Abstract
Cold water submersion can induce a high incidence of cardiac arrhythmias in healthy volunteers. Submersion and the release of breath holding can activate two powerful and antagonistic responses: the ‘cold shock response’ and the ‘diving response’. The former involves the activation of a sympathetically driven tachycardia while the latter promotes a parasympathetically mediated bradycardia. We propose that the strong and simultaneous activation of the two limbs of the autonomic nervous system (‘autonomic conflict’) may account for these arrhythmias and may, in some vulnerable individuals, be responsible for deaths that have previously wrongly been ascribed to drowning or hypothermia. In this review, we consider the evidence supporting this claim and also hypothesise that other environmental triggers may induce autonomic conflict and this may be more widely responsible for sudden death in individuals with other predisposing conditions.

Michael Tipton (left) is Professor of Human & Applied Physiology, Extreme Environments Laboratory, Department of Sport & Exercise Science, University of Portsmouth, UK. He was educated at the Universities of Keele and London, and then joined the University of Surrey in 1986. After 12 years in the Robens Institute and European Institute of Health and Medical Science he moved to the University of Portsmouth in 1999. In addition to his university positions, Professor Tipton was based at the Institute of Naval Medicine (INM) from 1983 to 2004 and was Consultant Head of the Environmental Medicine Division of the INM from 1996. He has published over 350 papers, reviews, reports, chapters and books in the field of extreme environmental physiology. Michael Shattock (right) is Professor of Cellular Cardiology, Cardiovascular Division, King's College London. Mike graduated from the University of London with a BSc in Comparative Physiology in 1979 with dreams of becoming the next Jacques Cousteau. A final year project on the effects of potassium on the frog heart, however, sparked his interest in cardiac physiology and, as they say, the rest is history! This interest in cardiac electrophysiology and ion regulation was the basis of his PhD at St Thomas’ Hospital (1984), and a subsequent post-doc in California with Donald Bers (1985–88). He was appointed Professor of Cellular Cardiology at King's College London in 2004. His research interests focus on cellular electrophysiology and Na+ regulation in heart cells. His interest in marine and diving physiology has recently been rekindled by both undergraduate teaching and collaborative projects. He has published over 250 papers, abstracts and reviews.
Introduction: do all drowning victims drown?
In most countries of the world, immersion represents the second most common cause of accidental death in children and the third in adults (Bierens et al. 2002). Internationally, there are about half a million immersion-related deaths each year; about 500 of these occur in the UK where, on average, we lose about one child a week. Historically, death in cold water was generally ascribed to hypothermia; more recently, description of the initial ‘cold shock’ response (Tipton, 1989b) to immersion and other factors have refocused attention on drowning. Early summer is the most dangerous time of the year in the Northern Hemisphere for immersion deaths, with air temperatures rising in advance of water temperatures. In this review we describe a cardiac arrhythmogenic response that we believe may account for some immersion deaths but, due to its nature, largely goes unnoticed or detected. We have termed the trigger for this response ‘autonomic conflict’. It is inherent in our hypothesis that such autonomic conflict, particularly in the presence of other predisposing factors (see below), may provide the substrate for lethal rhythm disturbances and hence may explain some of the more puzzling statistics relating to cold water immersion, namely, 67% of drownings occur in strong swimmers and 55% of these are within 3 m of a safe haven (Home Office Report, 1977). These short-term deaths are usually ascribed to drowning and occasionally, when no water appears to have entered the lung, to hypothermia or ‘dry’ drowning, even though the period of immersion is too short (<30 min) for dangerous levels of hypothermia to occur, and the evidence for ‘dry’ drowning is unconvincing (Golden & Tipton, 2002). Electrical disturbances to the heart are usually not considered as a cause of death, primarily because they are undetectable post-mortem, and the incapacitation they cause may lead to agonal gasping, aspiration of water and death as a result of what appears to have been drowning (McDonough et al. 1987).
Autonomic conflict
The concept
Classically, the vagal and sympathetic drives to the heart are thought of as being exact opposites. The dogma in most textbooks is that there is a reciprocal relationship in which sympathetic and parasympathetic influences on the heart are not only in opposition, but are activated separately or sequentially. In 1979, Kollai and Koizumi pointed out that ‘… since Langley's time, reciprocal action between sympathetic and parasympathetic outflows to the heart has been taken for granted’ (Kollai & Koizumi, 1979). However, in this paper, Kollai and Koizumi recognised that this ‘alternate’ control is not the only pattern of autonomic outflow. In anaesthetised dogs, they simultaneously recorded from vagal and sympathetic nerves and observed that the pattern of autonomic activation was critically dependent on the nature of the experimental stimulus. For example, an acute rise in blood pressure activates the baroreflex in the classic ‘yin and yang’ reciprocal pattern; ie an immediate decrease in sympathetic activity and increase in vagal tone. However, Kollai and Koizumi also reported numerous instances in which simultaneous co-activation of the autonomic inputs to the heart can be evoked. These triggers, reviewed by Paton et al. (2005), include the activation of peripheral chemoreceptors, mild prolonged hypercapnia, the occulocardiac reflex, the startle response, somatic nociception and nasopharyngeal stimulation by formaldehyde. Paton et al. (2005) note that many of these triggers can generate cardiac arrhythmias and even, exceptionally, sudden death.
Sympathetic and parasympathetic drive during cold water immersion
Perhaps one of the most powerful and reproducible ways of inducing the autonomic conflict described above is by rapid submersion in cold water (<15°C) with attempted breath holding. This activates two powerful autonomic responses: (i) the cold shock response, and (ii) the diving response.
The cold shock response is a pattern of reflexes driven by cutaneous cold thermoreceptors, and characterised by sympathetically mediated tachycardia, a respiratory gasp, uncontrollable hyperventilation, peripheral vasoconstriction and hypertension (Tipton, 1989b).
The diving response features a profound sinus bradycardia driven by excitation of cardiac vagal motoneurones: an expiratory apnoea assisted by reflex inhibition of the central respiratory neurones and excitation of the sympathetic vasoconstrictor neurones producing vasoconstriction mainly in the trunk and limbs. As such, the primary function of the diving response is to conserve oxygen and extend underwater time. The appearance of the primary cardiovascular responses is dependent on there being no concomitant increase in activity of the lung stretch receptors; activation of these receptors can evoke secondary tachycardia and vasodilatation. The apnoea also leads to arterial hypoxaemia and hypercapnia and thus, after a delay, to the stimulation of the peripheral arterial chemoreceptors which reinforce the more immediate cardiovascular effects. There is a negative inotropic effect on the ventricles (Bert, 1870; de Burgh Daly & Angell-James, 1979). The response occurs in all marine and terrestrial vertebrates thus far examined, including man. It appears to be initiated partly consciously and partly by a reflex evoked by the cooling of the cold receptors of the face innervated by the ophthalmic and maxillary division of the trigeminal nerve. Stimulation of vagal receptors in the pharynx and larynx can give rise to similar responses (Angell-James & de Burgh Daly, 1975).
In man, any bradycardia observed during submersion in water is generally considered to be due to a combination of ‘neural’ and ‘mechanical’ factors. Cold facial immersion activates a classic diving response via the trigeminal innervation of the face while breath holding activates stretch receptors in the lung (Paulev, 1968). Mechanical factors suggested to contribute to the bradycardia include the cephalic redistribution of blood volume associated with immersion and changes in transmural and thoracic pressures with the release of breath holding on surfacing (Risch et al. 1978). While humans exhibit diving bradycardia and vasoconstriction on breath hold immersion, it is attenuated and slower to develop compared with that seen in diving mammals (Lin, 1988). Perhaps revealingly, arrhythmias are rarely observed in diving animals that, while possessing a strong diving response, may lack a significant cold shock response. This would be consistent with our proposal that a combination of the two responses (as occurs in man) is required for the occurrence of autonomic conflict and a consequent high incidence of arrhythmias.
In most individuals, the heart rate response to sudden total submersion is dominated by cold shock, resulting in a tachycardia. However, in many circumstances a slight amelioration of the cold shock response (due, for example, to clothing (Tipton, 1989a) cold habituation, breath holding or orientation on submersion) can, we hypothesise, bring the autonomic control of the heart into conflict.
Cold water immersion and cardiac arrhythmias
Studies in healthy volunteers
Cardiac arrhythmias have often been reported during breath hold diving, especially in cold water. For example, the incidence of cardiac arrhythmias during dives undertaken by Korean women (similar to the Japanese Ama) increases from 43% in the summer to 72% in the winter (Hong, 1976). Wyss (1956) reported the first rhythm changes, including nodal extrasystoles, in man during immersion and underwater swimming. Olsen et al. (1962) observed cardiac arrhythmias during breath holding in air and during shallow submersions (1.4 m). Greater bradycardia and incidence of arrhythmias were seen during submersion (Olsen et al. 1962). With the exception of atrial, nodal and ventricular premature contractions, the most common arrhythmias observed were inhibitory in nature (e.g. sinus arrest, atrioventricular (AV) nodal block). In general, the arrhythmias are thought to be associated with enhanced cardiac vagal activity, but relatively little specific investigation of the mechanism producing these arrhythmias has been undertaken.
Indeed, cardiac arrhythmias have probably not received the attention they deserve as a cause of death on immersion because firstly, they can go undetected and secondly, the vast majority of studies conducted on cold water immersion and submersion have examined young healthy participants. When this cohort undertakes free-breathing head-out immersions in cold water laboratory tests, arrhythmias are seen in about 2% of immersions (Datta & Tipton, 2006). This predominantly activates the sympathetically driven cold shock response. Away from the laboratory, vagal arrest of the heart may occur in rare instances, for example by mechanical stimulation of laryngeal and pharyngeal receptors, but is reversed with expansion of the lung during resuscitation (Wolf, 1964; de Burgh Daly et al. 1979). Thus, both sympathetic and parasympathetic stimulation of the heart can, independently, induce cardiac arrhythmias. However, co-incidental stimulation of both divisions of the autonomic nervous supply to the heart, as occurs during cold water submersion, with breath holding, produces a much higher incidence of arrhythmias (62–82%) in young, fit and healthy participants (Fig. 1). In other words, we argue that in such circumstances there is ‘autonomic conflict’: simultaneous and conflicting positive and negative chronotropic drives to the heart (Tipton et al. 1994).
Figure 1. ECG trace during submersion.
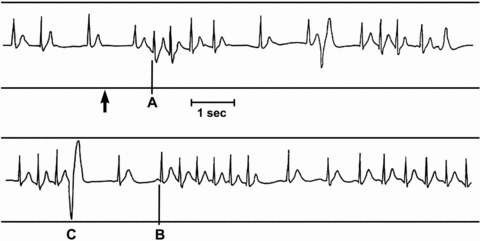
The end of breath hold is indicated by the arrow: supraventricular extrasystoles (A), run of supraventricular tachycardia (B) and premature ventricular complex (C) (reproduced with permission from the Undersea & Hyperbaric Medical Society, from Tipton et al. (1994)).
The arrhythmias that occur in such circumstances, when there is dual activation of the sympathetic and parasympathetic drives, are predominantly supraventricular and junctional (Tipton et al. 1994; Datta & Tipton, 2006). These include short bursts of ventricular tachycardia interposed between periods of bradycardia, supraventricular ectopics and even AV block. That they are usually asymptomatic suggests they are haemodynamically effective. They are idiosyncratic in nature, with the same person demonstrating the same ECG waveform on repeated exposures. The arrhythmias are often linked to respiration and tend to occur when the heart rhythm changes between tachycardia (sympathetic predominance) to bradycardia (vagal predominance) and within 10 s of the cessation of breath holding (Datta & Tipton, 2006).
That arrhythmias are also observed on the cessation of breath holding on immersion without face immersion (Datta et al. 2005) suggests that the release of breath holding is, in itself, an arrhythmogenic trigger. It remains unclear if this is due to ‘mechanical’ (changes in intrathoracic pressure and venous return) or ‘neural’ responses associated with the release of the stimulation of the cardiac vagal neurones at the end of breath holding.
Having seen arrhythmias (predominantly supraventricular and junctional) just after maximal breath holding in 60% of their head-out cold water immersions, Datta et al. (2005) concluded that the arrhythmias were due to the interaction of release of breath holding in a cold milieu (Datta et al. 2005). As in the earlier study by Tipton et al. (1994), it was also notable that the arrhythmias were often time linked to respiration; this was interpreted as suggesting that the arrhythmias were, in part, due to a cyclical vagal stimulus to the heart (Tipton et al. 1994). Given that the central respiratory rhythm appears to continue during breath holding (Cooper et al. 2004), it is interesting to speculate whether the appearance of arrhythmias is related to the timing or magnitude of this rhythm in relation to the cessation of breath holding. Certainly, evidence suggesting that afferents arising from the diaphragm at the end of breath holding, and associated with the sensation of distress, are transmitted in the vagus (Guz et al. 1966; Parkes, 2006) raises interesting possibilities, and supports the contention that the arrhythmias seen post breath holding are neural rather than mechanical in origin.
Alternative explanations for the arrhythmias seen on submersion have been offered, including hypoxaemia, respiratory acidosis and hydrostatic changes associated with immersion and consequent cardiac distension (Ferrigno & Lundgren, 2003). However, that the arrhythmias are seen after short breath holds (Tipton et al. 2010), during horizontal immersion (Datta & Tipton, 2006) and in isolated hearts (see below) does not support a major role for chemical or hydrostatic factors in their genesis. The present data suggest that arrhythmias are most likely to occur in a situation where there is varying vagal stimulation to the heart against a background of sympathetic stimulation i.e. autonomic conflict.
From a practical perspective cardiac arrhythmias typical of those seen with autonomic conflict have recently been reported during helicopter underwater escape training (Tipton et al. 2010). This is undertaken by organisations such as the military and oil industry employees and involves an inversion into water (usually warm) and a short breath hold (approximately 10 s). Interestingly, the arrhythmias are more apparent in less aerobically fit individuals; it has been concluded that the physiology of the cardiac vagus nerves are modified by fitness training (Arnold, 1985) and Bove et al. (1968) suggested that fitness training augments diving bradycardia. In addition, facial immersion in chilled water during breath holding has been reported to be the best method of identifying individuals who later develop arrhythmias whilst diving (McDonough et al. 1987). Finally, as noted earlier, analysis of reports of fatal immersions reveals that, in the absence of clear evidence to the contrary, some deaths have been wrongly ascribed to hypothermia. Such mis-diagnoses of the cause of death have resulted in inappropriate legal action and much upset. It is pertinent to note that electrical disturbances to the heart that result in fatal arrhythmias are undetectable post-mortem.
Studies in vitro in isolated hearts
A crude form of autonomic conflict can be simulated in isolated hearts by the simultaneous perfusion of autonomic agonists. The advantage of this approach is that it removes many of the confounding factors previously suggested as contributing to the production of arrhythmias on immersion; these variables include intrapleural pressure and hydrostatic pressure, for example. Figure 2 shows such an experiment. In this previously unpublished example, an isolated Langendorff-perfused rat heart was subjected to a basal low level of sympathetic tone (75 nm adrenaline + 313 nm noradrenaline: Adr–NA). These concentrations were chosen to be similar to those measured in conscious rats (Curtis et al. 1985) and are comparable to those measured in man during moderate stress (Baumgartner et al. 1985; Ratge et al. 1986; Coplan et al. 1989). This moderate level of sympathetic drive was sufficient to raise the basal heart rate from 298 ± 2 beats min−1 to 394 ± 15 beats min−1 but did not maximally stimulate the hearts. After stabilisation, and in the maintained presence of Adr–NA, hearts were then exposed to 3 × 30 s pulses of ACh (30 μm) (to mimic bursts of parasympathetic drive as occur, for example, during break of breath hold) interspersed with 4 min washout in Adr–NA alone. Epicardial ECG was recorded throughout and arrhythmias quantified using the Lambeth Conventions (Walker et al. 1988) and given an arrhythmia score depending on the type and number of the arrhythmia observed. Figure 2 shows that superimposing ACh on a background of sympathetic agonists (Adr–NA) induces a wide range of arrhythmias including AV block, tachycardia, bradycardia, extrasystoles and, most surprisingly, a rhythm resembling torsades de pointes.
Figure 2. An example of an epicardial ECG recorded from an isolated rat heart during simulated autonomic conflict.
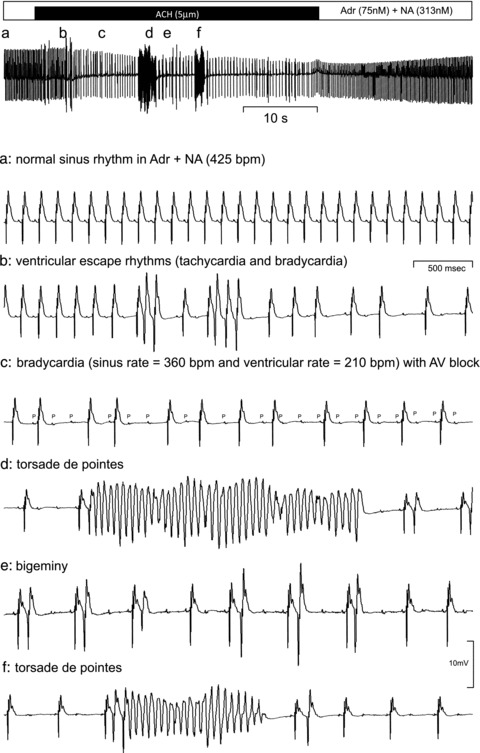
The heart was perfused with a constant background concentration of adrenaline (75 nm) and noradrenaline (313 nm) and a 1 min period of acetylcholine (ACh: 5 μm) was superimposed as indicated. The top trace shows a slow time-base recording and the arrhythmias recorded at the points marked a–f on this trace are expanded below.
Figure 2 demonstrates two striking features of these in vitro observations: (i) the pattern of arrhythmias recorded in the rat heart, alternating brady- and tachycardia, AV block etc., is similar to that induced during cold water immersion in human participants (see Fig. 1), and (ii) to our knowledge this is the first report of a model which induces torsades de pointes in an isolated rat heart. As can be seen in Fig. 3, the arrhythmia scores were consistently increased in hearts that were exposed to repeated bouts of simulated autonomic conflict.
Figure 3. Total arrhythmia score for three successive periods of simulated autonomic conflict in isolated rat hearts.
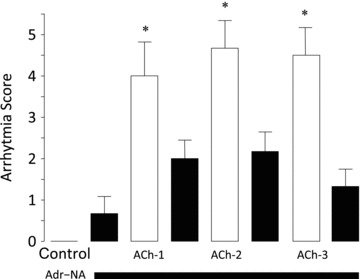
Hearts were perfused with a constant background concentration of adrenaline (75 nm) and noradrenaline (313 nm) and then three 1-min periods of acetylcholine (ACh) were superimposed separated by 3 min of ACh washout. Arrhythmia scoring: (1) AV block, (2) ventricular premature beats, (3) bigeminy, (4) salvo, (5) ventricular tachycardia, (6) ventricular fibrillation, (7) torsades de pointes. *P < 0.05 when compared with the immediately preceding adrenaline/noradrenaline perfusion period. Data are means ± SEM (n= 6).
Predisposing factors
QT hysteresis and cold water immersion
The QT interval of the ECG approximates to the underlying ventricular action potential duration. When heart rate increases (i.e. the RR interval of the ECG decreases), both action potential duration and the QT interval decrease and, conversely, when heart rate slows the action potential and the QT interval are prolonged. We have recently observed a phenomenon that may predispose individuals to arrhythmias during rapid changes in heart rate such as occur during autonomic conflict. Figure 4A shows heart rate recorded in healthy human volunteers during the activation of a diving response by facial immersion in water at 12°C. Heart rate falls from a baseline of an average (SEM) 96 (7) beats min−1 to 56 (3) beats min−1 within 30 s. However, despite this bradycardia, there is no concomitant prolongation of the QT interval (Fig. 4B). Although an RR/QT hysteresis has been described before, (Arnold et al. 1982; Lau et al. 1988) in the example shown in Fig. 4 there is a striking failure of the QT interval (action potential duration) to prolong in response to the significant bradycardia even after 30 s. Figure 4C shows a linear nomogram relating the QT to the prevailing RR interval under steady-state conditions and the deviation from this relationship after 30 s of facial immersion in cold water (Wong et al. 2009).
Figure 4. Heart rate changes (A) and QT interval (B) measured during the activation of the diving response elicited by a 30 s facial immersion in cold water (12°C) with breath-hold.
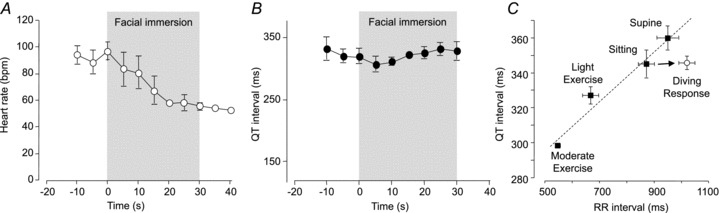
Participants were young, healthy volunteers (n= 4). C, relationship between the QT interval of the ECG and prevailing hearts rate (RR interval) in healthy male participants during mild and moderate exercise, while sitting, supine or after 30 s of facial immersion with apnoea (diving response). The nomogram described by the filled symbols is fitted with a straight line: QT (ms) = (0.14 × RR) + 225. Data are expressed as means ± SEM (n= 10) and r2 for the regression line is 0.9592. C reproduced with permission from Wong et al. (2009).
That the vagally induced bradycardia associated with activation of the diving response in humans is not accompanied by a prolongation of the QT interval has also been observed by others (Davidowski & Wolf, 1984). There may be two explanations for this. First, the latency of QT/RR hysteresis – that is, during the early period of the diving response (i.e. the first 30 s) – the QT interval (action potential duration) does not have time to ‘catch-up’ with the prevailing heart rate (Arnold et al. 1982; Franz et al. 1988; Lau et al. 1988; Carmeliet, 2006). Eisner et al. (2009) review the mechanisms underlying the slow response of the action potential duration/QT interval to abrupt changes in rate and describe three phases: (i) an early phase (milliseconds) – due to restitution of ion channels; (ii) a slower phase (seconds to minutes) – due to changes in transmembrane ion gradients; and (iii) a very slow phase (hours to days) – the so-called cardiac memory (Eisner et al. 2009). Thus, while the QT interval may take many minutes or even hours to respond to completion, the very rapid early phase changes in QT interval should have been seen during a 30 s facial immersion. Extrapolating from the data of Lau et al. (1988) suggests that an abrupt pacing-induced change in rate from 110 to 50 beats min−1 (comparable to that seen during the diving response) results in approximately a 20% (80 ms) prolongation of QT interval within 30 s (Lau et al. 1988). This prolongation should have been easily detectable in the data shown in Fig. 4 but was not observed. The second possible explanation for the failure of the QT interval to prolong within 30 s of a diving response is that facial immersion may exert some unique effect that differs from a pure pacing-induced heart rate change. Indeed, Davidowski and Wolf (1984) have suggested that this failure of the QT interval to prolong in response to an abrupt change in heart rate may be a general feature of vagal activation.
Whatever the mechanism of the failure of the action potential to adapt to an abrupt change in rate, this phenomenon may seriously predispose to ventricular arrhythmias during periods of autonomic conflict. If the action potential remains long following a rapid increase in heart rate, then the myocardial cell will spend a greater proportion of the cardiac cycle depolarised. This, in the face of a rate-dependent increase in calcium influx, will increase the likelihood of cellular calcium overload – a known trigger for ventricular automaticity and arrhythmias. Conversely, on a rapid reduction in heart rate, if the action potential duration remains short as the diastolic interval prolongs, then the relative refractory period will decrease leaving the heart more vulnerable to re-entrant arrhythmias.
Heterogeneity of cardiac innervation
The proarrhythmic potential of autonomic conflict may be exacerbated by the heterogeneity of autonomic innervation to the heart. While parasympathetic nerves are found predominantly in the atria (and particularly the sinoatrial (SA) and AV nodes) ventricular innervation is dominated by sympathetic neurones (Kawano et al. 2003). Even within the left ventricle it is clear there is a gradient of sympathetic innervation which becomes increasing sparse from base to apex (Kawano et al. 2003). This heterogeneity is also seen post-synaptically with an uneven distribution of myocardial adrenergic and muscarinic receptors. Muscarinic receptors are, for example, relatively poorly expressed in the ventricular myocardium but are strongly expressed in atrial and nodal tissues (Brodde et al. 2001). β-Adrenergic receptors, on the other hand, are reported to be either evenly distributed (Brodde et al. 2001) or more strongly expressed in the ventricle with a decreasing gradient from apex to base (Mori et al. 1993). These regional variations are likely to compound electrical inhomogeneity in circumstances where both limbs of the autonomic nervous system are strongly co-activated. It is therefore possible that the strong activation of the parasympathetic inputs will exert its effects predominantly supraventricularly; that is, it may cause a strong bradycardia without significant effects on the ventricle. Conversely, the simultaneous activation of sympathetic inputs to the heart may have little chronotropic effect (when the vagal inhibition is strong) but may have strong effects in the ventricle. Kollai and Koizumi have suggested that these reciprocal autonomic inputs are actually physiologically beneficial and synergistic, allowing for increased ventricular filling (mediated by the vagally induced bradycardia) and increased ventricular inotropy (mediated by the activation of ventricular β-receptors). Similarly, Paton and colleagues have suggested that during a vagally mediated bradycardia, a concomitant sympathetic stimulation of the ventricle may counteract the rate-dependent decrease in cardiac contractility mediated by the Bowditch effect (Paton et al. 2005).
Since, at rest, atropine increases and beta blockers decrease heart rate, it is clear that the heart is subject to simultaneous and tonic inputs from both limbs of the autonomic nervous system; that is, the heart normally functions with “one foot gently on the brake, the other gently on the accelerator”. As argued by Paton et al. (2005), this is proposed to be the normal state of affairs for autonomic control of cardiac function. In this review we propose that in extreme situations, such as rapid cold water submersion, the proverbial cardiac accelerator and brake are vigorously and simultaneously applied – the accelerator being strongly pressed while the brakes are cyclically ‘pumped’. This strong co-activation of both limbs of the autonomic nervous system may precipitate arrhythmias as a consequence of increased regional electrical heterogeneity. In the presence of other predisposing factors (such as those noted below), these arrhythmias may be lethal (see Fig. 5).
Figure 5. Autonomic conflict.
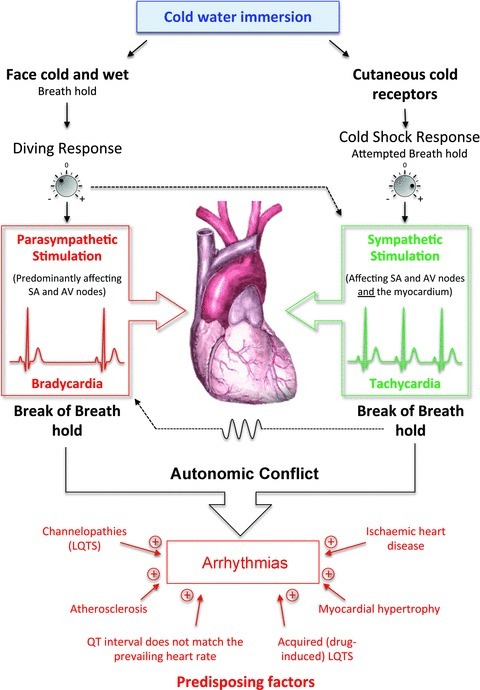
Cold water immersion activates two powerful reflexes – the diving response (on facial immersion) and the cold shock response (on the activation of cutaneous cold receptors). The magnitudes of these responses can vary with a range of factors including water temperature, clothing and habituation. Individually, the diving response triggers a parasympathetically driven bradycardia while cold shock activates a sympathetically driven tachycardia. We hypothesise that together these conflicting inputs to the heart can lead to arrhythmias – particularly at the break of breath hold which increases parasymypathetic tone that varies with respiration. The substrate for arrhythmias is enhanced by various predisposing factors including the failure of the QT interval to match the rapid and transient changes in heart rate. In circumstances other than cold water immersion these may additionally include awakening, anger, stress, arousal etc.
Little is known about the neural pathways that might be involved in the production of autonomic conflict. Using c-fos protein immunohistochemistry we have identified in the rat the neuronal cell groups activated by cold shock in the nucleus tractus solitarii, area postrema and dorsal motor nucleus, areas known to process cardiovascular and respiratory afferents (Datta & Tipton, 2006). The fact that arrhythmias are often linked to changes in respiration or lung inflation and occur when the heart changes between vagally dominated bradycardia and sympathetically dominated tachycardia indicates that they reflect complex interactions between central respiratory neurones, cardiac vagal neurones and sympathetic pre-motor neurones supplying the heart. However, it seems likely that it is distally, at the level of the heart, that efferent pathways converge to induce arrhythmias.
Heritable and acquired LQTS
There is a strong association between swimming and sudden cardiac arrest in children with heritable long QT syndrome (LQTS) (Brookfield et al. 1988; Ackerman et al. 1999; Batra & Silka, 2002; Choi et al. 2004). Ishikawa et al. (1992) reported that 51 of 64 children with known LQTS developed significant arrhythmias while swimming or diving. Furthermore, in children with a high probability of sporadic LQTS, cold water face immersion results in a much longer QT interval than in control children (Yoshinaga et al. 1999). Interestingly, a recent post-mortem study using DNA sequencing has revealed that nearly 30% of the victims of seemingly unexplained drowning had cardiac ion channel mutations (Tester et al. 2011). Certain drugs may also prolong QT interval e.g. antihistamines (terfenadine, astemizole), class Ia antiarrhythmics (quinidine, procainamide), class III antiarrhythmics (amiodarone, sotalol), antibiotics (erythromycin, clarithromycin), gastrointestinal prokinetics (cisapride, domperidone) and antipsychotics (chlorpromazine, haloperidol, thioridazine, mesoridazine), and all may predispose patients to lethal arrhythmias, particularly torsades de pointes. We would hypothesize that either heritable or drug-induced QT prolongation may predispose to the pro-arrhythmic effects of autonomic conflict.
Ischaemic heart disease
During sympathetic/parasympathetic conflict, periods of sympathetic activation and associated, transient tachycardia will increase oxygen consumption. In the normal heart, although vasodilatation occurs with increasing rate, myocardial blood flow per beat is reduced (Tanaka et al. 1990; Colin et al. 2004). In patients with atherosclerosis, this rate-induced mismatch between oxygen demand and supply is exacerbated by the failure of the diseased vessels to dilate appropriately (Nabel et al. 1990). In cold water immersion, and most of the other complex responses described above, the relative decrease in myocardial perfusion will be associated with an increase in peripheral vasoconstriction and an increase in mean arterial blood pressure. This, in turn, will increase cardiac afterload and, in the specific case of cold water immersion, the hydrostatic effects will transiently increase venous return, pre-load and cardiac output. Furthermore, the associated hyperventilation and consequent hypocapnia may lead to coronary vasoconstriction. This combination is likely to exacerbate ischaemia and may thus amplify any concomitant pro-arrhythmic stimulus.
Autonomic conflict: a mediator of sudden cardiac death?
While cold water immersion may be a strong trigger for autonomic conflict other environmental triggers may, when in unfortunate combination, also trigger this scenario. Sudden cardiac death (SCD) out of hospital accounts for a large number of premature deaths per year in the UK and ∼460,000 deaths per year in the USA (Zheng et al. 2001). Drugs and lifestyle changes have had some impact on the overall heart disease death rate over the last 20 years, but death from lethal cardiac arrhythmia (the most likely terminal event in the majority of SCD victims) has proven resistant to prophylaxis. Since SCD is often the first symptom of heart disease, this is a major outstanding area of therapeutic need. A link between autonomic conflict and SCD risk in the human is difficult to examine and impossible to prove by clinical investigation alone. Nevertheless there are several pieces of evidence that make the link plausible. It has long been known that there is a circadian peak in SCD and life-threatening arrhythmias in the early morning (Muller et al. 1987; Willich et al. 1987; Lampert et al. 1994; Williams & Zipes, 2003). More recently, Muller et al. (2003) and others (Beard et al. 1982; Douglas et al. 1991), have shown there is also a profound seasonal variation with a peak in life-threatening arrhythmias in December–January. This ‘winter peak’ is apparent in both patients with and without ischaemic heart disease.
Although it is well established that a link appears to exist between SCD and ‘stress’ triggers including anger, sex, grief and fear, the nature of the link is unexplained (Kloner, 2006; Taggart et al. 2011). In general, however, chronic increased sympathetic outflow has been considered to be pro-arrhythmic while increased parasympathetic activity has been considered protective (Schwartz & Zipes, 2004). However, the situation is likely to be more complex. As reviewed recently by Taggart et al. (2011), different types of emotional stress may differentially affect the two limbs of the autonomic nervous system. Anger, for example, activates an increase in sympathetic tone in the face of a maintained parasympathetic tone and is the emotion most associated with ventricular fibrillation (Lampert et al. 2002; Rainville et al. 2006). Other less dangerous stresses (such as fear, happiness and sadness) are associated with an increased sympathetic tone in the face of decreased parasympathetic activity. In this review we hypothesise that strong and changing vagal stimulation, which with more moderate activation may be cardio-protective, may be pro-arrhythmic when combined acutely with a strong and co-incident sympathetic activation.
Increased SCD risk is notably linked with stress that is ‘unplanned’. Thus, sudden waking from sleep is recognised as one of the most well-established triggers of SCD (Kloner, 2006). Interestingly, the border between sleep and wakefulness is also marked by an autonomic shift from parasympathetic to sympathetic dominance (Baharav et al. 1995). A link between SCD and unplanned stress is also indicated by the observed increase in local SCD rate during the SCUD attacks by Iraq on Israel in 1991 (Sapolsky, 1999). Conversely, ‘planned’ stress, i.e. exercise training, makes the heart less prone to arrhythmogenesis, as demonstrated elegantly in an animal model of SCD in which ventricular fibrillation is triggered by acute ischaemia (Billman et al. 2006). Overall, these observations lead us to propose that a sudden interplay of sympathetic and parasympathetic control (i.e. autonomic conflict) is a more likely candidate mechanism for ‘stress’-related SCD than the maintained level of sympathetic or parasympathetic drive alone.
Conclusions
The co-activation of the sympathetic and parasympathetic divisions of the autonomic nervous system can reproducibly produce cardiac arrhythmias (Fig. 5). During cold water immersion, this powerful pro-arrhythmic stimulus occurs at a time when the QT interval does not match the underlying heart rate, further increasing the likelihood of problematic cardiac arrhythmias. The release of breath holding appears to be significantly involved, with many arrhythmias occurring within 10 s of the cessation of breath holding (81% in both Tipton et al. (1994) and Tipton et al. (2010)).
One can only speculate at the number of immersion-related deaths caused by autonomic conflict. We believe that the importance of cardiac problems on immersion tends to be underestimated because only young, fit and healthy individuals are usually studied in the laboratory and even if the primary problem on immersion is a cardiac one, aspiration of water during the terminal period is likely to mean a death is ascribed to drowning or, if fluid is not present in the lung ‘dry drowning’. It seems clear, however, that autonomic conflict is a common cause of cardiac arrhythmias on immersion and it is entirely possible that these arrhythmias could result in death, but be undiagnosed post-mortem. Thus, in situations where hypothermia and other cardiac problems have been excluded due to circumstantial or pathological evidence, autonomic conflict and cardiac arrhythmia should be considered as a potential cause of death. It should also be considered as a possible initiating event in some cases of apparent drowning.
Finally, we suggest that this concept, that is exemplified and perhaps amplified by cold water immersion, may not be unique to cold water. While it is clearly difficult to test, it is possible that autonomic conflict may be a common occurrence and may trigger SCD in association with a wide range of environmental factors: a large lunch, a narrow coronary artery, a breath of cold air, anger, a sudden shock, waking from sleep, an antihistamine, an undiagnosed sub-clinical channelopathy or cardiac hypertrophy, may all combine in such a way as to turn this usually benign autonomic co-activation into a life-threatening arrhythmia.
Acknowledgments
The discussion and advice of Drs Michael Curtis and Frank Golden is very gratefully acknowledged. Mr Darren Griffiths and Ms Stacey Hickson undertook the isolated heart perfusions in the course of student projects at King's College London. The students from the King's College London Extreme Physiology course (2006) are also very gratefully acknowledged for their original observation, and analysis, of the lack of QT interval changes during facial immersion.
References
- Ackerman MJ, Tester DJ, Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin Proc. 1999;74:1088–1094. doi: 10.4065/74.11.1088. [DOI] [PubMed] [Google Scholar]
- Angell-James JE, de Burgh Daly M. Some aspects of upper respiratory tract reflexes. Acta Otolaryngol. 1975;79:242–252. doi: 10.3109/00016487509124680. [DOI] [PubMed] [Google Scholar]
- Arnold RW. Extremes in human breath hold, facial immersion bradycardia. Undersea Biomed Res. 1985;12:183–190. [PubMed] [Google Scholar]
- Arnold L, Page J, Attwell D, Cannell M, Eisner DA. The dependence on heart rate of the human ventricular action potential duration. Cardiovasc Res. 1982;16:547–551. doi: 10.1093/cvr/16.10.547. [DOI] [PubMed] [Google Scholar]
- Baharav A, Kotagal S, Gibbons V, Rubin BK, Pratt G, Karin J, Akselrod S. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology. 1995;45:1183–1187. doi: 10.1212/wnl.45.6.1183. [DOI] [PubMed] [Google Scholar]
- Batra AS, Silka MJ. Mechanism of sudden cardiac arrest while swimming in a child with the prolonged QT syndrome. J Pediatr. 2002;141:283–284. doi: 10.1067/mpd.2002.126924. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Wiedermann CJ, Hortnagl H, Muhlberger V. Plasma catecholamines in arterial and capillary blood. Naunyn Schmiedebergs Arch Pharmacol. 1985;328:461–463. doi: 10.1007/BF00692916. [DOI] [PubMed] [Google Scholar]
- Beard CM, Fuster V, Elveback LR. Daily and seasonal variation in sudden cardiac death, Rochester, Minnesota, 1950–1975. Mayo Clin Proc. 1982;57:704–706. [PubMed] [Google Scholar]
- Bert P. Lecons sur la physiologie comparee de la respiration. Paris: Bailliere; 1870. [Google Scholar]
- Bierens JJ, Knape JT, Gelissen HP. Drowning. Curr Opin Crit Care. 2002;8:578–586. doi: 10.1097/00075198-200212000-00016. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kukielka M, Kelley R, Moustafa-Bayoumi M, Altschuld RA. Endurance exercise training attenuates cardiac β2-adrenoceptor responsiveness and prevents ventricular fibrillation in animals susceptible to sudden death. Am J Physiol Heart Circ Physiol. 2006;290:H2590–H2599. doi: 10.1152/ajpheart.01220.2005. [DOI] [PubMed] [Google Scholar]
- Bove AA, Lynch PR, Connell JV, Jr, Harding JM. Diving reflex after physical training. J Appl Physiol. 1968;25(1):70–72. doi: 10.1152/jappl.1968.25.1.70. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Bas Res Cardiol. 2001;96:528–538. doi: 10.1007/s003950170003. [DOI] [PubMed] [Google Scholar]
- Brookfield L, Bharati S, Denes P, Halstead RD, Lev M. Familial sudden death. Report of a case and review of the literature. Chest. 1988;94:989–993. doi: 10.1378/chest.94.5.989. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Action potential duration, rate of stimulation, and intracellular sodium. J Cardiovasc Electrophysiol. 2006;17:S2–S7. doi: 10.1111/j.1540-8167.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- Choi G, Kopplin LJ, Tester DJ, Will ML, Haglund CM, Ackerman MJ. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation. 2004;110:2119–2124. doi: 10.1161/01.CIR.0000144471.98080.CA. [DOI] [PubMed] [Google Scholar]
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308:236–240. doi: 10.1124/jpet.103.059717. [DOI] [PubMed] [Google Scholar]
- Cooper HE, Clutton-Brock TH, Parkes MJ. Contribution of the respiratory rhythm to sinus arrhythmia in normal unanesthetized subjects during positive-pressure mechanical hyperventilation. Am J Physiol Heart Circ Physiol. 2004;286:H402–H411. doi: 10.1152/ajpheart.00504.2003. [DOI] [PubMed] [Google Scholar]
- Coplan NL, Gleim GW, Nicholas JA. Exercise-related changes in serum catecholamines and potassium: effect of sustained exercise above and below lactate threshold. Am Heart J. 1989;117:1070–1075. doi: 10.1016/0002-8703(89)90864-8. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Macleod BA, Walker MJ. The effects of ablations in the central nervous system on arrhythmias induced by coronary occlusion in the rat. Br J Pharmacol. 1985;86:663–670. doi: 10.1111/j.1476-5381.1985.tb08943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Barwood M, Tipton MJ. ECG arrhythmias following breathhold during head-out cold water immersion: putative neural mechanisms and implications for sudden death on immersion. In: Holmer I, Kuklane K, Gao C, editors. Environmental Ergonomics XI. Ystad, Sweden: Lund University Press; 2005. pp. 247–250. [Google Scholar]
- Datta A, Tipton M. Respiratory responses to cold water immersion: neural pathways, interactions, and clinical consequences awake and asleep. J Appl Physiol. 2006;100:2057–2064. doi: 10.1152/japplphysiol.01201.2005. [DOI] [PubMed] [Google Scholar]
- Davidowski TA, Wolf S. The QT interval during reflex cardiovascular adaptation. Circulation. 1984;69:22–25. doi: 10.1161/01.cir.69.1.22. [DOI] [PubMed] [Google Scholar]
- de Burgh Daly M, Angell-James JE. The ‘diving response’ and its possible clinical implications. Int Med. 1979;1:12–19. [Google Scholar]
- de Burgh Daly M, Angell-James JE, Elsner R. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet. 1979;1:764–767. doi: 10.1016/s0140-6736(79)91218-2. [DOI] [PubMed] [Google Scholar]
- Douglas AS, al-Sayer H, Rawles JM, Allan TM. Seasonality of disease in Kuwait. Lancet. 1991;337:1393–1397. doi: 10.1016/0140-6736(91)93069-l. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Dibb KM, Trafford AW. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp Physiol. 2009;94:520–528. doi: 10.1113/expphysiol.2008.044008. [DOI] [PubMed] [Google Scholar]
- Ferrigno M, Lundgren CE. In: Bennett and Elliott's Physiology and Medicine of Diving. 5th ed. Brubakk AO, Neuman TS, editors. Edinburgh, New York: Saunders; 2003. p. xii. 779. [Google Scholar]
- Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988;82:972–979. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden F, Tipton MJ. Essentials of Sea Survival. Illinois: Human Kinetics; 2002. [Google Scholar]
- Guz A, Noble MI, Widdicombe JG, Trenchard D, Mushin WW, Makey AR. The role of vagal and glossopharyngeal afferent nerves in respiratory sensation, control of breathing and arterial pressure regulation in conscious man. Clin Sci. 1966;30:161–170. [PubMed] [Google Scholar]
- Home Office Report. HMSO, London: 1977. Report of the working party on water safety. [Google Scholar]
- Hong SK. The physiology of breath-hold diving. In: Strauss RH, editor. Diving Medicine. Orlando, Florida: Grune & Stratton Inc; 1976. [Google Scholar]
- Ishikawa H, Matsushima M, Nagashima M, Osuga A. Screening of children with arrhythmias for arrhythmia development during diving and swimming-face immersion as a substitute for diving and exercise stress testing as a substitute for swimming. Jpn Circ J. 1992;56(9):881–980. doi: 10.1253/jcj.56.881. [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Kloner RA. Natural and unnatural triggers of myocardial infarction. Prog Cardiovasc Dis. 2006;48:285–300. doi: 10.1016/j.pcad.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J Auton Nerv Syst. 1979;1:33–52. doi: 10.1016/0165-1838(79)90004-3. [DOI] [PubMed] [Google Scholar]
- Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–1805. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- Lampert R, Rosenfeld L, Batsford W, Lee F, McPherson C. Circadian variation of sustained ventricular tachycardia in patients with coronary artery disease and implantable cardioverter-defibrillators. Circulation. 1994;90:241–247. doi: 10.1161/01.cir.90.1.241. [DOI] [PubMed] [Google Scholar]
- Lau CP, Freedman AR, Fleming S, Malik M, Camm AJ, Ward DE. Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res. 1988;22:67–72. doi: 10.1093/cvr/22.1.67. [DOI] [PubMed] [Google Scholar]
- Lin YC. Applied physiology of diving. Sports Med. 1988;5(1):41–56. doi: 10.2165/00007256-198805010-00004. [DOI] [PubMed] [Google Scholar]
- McDonough JR, Barutt JP, Saffron JC. Cardiac arrhythmias as a precursor to drowning accidents. In: Lundgren CEG, Ferrigno M, editors. The Physiology of Breath-Hold Diving. Bethesda, MD: Undersea Hyperbaric Medical Society; 1987. pp. 212–229. [Google Scholar]
- Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–198. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- Muller D, Lampe F, Wegscheider K, Schultheiss HP, Behrens S. Annual distribution of ventricular tachycardias and ventricular fibrillation. Am Heart J. 2003;146(6):1061–1065. doi: 10.1016/S0002-8703(03)00426-5. [DOI] [PubMed] [Google Scholar]
- Nabel EG, Selwyn AP, Ganz P. Paradoxical narrowing of atherosclerotic coronary arteries induced by increases in heart rate. Circulation. 1990;81:850–859. doi: 10.1161/01.cir.81.3.850. [DOI] [PubMed] [Google Scholar]
- Olsen CR, Fanestil DD, Scholander PF. Some effects of breath holding and apneic underwater diving on cardiac rhythm in man. J Appl Physiol. 1962;17:461–466. doi: 10.1152/jappl.1962.17.3.461. [DOI] [PubMed] [Google Scholar]
- Parkes MJ. Breath-holding and its breakpoint. Exp Physiol. 2006;91:1–15. doi: 10.1113/expphysiol.2005.031625. [DOI] [PubMed] [Google Scholar]
- Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49:555–565. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Paulev P-E. Cardiac rhythm during breath-holding and water immersion in man. Acta Physiol Scand. 1968;73:139–150. doi: 10.1111/j.1748-1716.1968.tb04091.x. [DOI] [PubMed] [Google Scholar]
- Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Ratge D, Gehrke A, Melzner I, Wisser H. Free and conjugated catecholamines in human plasma during physical exercise. Clin Exp Pharmacol Physiol. 1986;13:543–553. doi: 10.1111/j.1440-1681.1986.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Risch WD, Koubenec HJ, Beckmann U, Lange S, Gauer OH. The effect of graded immersion on heart volume, central venous pressure, pulmonary blood distribution, and heart rate in man. Pflugers Archiv. 1978;374:115–118. doi: 10.1007/BF00581289. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why Zebras Don't Get Ulcers: an Updated Guide to Stress, Stress-Related Diseases, and Coping. New York: W.H. Freeman and Company; 1999. [Google Scholar]
- Schwartz PJ, Zipes DP. Autonomic regulation of cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology From bench to Bedside. Philadelphia: W.B. Saunders; 2004. pp. 300–314. [Google Scholar]
- Taggart P, Boyett MR, Logantha S, Lambiase PD. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol. 2011;2:67. doi: 10.3389/fphys.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Nozawa T, Yasumura Y, Futaki S, Hiramori K, Suga H. Heart-rate-proportional oxygen consumption for constant cardiac work in dog heart. Jpn J Physiol. 1990;40:503–521. doi: 10.2170/jjphysiol.40.503. [DOI] [PubMed] [Google Scholar]
- Tester DJ, Medeiros-Domingo A, Will ML, Ackerman MJ. Unexplained drownings and the cardiac channelopathies: a molecular autopsy series. Mayo Clin Proc. 2011;86:941–947. doi: 10.4065/mcp.2011.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton M. The effect of clothing on ‘diving bradycardia’ in man during submersion in cold water. Eur J Appl Physiol Occup Physiol. 1989a;59:360–364. doi: 10.1007/BF02389811. [DOI] [PubMed] [Google Scholar]
- Tipton MJ. The initial responses to cold-water immersion in man. Clin Sci. 1989b;77:581–588. doi: 10.1042/cs0770581. [DOI] [PubMed] [Google Scholar]
- Tipton MJ, Gibbs P, Brooks C, Roiz de Sa D, Reilly TJ. ECG during helicopter underwater escape training. Aviat Space Environ Med. 2010;81:399–404. doi: 10.3357/asem.2700.2010. [DOI] [PubMed] [Google Scholar]
- Tipton MJ, Kelleher PC, Golden FS. Supraventricular arrhythmias following breath-hold submersions in cold water. Undersea Hyperb Med. 1994;21:305–313. [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Williams ES, Zipes DP. The winter's tale – and toll. Am Heart J. 2003;146:941–943. doi: 10.1016/S0002-8703(03)00425-3. [DOI] [PubMed] [Google Scholar]
- Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- Wolf S. The bradycardia of the dive reflex – a possible mechanism of sudden death. Trans Am Clin Climatol Assoc. 1964;76:192–200. [PMC free article] [PubMed] [Google Scholar]
- Wong G, Clark JE, Shattock MJ. Failure of the QT interval of the electrocardiogram to prolong during a diving response-induced bradycardia in human subjects. Proc Physiol Soc. 2009;14:PC27. [Google Scholar]
- Wyss V. Swimming under water in apnea and the nature of the ECG. Boll Soc Ital Biol Sper. 1956;32:506–509. [PubMed] [Google Scholar]
- Yoshinaga M, Kamimura J, Fukushige T, Kusubae R, Shimago A, Nishi J, Kono Y, Nomura Y, Miyata K. Face immersion in cold water induces prolongation of the QT interval and T-wave changes in children with nonfamilial long QT syndrome. Am J Cardiol. 1999;83:1494–1497. doi: 10.1016/s0002-9149(99)00131-9. A1498. [DOI] [PubMed] [Google Scholar]
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]


