM containing 20% goat serum at 4°C overnight. Blocked oocytes were incubated for 1 hr in PHEM containing fluorescein isothiocyanate (FITC)-conjugated anti-α-tubulin monoclonal antibodies (1:50) and 5% goat serum to stain tubulin, and for 5 min in D-PBS with 10 μg/mL Hoechst 33342 to stain chromatin. Stained oocytes were observed with a laser scanning confocal microscope. Blue diode (405 nm) and argon (Ar; 488 nm) lasers were used to excite Hoechst and FITC respectively. Fluorescence was detected with bandpass emission filters 420–480 nm (Hoechst) and 505–540 nm (FITC), and the captured signals were recorded as blue and green, respectively.
Assay of H1 and MBP kinase activities
Nineteen oocytes from each treatment were washed, transferred to 10 μ of histone kinase buffer in a 1.5-mL microfuge tube, and stored frozen at −80°C. Frozen samples were subjected to freezing and thawing 4–5 times to prepare lysates. Then, 10 μL of substrate buffer containing 2 mg/mL histone H1, 2 mM dithiothreitol, and 20 μCi γ-32P]adenosine triphosphate (ATP) was added, and the reaction was carried out for 50 min at 37°C. Finally, an equal volume of double-strength sodium dodecyl sulfate (SDS) sample buffer containing β-mercaptoethanol was added, and the mixture was boiled for 3–5 minutes. Kinase reaction products were separated by 12% linear gradient SDS-polyacrylamide gel electrophoresis (PAGE). Gels were exposed to phosphor screens. Data acquisition was the actual scanning of sample images with the Cyclone® Plus Storage Phosphor System to create an image file that can be analyzed by the OptiQuant™ Image Analysis Software. The H1 kinase activity values of freshly in vitro matured oocytes were arbitrarily set as 100%, and the other values were expressed relative to this activity. The same procedures were repeated for assay of MBP kinase activity, except that histone H1 was replaced with 1 mg/mL MBP in the substrate buffer.
Calcium measurement
Intracellular Ca2+ in goat and mouse oocytes was measured using the Ca2+-sensitive dyes Oregon Green BAPTA 488-dextran and fluo-3/acetoxymethyl (AM), respectively. For loading, goat oocytes were microinjected with 10 pL of 1 mM Oregon Green BAPTA 488-dextran in HEPES-buffered KCl medium (Yu et al., 2008) and then cultured at 38.5°C for 30 min. Mouse oocytes were loaded by incubation for 20 min at 37°C with 30 μM of fluo-3/AM made up in CZB medium supplemented with 0.02% pluronic F-127. After loading, oocytes were subjected to ICSI or ROSI. For calcium measurement, drops of D-PBS containing 4 mg/mL BSA (for goat oocytes) or HCZB (for mouse oocytes) were made under paraffin oil in a Fluoro dish (World Precision Instruments, Inc.) with its base coated with phytoagglutinin. The drops were equilibrated overnight in a CO2 incubator. After ROSI or ICSI, oocytes were washed with D-PBS or HCZB and placed in the drops, and the dish was transferred to a heated stage (38.5°C for goat oocytes and 37°C for mouse oocytes) of a Leica laser-scanning confocal microscope (TCS SP2; Leica Microsystems). To measure Ca2+ oscillations in ionomycin treatment, oocytes were placed in drops of D-PBS and ionomycin was injected into the drops to produce a final concentration of 10 μM. At 2 min after ionomycin injection, the activation treatment was terminated by diluting the drop with D-PBS. For both dyes, an argon laser was used for excitation at 488 nm, and signals emitted at 505–540 nm were collected by the laser scanning confocal imaging system. Traces of calcium oscillations were plotted using SigmaPlot 2000 software.
Data analysis
There were at least three replicates for each treatment. Percentage data were arc sine transformed and analyzed with analysis of variance (ANOVA); a test of Duncan multiple comparisons was used to locate differences. The software used was Statistics Package for Social Science (SPSS 11.5; SPSS Inc., Chicago, IL, USA). Data were expressed as mean±standard error of the mean (SEM), and P<0.05 was considered significant.
Results
Goat ROS did not activate goat oocytes after ROSI, but ionomycin treatment significantly improved activation of ROSI oocytes
Goat oocytes were injected with ROS, ejaculated spermatozoa, or PBS medium and treated with or without ionomycin. Oocytes showing one or more pronuclei were considered activated and those showing two or more pronuclei fertilized. Without ionomycin, whereas 71% of the oocytes injected with spermatozoa were activated, only 13–14% of the oocytes injected with ROS or PBS were activated following culture (Table 1). With ionomycin, however, activation rates of oocytes injected with either ROS or PBS increased to over 90%. Because oocytes injected with ROS or PBS showed similar low activation rates without ionomycin, it is concluded that goat ROS could not activate goat oocytes and ionomycin treatment significantly improved activation/fertilization of ROSI oocytes.
Table 1.
Effects of Ionomycin Treatment on Activation and Fertilization of Goat Oocytes after ROSI
| Injected with | Ionomycin | Oocytes injected | % Activated oocytes | % 2PN activated oocytes |
|---|---|---|---|---|
| Sperm | − | 58 | 70.6±4.2a | 94.9±3.2a |
| ROS | − | 37 | 14.2±3.8a | 100.0±0.0a |
| ROS | + | 39 | 92.5±3.8b | 92.5±4.5a |
| PBS | − | 53 | 12.7±4.0b | 0.0±0.0b |
| PBS | + | 44 | 95.0±2.5b | 14.8±1.9c |
Values without a common letter in their superscripts differ (P<0.05) in the same column. Oocytes showing one or more pronuclei (PN) were considered activated and those showing two PN were considered fertilized.
ROS, round spermatids; ROSI, round spermatids injection; PBS, phosphate-buffered saline.
Ionomycin treatment promoted pronuclear formation while decreasing premature chromosome condensation of goat ROSI oocytes
Without ionomycin treatment (ROSI−I), 80–90% of the ROS nuclei (ROSN) underwent premature chromosome condensation (PCC) (Fig. 1D) following nuclear envelope breakdown (NEBD; Fig. 1C), but only 16% formed pronuclei when observed at 6 hr after ROSI (Table 2). With ionomycin treatment (ROSI+I), no ROSN showed NEBD and few displayed PCC, but 80% formed well-developed pronuclei (Fig. 1F) when observed at 4 h after ROSI. The PCC in ROSI+I oocytes was different from that observed in ROSI−I oocytes. After ionomycin treatment, the PCC chromosomes were separated into two groups and moved to opposite poles of the oocyte (Fig. 1E).
Table 2.
Effects of Ionomycin Treatment on Nuclear Remodeling of Goat Oocytes after ROSI
| |
|
% Oocytes with ROSN at |
|||
|---|---|---|---|---|---|
| Time postinjection | Oocytes observed | Intact NE | NEBD | PCC | MPN |
| Without ionomycin | |||||
| 0 min | 57 | 100.0±0.0a | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a |
| 20 min | 52 | 69.3±2.7b | 30.7±2.7b | 0.0±0.0a | 0.0±0.0a |
| 40 min | 55 | 31.0±3.6c | 67.1±2.6c | 1.9±1.9a | 0.0±0.0a |
| 1 hr | 47 | 6.3±2.1d | 38.2±1.3d | 55.5±2.9b | 0.0±0.0a |
| 1.5 hr | 47 | 4.2±2.4d | 4.1±2.4a | 91.7±3.7c | 0.0±0.0a |
| 6 hr | 43 | 2.4±2.4d | 0.0±0.0a | 81.4±2.2d | 16.2±1.9b |
| With ionomycin | |||||
| 1 hr | 32 | 100.0±0.0a | 0.0±0.0a | 0.0±0.0a | 0.0±0.0a |
| 2 hr | 43 | 76.8±1.8b | 0.0±0.0a | 16.2±1.9d | 7.0±0.1a |
| 3 hr | 43 | 39.6±3.2c | 0.0±0.0a | 11.4±1.6d | 49.0±2.9c |
| 4 hr | 42 | 19.1±2.6e | 0.0±0.0a | 2.6±2.6a | 78.3±4.8d |
| 6 hr | 44 | 11.2±1.7e | 0.0±0.0a | 4.3±2.2a | 84.5±3.9d |
Values without a common letter in their superscripts differ (P<0.05) within ionomycin treatment groups in the same column.
ROSI, round spermatids injection; ROSN, ROS nuclei; NE, nuclear envelope; NEBD, nuclear envelope breakdown; MPN, male pronuclei.
Ionomycin treatment improved microtubule assembly of ROSI goat oocytes
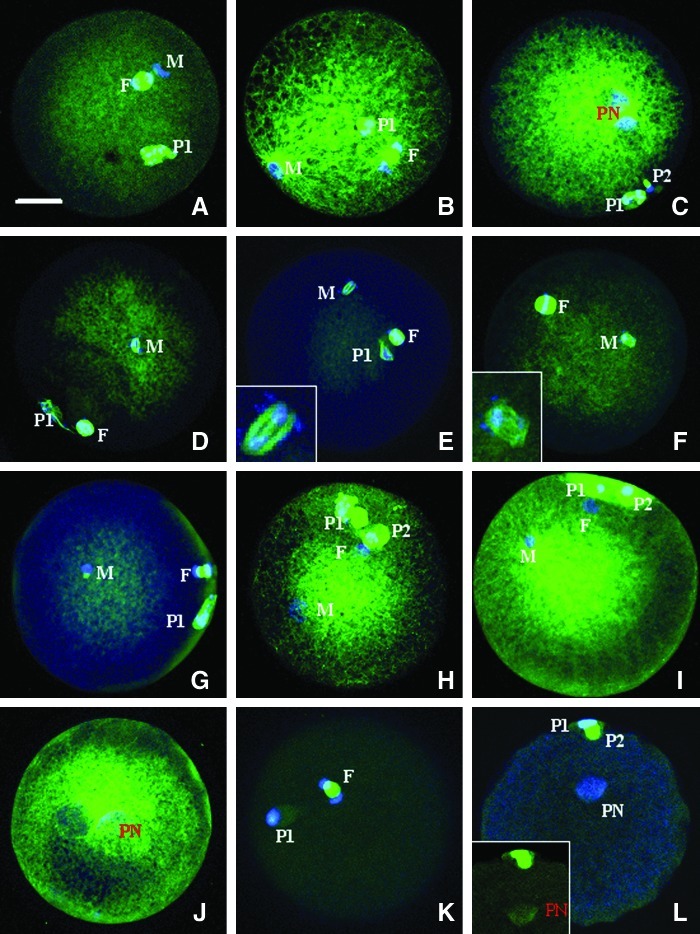
Goat oocytes showed decondensing sperm heads at 2 hr after ICSI (Fig. 2A). At 3 hr after ICSI, sperm asters formed around the decondensing sperm chromatin with microtubules emanating to approach the oocyte spindle (Fig. 2B) in 53% (17/32) of oocytes observed. By 6 hr after ICSI, the microtubule network expanded to the entire ooplasm, with more microtubules surrounding the two apposed pronuclei (Fig. 2C). Oocytes remained arrested at the metaphase II stage until 6 hr after ROSI-I, and the prematurely condensed spermatid chromatin was always associated with microtubules and formed spindle-like structures (Fig. 2D, E, F). At 2 hr after ROSI+I, most of the oocytes showed intact ROSN without microtubular asters, and 13% (2/15) displayed asters attached to the intact ROSN (Fig. 2G). At 4 h, 26% (8/31) of the oocytes showed ROSN-associated asters that enlarged to fill the ooplasm (Fig. 2H, I), and the rest of the oocytes showed limited microtubules surrounding the ROSN-derived pronuclei. By 6 h, microtubule networks filled the entire ooplasm with denser microtubules around the apposed pronuclei (Fig. 2J) in all (23/23) of the activated oocytes. No microtubule network was observed in parthenogenetic oocytes activated with ionomycin alone; only a limited amount of microtubules were concentrated in the pronuclear area at 4 and 6 h after ionomycin activation (Fig. 2K, L).
FIG. 2.
Confocal micrographs of goat oocytes after ICSI, ROSI, or ROSI followed by ionomycin activation (ROSI+I). The α-tubulin and chromatin are pseudo-colored green and blue, respectively. (A–C) Oocytes observed at 2, 3, and 6 h after ICSI, respectively. (A) Oocyte anaphase II spindle (F) and the decondensing sperm head (M). (B) Aster formation around the decondensed sperm chromatin (M) and the oocyte spindle in telophase II (F). (C) Microtubule network surrounding the apposed male and female pronuclei (PN). (D–F) Oocytes observed at 1, 1.5, and 6 h after ROSI, respectively, with D showing the prematurely condensed spermatid chromatin surrounded with microtubules (M) and the oocyte metaphase II spindle (F), and E and F showing oocyte metaphase II spindle (F) and the spindle-like structure around the spermatid condensed chromatin (M). (Insets, E and F) Two times amplifications of the spermatid spindle-like structures. (G–J) Oocytes observed at 2, 4, and 6 h after ROSI+I, with G showing oocyte spindle in telophase II (F) and intact ROSN with an aster-like structure (M), H and I showing spermatid (M) and oocyte (F) pronuclei and the microtubule network emerging from the spermatid pronucleus, and J showing well-developed ooplasmic microtubule network surrounding the apposed pronuclei (PN). (K and L) Oocytes observed at 2 and 6 h, respectively, after ionomycin treatment for parthenogenetic activation, with K showing oocyte anaphase II spindle (F) and L showing microtubules observed only in the pronucleus (PN) area. (Inset, L) Image before merging to show only the α-tubulin (pseudo-colored green). First (P1) and second (P2) polar bodies are often observed. Scale bar, 15 μm.
Artificial activation improved embryo development of goat ROSI oocytes
Observation of in vitro embryo development showed that 29% and 26% of oocytes developed into blastocysts following ICSI and I+ROSI, respectively (Table 3). Although none of the ROSI−I oocytes formed blastocysts, 20% of the ROSI+I oocytes did. When double ionomycin treatment (I+ROSI+I) and I+ROSI+I combined with 6-DMAP (I+ROSI+I+6D) was performed, rates of blastocysts increased to 33% and 40%, respectively.
Table 3.
Effects of Activation Protocols on Embryo Development of ROSI Goat Oocytes
| Protocols | Oocytes observed | % Two-cell embryos | % Blastocysts/two-cell embryos |
|---|---|---|---|
| ICSI | 42 | 66.1±4.3a | 28.6±0.8a |
| ROSI−I | 41 | 9.9±2.8b | 0.0±0.0b |
| I+ROSI | 36 | 86.3±2.3c | 25.8±3.0ac |
| ROSI+I | 41 | 88.0±1.9c | 19.8±3.8c |
| I+ROSI+I | 43 | 93.0±0.3c | 32.5±2.1a |
| I+ROSI+I + 6D | 87 | 95.4±1.9c | 39.7±1.6d |
Values without a common letter in their superscripts differ (P<0.05) in the same column.
ROSI, round spermatids injection; ICSI, intracytoplasmic sperm injection; I, ionomycin.
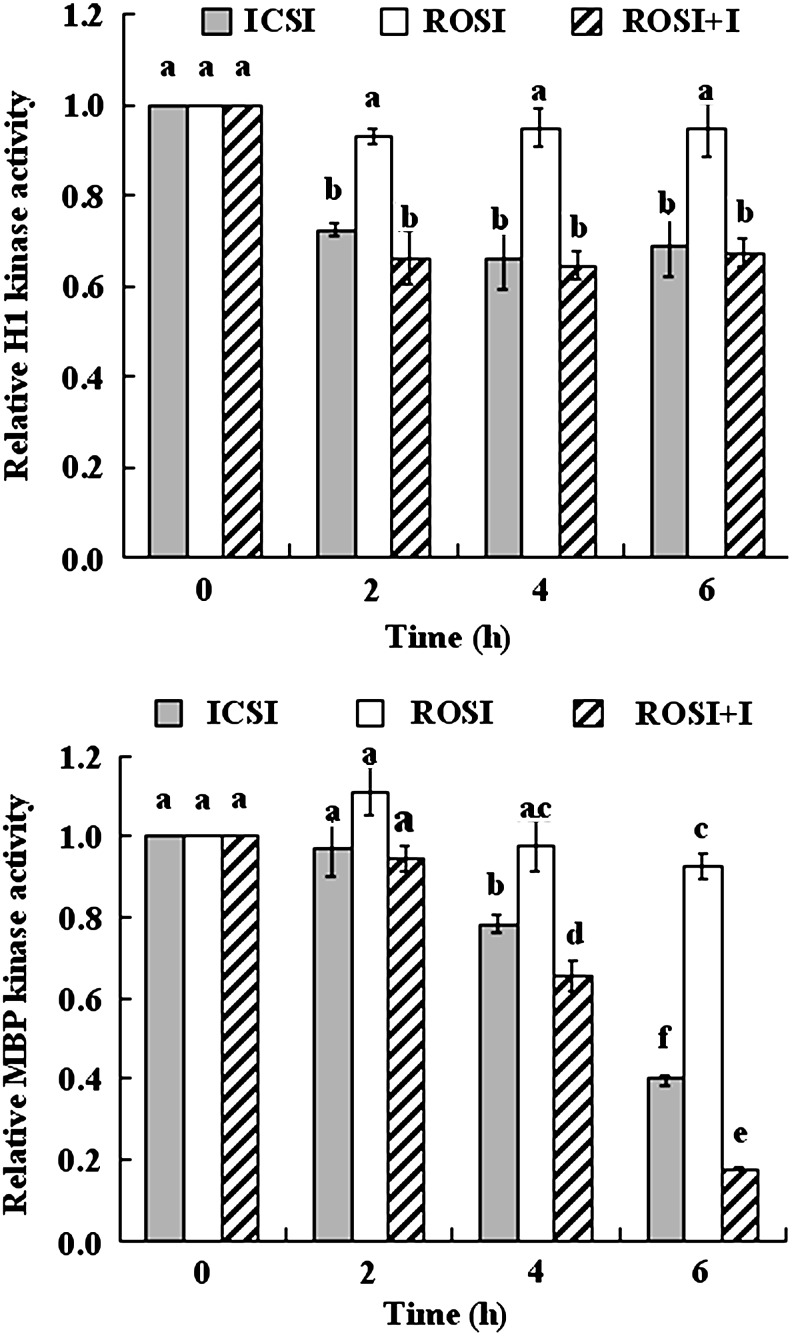
Changes in the MPF and MAPK activities after ROSI of goat oocytes
Although both MPF and MAPK activities remained constant up to 6 h following ROSI−I, they declined significantly after ICSI or ROSI+I (Fig. 3). Thus, MPF activity decreased significantly at 2 h and after that remained constant until 6 hr after ICSI or ROSI+I. MAPK activity decreased in a time-dependent manner from 4 to 6 h after ICSI and ROSI+I.
FIG. 3.
Relative MPF (histone H1 kinase) and MAPK (MBP kinase) activity of goat oocytes at different times after ICSI, ROSI, or ROSI+I. Values without a common letter above their bars differ significantly (P<0.05).
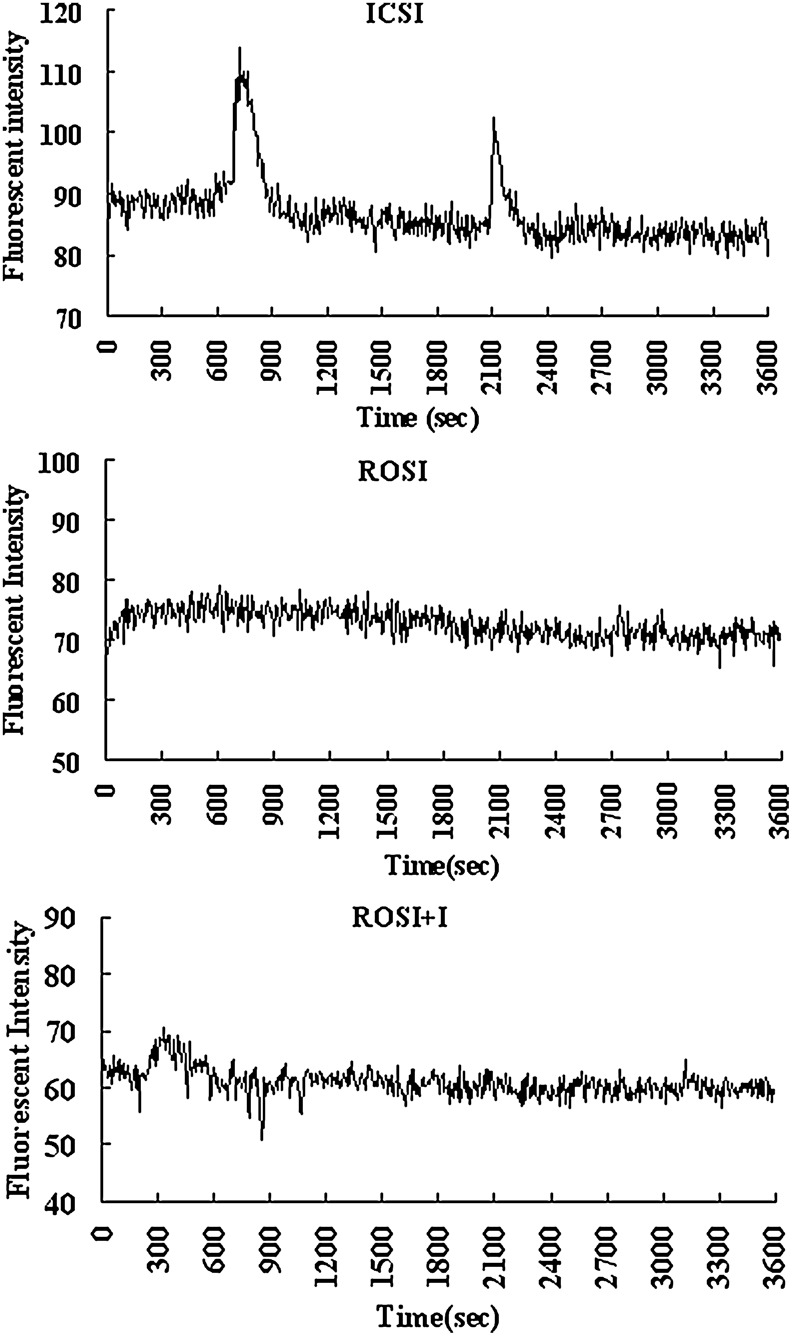
Goat ROS did not induce intracellular Ca2+ oscillations in goat oocytes after ROSI
Goat oocytes were measured for Ca2+ transients for 60 min immediately after ICSI or ROSI. To observe effects of ionomycin activation on Ca2+ oscillations, ROSI oocytes were treated with 10 μM ionomycin for 2 min, and Ca2+ transients were measured for 60 min during and after ionomycin treatment. Although 54% (15/28) of the ICSI oocytes showed one to three Ca2+ rises, none (0/27) of the ROSI−I oocytes displayed any Ca2+ increase (Fig. 4). A single mild Ca2+ increase was observed in 60% (18/30) of ROSI+I oocytes immediately following ionomycin exposure.
FIG. 4.
Dynamics of intracellular concentration of free calcium ion in goat oocytes. Ca2+ rises were observed after ICSI or ROSI+I, as shown in the top panel and the bottom panel, respectively. No Ca2+ rises were observed after ROSI, as shown in the middle panel.
Goat ROS could not activate goat oocytes due to their limited contents of SOAF
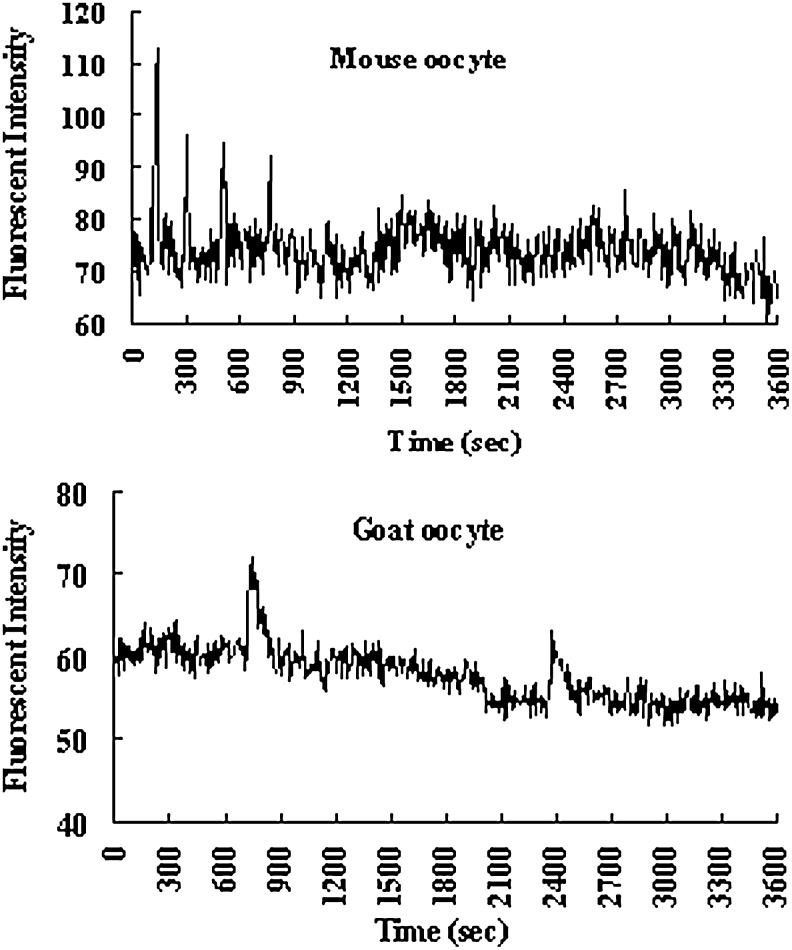
Different numbers of goat ROS were injected into mouse or goat oocytes to observe pronuclear formation and Ca2+ oscillations. When a single ROS was injected, 65% of mouse oocytes were activated (forming pronuclei), but only 16% of the goat oocytes formed pronuclei (Table 4). However, pronuclear formation rates of goat oocytes increased to 73% when four ROS were injected. Both mouse oocytes injected with one ROS and goat oocytes injected with four ROS displayed Ca2+ oscillations (Fig. 5).
Table 4.
Activation of Goat and Mouse Oocytes Following ROSI with Different Numbers of Goat ROS
| Injected with | Oocytes from | Oocytes injected | % Activated oocytes* |
|---|---|---|---|
| 1 ROS | Goat | 41 | 15.9±3.3a |
| 1 ROS | Mouse | 43 | 64.7±6.0b |
| HCZB medium | Mouse | 40 | 9.7±1.4a |
| 2 ROS | Goat | 51 | 56.2±3.4b |
| 4 ROS | Goat | 40 | 72.6±4.3c |
Oocytes showing one or more pronuclei were considered activated.
Values without a common letter in their superscripts differ (P<0.05) in the same column.
ROSI, round spermatids injection.
FIG. 5.
Dynamics of intracellular concentration of free calcium ion in mouse and goat oocytes. (Upper panel) Pattern of Ca2+ oscillations in mouse oocytes after ROSI with one ROS. (Lower panel) Ca2+ rises in goat oocytes were observed only after ROSI with four ROS as demonstrated here.
Discussion
It has been shown in most species studied so far that activation of the oocyte rarely occurs after ROSI alone (Kimura and Yanagimachi, 1995; Ogonuki et al., 2003; Tachibana et al., 2009; Vanderzwalmen et al., 1997; Yazawa et al., 2000). However, the mechanism for the inability of ROS to activate oocytes is largely unknown. This study showed that goat ROS activated mouse oocytes efficiently although they could not activate goat oocytes. Similarly, although rabbit (Tachibana et al., 2009) and human (Vanderzwalmen et al., 1997) ROS could not activate oocytes of the same species effectively, they activated mouse oocytes efficiently after ROSI (Yanagida et al., 2000; Yazawa et al., 2000). Because oocytes from rabbit, human, and goat are much larger than mouse oocytes, we hypothesized that goat ROS might have contained too little SOAF to activate the large goat oocytes, but enough to activate the small mouse oocytes. To test this hypothesis, we increased the number of goat ROS injected into goat oocytes. When four ROS were injected, pronuclear formation rates of goat oocytes increased to 73%, and typical Ca2+ oscillations reminiscent of those seen in ICSI oocytes were observed. Thus, it is suggested that ROS do contain some SOAF, but the amount is much less than that contained in mature spermatozoa.
Phospholipase C (PLC) zeta has been considered a candidate SOAF (Swann et al., 2006). According to Fissore (2009), there is a balance between sperm PLC zeta activity and oocyte inositol trisphosphate (IP3) receptor responsiveness. In species in which sperm has a strong PLC zeta activity, the oocyte IP3 receptor is less responsive. On the other hand, species in which sperm has a weak PLC zeta activity, the oocyte IP3 receptor is highly responsive. Interestingly, the IP3 receptor in mouse oocytes is highly responsive to PLC zeta. This could help to explain why Ca2+ oscillations were observed in mouse oocytes, but not observed in goat oocytes in the present study after injection of a single goat ROS.
It is well known that sperm activate oocytes by inducing a series of Ca2+ spikes. The Ca2+ signal activates the anaphase-promoting complexes (APC), leading to the destruction of key proteins necessary for meiotic arrest (Chang et al., 2004). Calmodulin-dependent protein kinase II (CaMKII) was found to trigger cell-cycle resumption in mouse eggs and to act downstream of sperm-induced Ca2+ release (Madgwick et al., 2005). However, although studies have been conducted on Ca2+ responses (Sato et al., 1998; Yanagida et al., 2000; Yazawa et al., 2000, 2001), no study has been reported on MPF/MAPK dynamics in ROSI oocytes. The present results showed that MPF and MAPK activities declined significantly after ICSI or ROSI+I, but both kinase activities remain constant following ROSI−I. Whereas ICSI and ROSI+I oocytes showed obvious Ca2+ increases, ROSI−I oocytes did not. Therefore, this is the first report that correlates Ca2+ responses with MPF/MAPK dynamics in ROSI oocytes.
In this study, although ICSI goat oocytes displayed typical Ca2+ oscillations, the ROSI+I oocytes showed only mild Ca2+ rises; however, cell cycle resumption was successful in both oocyte groups. Dissociation between oocyte meiotic resumption and Ca2+ oscillation-inducing abilities of spermatids has been reported previously (Yazawa et al., 2000, 2001). Although mouse elongated spermatid (ELS) could induce meiotic resumption of injected mouse oocytes, most of the ELS-injected oocytes did not show Ca2+ oscillations but only several transient Ca2+ rises (Yazawa et al., 2001). Similarly, although hamster, rabbit, and human ROS could induce mouse oocyte meiotic resumption efficiently, most of the Ca2+ patterns of injected oocytes were transient ones (Yazawa et al., 2000). In this study, however, goat oocytes injected with four ROS showed both cell-cycle resumption and typical Ca2+ oscillations. Together, these data suggest that: (1) oocyte meiotic resumption is more easily induced than Ca2+ oscillations and (2) spermatozoa contain more SOAF than spermatids do. Because this study showed the same degree of MPF/MAPK decline following goat ROSI+I and ICSI, and because healthy offspring have been produced after ROSI in several species (Gianaroli et al., 1999; Hirabayashi et al., 2002; Kimura and Yanagimachi, 1995; Haigo et al., 2004; Hirabayashi et al., 2009; Ogonuki et al., 2003), whether typical Ca2+ oscillations are necessary for the destruction of MPF/MAPK or for the support of normal development needs further investigation.
This study showed that 80–90% of ROSN underwent NEBD and PCC, with only 16% forming pronuclei after ROSI−I. In contrast, 80% of ROSN formed well-developed pronuclei without NEBD and only 16% PCC was observed following ROSI+I. In mice, small ROS-derived pronuclei were observed after ROSI. These small male pronuclei supported embryo development less efficiently than the regular-sized male pronuclei (Kimura and Yanagimachi, 1995). The formation of small pronuclei was probably due to the persistence of ROS nuclear membrane because it was observed more frequently when ROSN were injected into oocytes at more advanced stages in which the cytoplasmic MPF level had decreased. In the present study, small ROS-derived pronuclei were also observed in about 10% of goat oocytes treated with I+ROSI or I+ROSI+I protocols, but none of the ROSI+I oocytes formed small pronuclei (data not shown). Furthermore, significantly more ROSI+I goat oocytes (36%) formed three or more pronuclei than oocytes activated by I+ROSI (14%) or I+ROSI+I (12%) protocols. When ROSI mouse oocytes were left unactivated for 30–90 min, many ROSN underwent PCC, with chromosomes sometimes spreading, and three or more pronuclei were formed after activation of such oocytes (Kimura and Yanagimachi, 1995). This suggests that more goat ROSN had undergone NEBD and PCC after ROSI+I than we had actually observed in this study. However, the possibility cannot be excluded that goat ROS were transformed directly into pronuclei without undergoing NEBD and PCC because we did not observe NEBD in goat oocytes at any time points after ROSI+I.
The present results demonstrated that development of ROSI goat oocytes could be improved by optimizing activation protocols. Thus, whereas only 20% of ROSI+I oocytes developed into blastocysts, 33% of I+ROSI+I oocytes formed blastocysts. Blastocyst rates increased to 40% when ROSI oocytes were activated with I+ROSI+I in combination with 6-DMAP. In mice, the rate of normal fertilization was higher with pre-ROSI activation than with post-ROSI activation (Kimura and Yanagimachi, 1995). In bovine (Ock et al., 2006) and rabbit (Hirabayashi et al., 2009), embryo development was better in I+ROSI+I oocytes than in I+ROSI or ROSI+I oocytes, and incubation with cycloheximide and/or 6-DMAP after I+ROSI+I showed beneficial effects. In addition, both Ock et al. (2006) and the present study showed that I+ROSI produced higher blastocyst rates than ROSI+I. The present results that many ROSI+I goat oocytes formed multiple pronuclei suggests incidence of PCC. Few I+ROSI and I+ROSI+I oocytes formed small male pronuclei, which is detrimental to development. These results would would help to explain why pre-ROSI activation is better for development than post-ROSI activation. Furthermore, repeated ionomycin treatment causes multiple Ca2+ rises, which may be good for embryo development after fertilization (Ajduk et al., 2008), although typical Ca2+ oscillations may not be essential for oocyte activation.
Because ROS have not yet developed mature centriolar complexes with spindles, whether their injection into oocytes would lead to the formation of functional microtubule asters is not known. In this study, we observed for the first time in ROSI+I goat oocytes the organization of functional ROS asters that nucleated a radial microtubule network to fill the ooplasm, but it was not seen in ROSI−I oocytes. Small ROS asters were observed in 30% of the porcine oocytes, but they did not enlarge nor fill the ooplasm, whether after ROSI alone or ROSI plus electrical stimulation (Lee et al., 1998). Instead, a dense network of microtubules in the ooplasm was organized from the cortex. Rates of ROS aster formation in bovine oocytes were low (7% vs. 0%) regardless of ionomycin-6-DMAP activation, but ooplasmic aster formation was significantly increased by the addition of an activation step (Kani et al., 2008). It should be noted that in this study, whereas ROS asters were observed in only 30% of oocytes at 4 h, microtubule networks filling the ooplasm were observed in all of the activated oocytes by 6 h after ROSI+I. This suggests the possibility that oocyte-derived microtubules were organized into functional network very quickly between 4 and 6 h after ROSI+I. Kani et al. (2008) made only a single observation at 6 h after ROSI, thus they might have missed some ROS asters that had appeared at early stages, but observed both the ROS- and oocyte-derived network of microtubules in activated oocytes. Together, these data suggest that the ROSI zygotes of ruminants are similar in having microtubule networks from both ROS- and oocyte-derived asters. In addition, both our previous study (Lan et al., 2005) and the present one observed no microtubule network in pronuclear parthenogenetic goat oocytes activated with ionomycin alone. This suggests that a paternally derived factor(s) may be essential for organization of a functional microtubule network to support pronuclear migration in goat oocytes.
Oocytes in humans, mice, and other mammals lack identifiable centrioles (Sun and Schatten, 2007). The proximal centriole brought in by the fertilizing sperm in humans and most other mammals appears to give rise to the centrioles at the spindle poles in the zygote, and this is believed to indicate that centrioles are inherited through the paternal lineage in these species (Schatten et al., 1986). However, both the proximal and distal sperm centrioles degenerate in mice and other rodents. A bipolar mitotic spindle nucleates from multiple centrosome-like structures in the mouse zygote, and centrioles are not seen until the blastocyst stage, suggesting that centrioles are inherited through the maternal lineage in mice (Sun and Schatten, 2007). It is not known, however, whether the centriole in the goat is of paternal or maternal origin. According to Shin et al. (1998), the behavior of the centrosome can be inferred from the patterns of microtubules observed throughout the fertilization process. In the present study, we observed sperm asters organizing microtubule networks in some of the ROSI+I goat oocytes and in almost all the activated oocytes following ICSI. This indicates that the centriole in the goat is of paternal origin. The hamster was considered to have a maternal pattern of centrosome inheritance because no microtubule asters were found associated with fertilizing spermatozoa or ROSI (Shin et al., 1998).
In summary, we have conducted a systematic study on ROSI in the goat, and the results suggest that activation is the critical procedure for improving fertilization and development of ROSI oocytes. Goat ROS contain too little oocyte-activating factor to induce intracellular Ca2+ rises and thus to inhibit MPF and MAPK activities. Goat ROS can organize functional microtubular asters in activated oocytes. A paternally derived factor(s) may be essential for organization of a functional microtubule network to unite pronuclei in goat oocytes. Goat centrosome is of paternal origin.
Acknowledgments
This study was supported by grants from the National Basic Research Program of China (Nos. 2012CB944403 and 2007CB947403), the China National Natural Science Foundation (Nos. 30972096 and 30771556),and the China National Project of Transgenics (Nos. 2009ZX08008-006B).
Author Disclosure Statement
No competing financial interests exist.
References
- Ajduk A. Małagocki A. Maleszewski M. Cytoplasmic maturation of mammalian oocytes: Development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod. Biol. 2008;8:3–22. doi: 10.1016/s1642-431x(12)60001-1. [DOI] [PubMed] [Google Scholar]
- Al-Hasani S. Ludwig M. Palermo I. Küpker W. Sandmann J. Johannisson R. Fornara P. Sturm R. Bals-Pratsch M. Bauer O. Diedrich K. Intracytoplasmic injection of round and elongated spermatids from azoospermic patients: results and review. Hum. Reprod. 1999;14(Suppl 1):97–107. doi: 10.1093/humrep/14.suppl_1.97. [DOI] [PubMed] [Google Scholar]
- Balaban B. Urman B. Isiklar A. Alatas C. Aksoy S. Mercan R. Nuhoglu A. Progression to the blastocyst stage of embryos derived from testicular round spermatids. Hum. Reprod. 2000;15:1377–1382. doi: 10.1093/humrep/15.6.1377. [DOI] [PubMed] [Google Scholar]
- Chang H.Y. Levasseur M. Jones K.T. Degradation of APCcdc20 and APCcdh1 substrates during the second meiotic division in mouse eggs. J. Cell Sci. 2004;117(Pt 26):6289–6296. doi: 10.1242/jcs.01567. [DOI] [PubMed] [Google Scholar]
- Craft I. Bennett V. Nicholson N. Fertilizing ability of testicular spermatozoa. Lancet. 1993;342:864. doi: 10.1016/0140-6736(93)92722-6. [DOI] [PubMed] [Google Scholar]
- Craft I. Tsirigotis M. Bennett V. Taranissi M. Khalifa Y. Hogewind G. Nicholson N. Percutaneous epididymal sperm aspiration and intracytoplasmic sperm injection in the management of infertility due to obstructive azoospermia. Fertil. Steril. 1995;63:1038–1042. doi: 10.1016/s0015-0282(16)57544-x. [DOI] [PubMed] [Google Scholar]
- Edwards R.G. Tarin J.J. Dean N. Hirsch A. Tan S.L. Are spermatid injections into human oocytes now mandatory? Hum. Reprod. 1994;9:2217–2219. doi: 10.1093/oxfordjournals.humrep.a138426. [DOI] [PubMed] [Google Scholar]
- Fishel S. Aslam I. Tesarik J. Spermatid conception: A stage too early, or a time too soon? Hum. Reprod. 1996;11:1371–1375. doi: 10.1093/oxfordjournals.humrep.a019402. [DOI] [PubMed] [Google Scholar]
- Fissore R.A. Activating the egg: A tale of two molecules. Biol. Reprod. 2009;81(Suppl) Abstract 65. [Google Scholar]
- Gianaroli L. Selman H.A. Magli M.C. Colpi G. Fortini D. Ferraretti A.P. Birth of a healthy infant after conception with round spermatids isolated from cryopreserved testicular tissue. Fertil. Steril. 1999;72:539–541. doi: 10.1016/s0015-0282(99)00285-x. [DOI] [PubMed] [Google Scholar]
- Haigo K. Yamauchi Y. Yazama F. Yanagimachi R. Horiuchi T. Full-term development of hamster embryos produced by injection of round spermatids into oocytes. Biol. Reprod. 2004;71:194–198. doi: 10.1095/biolreprod.104.027706. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M. Kato M. Aoto T. Ueda M. Hochi S. Rescue of infertile transgenic rat lines by intracytoplasmic injection of cryopreserved round spermatids. Mol. Reprod. Dev. 2002;62:295–299. doi: 10.1002/mrd.10127. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M. Kato M. Kitada K. Ohnami N. Hirao M. Hochi S. Activation regimens for full-term development of rabbit oocytes injected with round spermatids. Mol. Reprod. Dev. 2009;76:573–579. doi: 10.1002/mrd.20984. [DOI] [PubMed] [Google Scholar]
- Huang Z. Tamuram M. Sakurai T. Chuma S. Saito T. Nakatsuji N. In vivo transfection of testicular germ cells and transgenesis by using the mitochondrially localized jellyfish fluorescent protein gene. FEBS Lett. 2000;487:248–251. doi: 10.1016/s0014-5793(00)02271-7. [DOI] [PubMed] [Google Scholar]
- Kani C. Takenaka M. Kamihata M. Ikegami M. Ochi M. Horiuchi T. Sperm aster formation and blastocyst development of bovine oocytes injection with spermatogenic cells. Biol. Reprod. 2008;78(Suppl):124. Abstract 257. [Google Scholar]
- Kimura Y. Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- Lan G.C. Han D. Wu Y.G. Han Z.B. Ma S.F. Liu X.Y. Chang C.L. Tan J.H. Effects of duration, concentration, and timing of ionomycin and 6-dimethylaminopurine (6-DMAP) treatment on activation of goat oocytes. Mol. Reprod. Dev. 2005;71:380–388. doi: 10.1002/mrd.20267. [DOI] [PubMed] [Google Scholar]
- Lavitrano M. Bacci M.L. Forni M. Lazzereschi D. Di Stefano C. Fioretti D. Giancotti P. Marfé G. Pucci L. Renzi L. Wang H. Stoppacciaro A. Stassi G. Sargiacomo M. Sinibaldi P. Turchi V. Giovannoni R. Della Casa G. Seren E. Rossi G. Efficient production by sperm-mediated gene transfer of human decay accelerating factor (hDAF) transgenic pigs for xenotransplantation. Proc. Natl. Acad. Sci. USA. 2002;99:14230–14235. doi: 10.1073/pnas.222550299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W. Kim N.H. Lee H.T. Chung K.S. Microtubule and chromatin organization during the first cell-cycle following intracytoplasmic injection of round spermatid into porcine oocytes. Mol. Reprod. Dev. 1998;50:221–228. doi: 10.1002/(SICI)1098-2795(199806)50:2<221::AID-MRD13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Levran D. Nahum H. Farhi J. Weissman A. Poor outcome with round spermatid injection in azoospermic patients with maturation arrest. Fertil. Steril. 2000;74:443–449. doi: 10.1016/s0015-0282(00)00698-1. [DOI] [PubMed] [Google Scholar]
- Madgwick S. Levasseur M. Jones K.T. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 2005;118(Pt 17):3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- Maione B. Lavitrano M. Spadafora C. Kiessling A.A. Sperm-mediated gene transfer in mice. Mol. Reprod. Dev. 1998;50:406–409. doi: 10.1002/(SICI)1098-2795(199808)50:4<406::AID-MRD4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Meng X. Akutsu H. Schoene K. Reifsteck C. Fox E.P. Olson S. Sariola H. Yanagimachi R. Baetscher M. Transgene insertion induced dominant male sterility and rescue of male fertility using round spermatid injection. Biol. Reprod. 2002;66:726–734. doi: 10.1095/biolreprod66.3.726. [DOI] [PubMed] [Google Scholar]
- Ock S.A. Kwack D.O. Lee S.L. Cho S.R. Jeon B.G. Kumar B.M. Choe S.Y. Rho G.J. In vitro development of bovine oocytes reconstructed with round spermatids. Theriogenology. 2006;65:1242–1253. doi: 10.1016/j.theriogenology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ogonuki N. Sankai T. Yagami K. Shikano T. Oda S. Miyazaki S. Ogura A. Activity of a sperm-borne oocyte-activating factor in spermatozoa and spermatogenic cells from cynomolgus monkeys and its localization after oocyte activation. Biol. Reprod. 2001;65:351–357. doi: 10.1095/biolreprod65.2.351. [DOI] [PubMed] [Google Scholar]
- Ogonuki N. Mochida K. Inoue K. Matsuda J. Yamamoto Y. Takano K. Ogura A. Fertilization of oocytes and birth of normal pups following intracytoplasmic injection with spermatids in mastomys (Praomys coucha) Biol. Reprod. 2003;68:1821–1827. doi: 10.1095/biolreprod.102.011726. [DOI] [PubMed] [Google Scholar]
- Ogura A. Yamamoto Y. Suzuki O. Takano K. Wakayama T. Mochida K. Kimura H. In vitro fertilization and microinsemination with round spermatids for propagation of nephrotic genes in mice. Theriogenology. 1996;45:1141–1149. doi: 10.1016/0093-691x(96)00070-2. [DOI] [PubMed] [Google Scholar]
- Ogura A. Yanagimachi R. Round spermatid nuclei injected into hamster oocytes from pronuclei and participate in syngamy. Biol. Reprod. 1993;48:219–225. doi: 10.1095/biolreprod48.2.219. [DOI] [PubMed] [Google Scholar]
- Practice Committee, Society for Assisted Reproductive Technology; Practice Committee, American Society for Reproductive Medicine. Round spermatid nucleus injection (ROSNI) Fertil. Steril. 2003;80:687–689. doi: 10.1016/s0015-0282(03)00969-5. [DOI] [PubMed] [Google Scholar]
- Rosenkrans C.F., Jr. Zeng G.Q. McNamara G.T. Schoff P.K. First N.L. Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod. 1993;49:459–462. doi: 10.1095/biolreprod49.3.459. [DOI] [PubMed] [Google Scholar]
- Runft L.L. Jaffe L.A. Mehlmann L.M. Egg activation at fertilization: Where it all begins. Dev. Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Sato Y. Miyazaki S. Shikano T. Mitsuhashi N. Takeuchi H. Mikoshiba K. Kuwabara Y. Adenophostin, a potent agonist of the inositol 1,4,5-trisphosphate receptor, is useful for fertilization of mouse oocytes injected with round spermatids leading to normal offspring. Biol. Reprod. 1998;58:867–873. doi: 10.1095/biolreprod58.3.867. [DOI] [PubMed] [Google Scholar]
- Schatten G. The centrosome and its mode of inheritance: The reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Schatten H. Schatten G. Mazia D. Balczon R. Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl. Acad. Sci. USA. 1986;83:105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoysman R. Vanderzwalmen P. Bertin G. Nijs M. Van Damme B. Oocyte insemination with spermatozoa precursors. Curr. Opin. Urol. 1999;9:541–545. doi: 10.1097/00042307-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Shin T.Y. Noguchi Y. Yamamoto Y. Mochida K. Ogura A. Microtubule organization in hamster oocytes after fertilization with mature spermatozoa and round spermatids. J. Reprod. Devel. 1998;44:185–189. [Google Scholar]
- Silber S.J. Van Steirteghem A.C. Liu J. Nagy Z. Tournaye H. Devroey P. High fertilization and pregnancy rate after intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy. Hum. Reprod. 1995;10:148–152. doi: 10.1093/humrep/10.1.148. [DOI] [PubMed] [Google Scholar]
- Sousa M. Barros A. Tesarik J. Current problems with spermatid conception. Hum. Reprod. 1998;13:255–258. doi: 10.1093/humrep/13.2.255. [DOI] [PubMed] [Google Scholar]
- Sun Q.Y. Schatten H. Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv. Exp. Med. Biol. 2007;591:58–71. doi: 10.1007/978-0-387-37754-4_4. [DOI] [PubMed] [Google Scholar]
- Swann K. Saunders C.M. Rogers N.T. Lai F.A. PLCzeta(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Terada Y. Ogonuki N. Ugajin T. Ogura A. Murakami T. Yaegashi N. Okamura K. Functional assessment of centrosomes of spermatozoa and spermatids microinjected into rabbit oocytes. Mol. Reprod. Dev. 2009;76:270–277. doi: 10.1002/mrd.20951. [DOI] [PubMed] [Google Scholar]
- Temple-Smith P.D. Southwick G.J. Yates C.A. Trounson A.O. de Kretser D.M. Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J. In Vitro Fert. Embryo Transf. 1985;2:119–122. doi: 10.1007/BF01131497. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Mendoza C. Spermatid injection into human oocytes. I. Laboratory techniques and special features of zygote development. Hum. Reprod. 1996;11:772–779. doi: 10.1093/oxfordjournals.humrep.a019253. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Mendoza C. Testart J. Viable embryos from injection of round spermatids into oocytes. N. Engl. J. Med. 1995;333:525. doi: 10.1056/NEJM199508243330819. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Cruz-Navarro N. Moreno E. Canete M.T. Mendoza C. Birth of healthy twins after fertilization with in vitro cultured spermatids from a patient with massive in vivo apoptosis of post-meiotic germ cells. Fertil. Steril. 2000a;74:1044–1046. doi: 10.1016/s0015-0282(00)01569-7. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Mendoza C. Greco E. The activity (calcium oscillator?) responsible for human oocyte activation after injection with round spermatids is associated with spermatid nuclei. Fertil. Steril. 2000b;74:1245–1247. doi: 10.1016/s0015-0282(00)01598-3. [DOI] [PubMed] [Google Scholar]
- Vanderzwalmen P. Zech H. Birkenfeld A. Yemini M. Bertin G. Lejeune B. Nijs M. Segal L. Stecher A. Vandamme B. van Roosendaal E. Schoysman R. Intracytoplasmic injection of spermatids retrieved from testicular tissue: Influence of testicular pathology, type of selected spermatids and oocyte activation. Hum. Reprod. 1997;12:1203–1213. doi: 10.1093/humrep/12.6.1203. [DOI] [PubMed] [Google Scholar]
- Vicdan K. Isik A.Z. Delilbasi L. Development of blastocyst-stage embryos after round spermatid injection in patients with complete spermiogenesis failure. J. Assist. Reprod. Genet. 2001;18:78–86. doi: 10.1023/A:1026578507736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K. Yazawa H. Katayose H. Kimura Y. Hayashi S. Sato A. Oocyte activation induced by spermatids and the spermatozoa. Int. J. Androl. 2000;23(Suppl 2):63–65. doi: 10.1046/j.1365-2605.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- Yazawa H. Yanagida K. Katayose H. Hayashi S. Sato A. Comparison of oocyte activation and Ca2+ oscillation-inducing abilities of round/elongated spermatids of mouse, hamster, rat, rabbit and human assessed by mouse oocyte activation assay. Hum. Reprod. 2000;15:2582–2590. doi: 10.1093/humrep/15.12.2582. [DOI] [PubMed] [Google Scholar]
- Yazawa H. Yanagida K. Sato A. Oocyte activation and Ca(2+) oscillation-inducing abilities of mouse round/elongated spermatids and the developmental capacities of embryos from spermatid injection. Hum. Reprod. 2001;16:1221–1228. doi: 10.1093/humrep/16.6.1221. [DOI] [PubMed] [Google Scholar]
- Yu Y. Saunders C.M. Lai F.A. Swann K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase C zeta. Hum. Reprod. 2008;23:365–373. doi: 10.1093/humrep/dem350. [DOI] [PubMed] [Google Scholar]
- Zhao B.T. Han D. Xu C.L. Luo M.J. Chang Z.L. Tan J.H. Protocol optimization for long-term liquid storage of goat semen in a chemically defined extender. Reprod. Domest. Anim. 2009;44:865–872. doi: 10.1111/j.1439-0531.2008.01101.x. [DOI] [PubMed] [Google Scholar]