Abstract
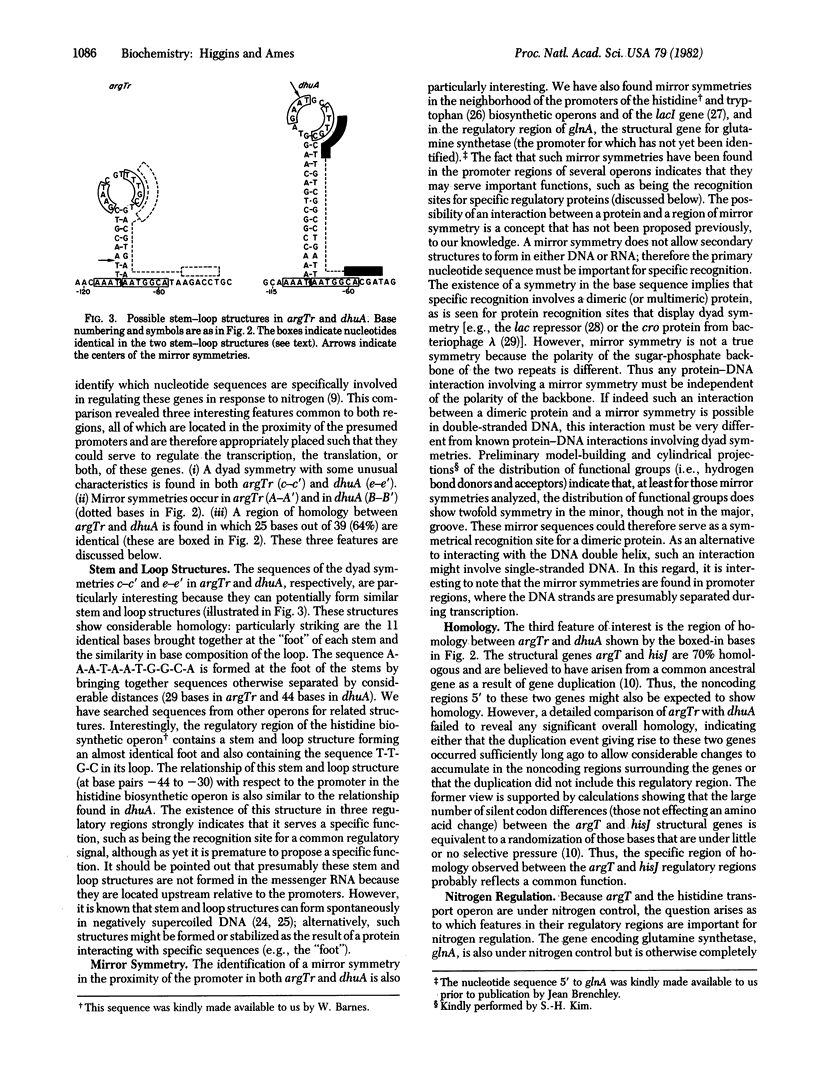
We have determined the nucleotide sequences of the regulatory regions from two amino acid transport operons from Salmonella typhimurium: dhuA, which regulates the histidine transport operon, and argTr, which regulates argT, the gene encoding the lysine-arginine-ornithine-binding protein, LAO. The promoter for the histidine transport operon has been identified from the sequence change in the promoter-up mutation dhuA1. Neither regulatory region has any of the features typical of the regulatory regions of the amino acid biosynthetic operons, indicating that regulation of at least these transport genes does not involve a transcription attenuation mechanism. We have identified three interesting features, present in both of these sequences, which may be of importance in the regulation of these and other operons: a "stem-loop-foot" structure, a region of specific homology, and a mirror symmetry. The region of mirror symmetry may be a protein recognition site important is regulating expression of these and other operons in response to nitrogen availability. Mirror symmetry as a structure for DNA-protein interaction sites has not been proposed previously.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Identification of a membrane protein as a histidine transport component in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5447–5451. doi: 10.1073/pnas.75.11.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Noel K. D., Taber H., Spudich E. N., Nikaido K., Afong J. Fine-structure map of the histidine transport genes in Salmonella typhimurium. J Bacteriol. 1977 Mar;129(3):1289–1297. doi: 10.1128/jb.129.3.1289-1297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. F., Ohlendorf D. H., Takeda Y., Matthews B. W. Structure of the cro repressor from bacteriophage lambda and its interaction with DNA. Nature. 1981 Apr 30;290(5809):754–758. doi: 10.1038/290754a0. [DOI] [PubMed] [Google Scholar]
- Ardeshir F., Ames G. F. Cloning of the histidine transport genes from Salmonella typhimurium and characterization of an analogous transport system in Escherichia coli. J Supramol Struct. 1980;13(1):117–130. doi: 10.1002/jss.400130111. [DOI] [PubMed] [Google Scholar]
- Ardeshir F., Higgins C. F., Ames G. F. Physical map of the Salmonella typhimurium histidine transport operon: correlation with the genetic map. J Bacteriol. 1981 Aug;147(2):401–409. doi: 10.1128/jb.147.2.401-409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Funanage V. L., Ayling P. D., Dendinger S. M., Brenchley J. E. Salmonella typhimurium LT-2 mutants with altered glutamine synthetase levels and amino acid uptake activities. J Bacteriol. 1978 Nov;136(2):588–596. doi: 10.1128/jb.136.2.588-596.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Calvo J. M. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, his, thr, and leu operons. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6186–6190. doi: 10.1073/pnas.76.12.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Anderson J. J., Mayo M. M., Gunsalus R. P., Mavromara P., Daniels C. J., Oxender D. L. Regulation of high-affinity leucine transport in Escherichia coli. J Supramol Struct. 1980;14(4):527–537. doi: 10.1002/jss.400140410. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Purification and properties of a component of histidine transport in Salmonella typhimurium. The histidine-binding protein J. J Biol Chem. 1972 Jul 10;247(13):4317–4326. [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Classical and postclassical modes of regulation of the synthesis of degradative bacterial enzymes. Prog Nucleic Acid Res Mol Biol. 1976;17:99–115. doi: 10.1016/s0079-6603(08)60067-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzari G., Yanofsky C. Naturally occurring promoter down mutation: nucleotide sequence of the trp promoter/operator/leader region of Shigella dysenteriae 16. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5580–5584. doi: 10.1073/pnas.75.11.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. DNA-binding site of lac repressor probed by dimethylsulfate methylation of lac operator. J Mol Biol. 1979 Aug 25;132(4):709–728. doi: 10.1016/0022-2836(79)90384-x. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]


