Abstract
Brown Norway (BN) rats have a relatively specific deficit in CO2 sensitivity. This deficit could be due to an abnormally weak carotid body contribution to CO2 sensitivity. Accordingly, we tested the hypothesis that CBD would have less of an effect on eupnoeic breathing and CO2 sensitivity in the BN rats compared to other rat strains. We measured ventilation and blood gases at rest (eupnoea) and during hypoxia ( = 0.12) or hypercapnia (
= 0.12) or hypercapnia ( = 0.07) before and up to 23 days after bilateral or Sham CBD in BN, Sprague–Dawley (SD) and Dahl Salt-Sensitive (SS) rats. In all three rat strains, CBD elicited eupnoeic hypoventilation (Δ
= 0.07) before and up to 23 days after bilateral or Sham CBD in BN, Sprague–Dawley (SD) and Dahl Salt-Sensitive (SS) rats. In all three rat strains, CBD elicited eupnoeic hypoventilation (Δ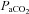 +8.7–11.0 mmHg) 1–2 days post-CBD (P < 0.05), and attenuated ventilatory responses to hypoxia (P < 0.05) and venous sodium cyanide (NaCN; P < 0.05), while sham CBD had no effect on resting breathing, blood gases or chemoreflexes (P > 0.05). In contrast, CBD had no effect on CO2 sensitivity (Δ
+8.7–11.0 mmHg) 1–2 days post-CBD (P < 0.05), and attenuated ventilatory responses to hypoxia (P < 0.05) and venous sodium cyanide (NaCN; P < 0.05), while sham CBD had no effect on resting breathing, blood gases or chemoreflexes (P > 0.05). In contrast, CBD had no effect on CO2 sensitivity (Δ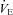 /Δ
/Δ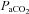 ) in all strains (P > 0.05). Eupnoeic
) in all strains (P > 0.05). Eupnoeic  returned to pre-CBD values within 15–23 days post-CBD. Thus, the effects of CBD in rats (1) further support an important role for the carotid bodies in eupnoeic blood gas regulation, (2) suggest that the carotid bodies are not a major determinant of CO2 sensitivity in rats, and (3) may not support the concept of an interaction among the peripheral and central chemoreceptors in rats.
returned to pre-CBD values within 15–23 days post-CBD. Thus, the effects of CBD in rats (1) further support an important role for the carotid bodies in eupnoeic blood gas regulation, (2) suggest that the carotid bodies are not a major determinant of CO2 sensitivity in rats, and (3) may not support the concept of an interaction among the peripheral and central chemoreceptors in rats.
Key points
Carbon dioxide (CO2) provides a major chemical stimulus to breathe, primarily through the activity of CO2/pH sensors called chemoreceptors in the brainstem and in the carotid body.
Carotid body denervation (CBD) causes hypoventilation at rest and reduces ventilatory sensitivity to CO2 in multiple mammalian species, suggesting an important role of the carotid bodies in determining levels of ventilation relative to the CO2 drive to breathe.
CBD in three strains of adult rats with large inherent differences in CO2 sensitivity causes hypoventilation at rest but has no effect on CO2 sensitivity.
These data from rats reinforce the concept that the carotid bodies provide a tonic facilitatory drive to breathe, but differ from other species suggesting a minimal contribution of the carotid bodies to CO2 sensitivity in rats.
Introduction
Brown Norway (BN), Dahl Salt-Sensitive (SS) and Sprague–Dawley (SD) rats demonstrate significant interstrain variation among ventilatory control phenotypes (Strohl et al. 1997; Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005). Although BN, SS, and SD rats show similar eupnoeic (resting) minute ventilation (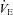 ) and equivalent ventilatory responses to mild exercise and hypoxia (
) and equivalent ventilatory responses to mild exercise and hypoxia (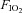 = 0.12), BN rats show a significantly blunted hypercapnic ventilatory response (HCVR) compared to SD and SS rats (Hodges et al. 2002). Thus, it appears that BN rats have a relatively specific deficit in CO2 sensitivity, perhaps due to dysfunctional central and/or carotid chemoreceptors, or altered mechanisms of interaction among peripheral and central chemoreceptors (Loeschcke et al. 1963; Day & Wilson 2008; Smith et al. 2010).
= 0.12), BN rats show a significantly blunted hypercapnic ventilatory response (HCVR) compared to SD and SS rats (Hodges et al. 2002). Thus, it appears that BN rats have a relatively specific deficit in CO2 sensitivity, perhaps due to dysfunctional central and/or carotid chemoreceptors, or altered mechanisms of interaction among peripheral and central chemoreceptors (Loeschcke et al. 1963; Day & Wilson 2008; Smith et al. 2010).
Despite the apparent segregation in function of peripheral (O2) and central (CO2/H+) chemoreceptors, there are several pieces of data that point to a significant role for the carotid bodies in CO2/H+ chemoreception in addition to a particularly important role in the regulation of eupnoeic  (Forster et al. 2007). A prime example is the data from studies of carotid body denervation (CBD), which attenuates or eliminates the hypoxic ventilatory response, causes hypoventilation at rest and during exercise in several mammalian species (Bisgard et al. 1976; Olson et al. 1988; Pan et al. 1998; Lowry et al. 1999; Serra et al. 2002), and reduces the ventilatory sensitivity to CO2 as much as 60% in goats (Pan et al. 1998). The effects of CBD on eupnoeic ventilation and CO2 sensitivity are unexpected based on the modest increase in carotid sinus nerve discharge when the carotid bodies are made hypercapnic at a constant level of
(Forster et al. 2007). A prime example is the data from studies of carotid body denervation (CBD), which attenuates or eliminates the hypoxic ventilatory response, causes hypoventilation at rest and during exercise in several mammalian species (Bisgard et al. 1976; Olson et al. 1988; Pan et al. 1998; Lowry et al. 1999; Serra et al. 2002), and reduces the ventilatory sensitivity to CO2 as much as 60% in goats (Pan et al. 1998). The effects of CBD on eupnoeic ventilation and CO2 sensitivity are unexpected based on the modest increase in carotid sinus nerve discharge when the carotid bodies are made hypercapnic at a constant level of 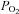 in cats (Biscoe et al. 1970; Lahiri et al.), rats (Lahiri & DeLaney 1981
1975; Vidruk et al. 2001; Day & Wilson 2005), and goats (Engwall et al. 1988). However, the tonic level of carotid body activity, and perhaps not the CO2 sensitivity of the carotid body itself, could be sufficient to alter the respiratory systems response to hypercapnia. This possibility is suggested by studies showing that acute physiological disfacilitation of isolated, extracorporeally perfused carotid bodies with hyperoxia and hypocapnia dampened the hypercapnic ventilatory response (Bisgard et al. 1980; Blain et al. 2009, 2010), while hypoxic stimulation of the carotid bodies augmented CO2 sensitivity in awake dogs (Blain et al. 2009, 2010). These and other data lead to the conclusion that peripheral chemoreceptor activity interacts synergistically with central chemoreceptors (hyper-additive) to affect CO2 sensitivity in the dog (Blain et al. 2009). Alternatively, others have shown in a perfused rat preparation a negative interaction among the peripheral and central chemoreceptor components, where decreasing central chemoreceptor activity (brain hypocapnia) increases the hypoxic ventilatory response (Day & Wilson 2009). Irrespective of the mode in which these chemoreceptor components interact (hyper-additive, additive, hypo-additive, etc.; Loeschcke et al. 1963;Cragg & Drysdale 1983; Day & Wilson 2007, 2009; Dahan et al. 2008; Blain et al. 2010; Cui et al. 2012), the overall conclusion is that there is a mechanism or mechanisms governing an interaction among peripheral and central chemoreceptors (Smith et al. 2010).
in cats (Biscoe et al. 1970; Lahiri et al.), rats (Lahiri & DeLaney 1981
1975; Vidruk et al. 2001; Day & Wilson 2005), and goats (Engwall et al. 1988). However, the tonic level of carotid body activity, and perhaps not the CO2 sensitivity of the carotid body itself, could be sufficient to alter the respiratory systems response to hypercapnia. This possibility is suggested by studies showing that acute physiological disfacilitation of isolated, extracorporeally perfused carotid bodies with hyperoxia and hypocapnia dampened the hypercapnic ventilatory response (Bisgard et al. 1980; Blain et al. 2009, 2010), while hypoxic stimulation of the carotid bodies augmented CO2 sensitivity in awake dogs (Blain et al. 2009, 2010). These and other data lead to the conclusion that peripheral chemoreceptor activity interacts synergistically with central chemoreceptors (hyper-additive) to affect CO2 sensitivity in the dog (Blain et al. 2009). Alternatively, others have shown in a perfused rat preparation a negative interaction among the peripheral and central chemoreceptor components, where decreasing central chemoreceptor activity (brain hypocapnia) increases the hypoxic ventilatory response (Day & Wilson 2009). Irrespective of the mode in which these chemoreceptor components interact (hyper-additive, additive, hypo-additive, etc.; Loeschcke et al. 1963;Cragg & Drysdale 1983; Day & Wilson 2007, 2009; Dahan et al. 2008; Blain et al. 2010; Cui et al. 2012), the overall conclusion is that there is a mechanism or mechanisms governing an interaction among peripheral and central chemoreceptors (Smith et al. 2010).
Given the data supporting the hypothesis that the carotid bodies contribute to ventilatory CO2 sensitivity, we sought to determine whether the effects of CBD would be uniform among rat strains differing in CO2 sensitivity. We hypothesized that as in other species, CBD in BN, SS, and SD rats will cause hypoventilation at rest and a reduction in ventilatory CO2 sensitivity. We further hypothesized that the inherent differences in CO2 sensitivity among these strains was due to differences in the contribution of the carotid chemoreceptors, and thus the resulting hypoventilation and reduction in CO2 sensitivity following CBD would be greatest in the most CO2 sensitive strains and least in the CO2 insensitive BN rats.
Methods
In-house adult (6–12 weeks of age) male Brown Norway (BN/Mcwi; n= 15), Dahl Salt-Sensitive (SS/Mcwi; n= 14), and commercially available Sprague–Dawley ((Harlan) SD; n= 12) rats were used in this study. All rats were housed in the Biomedical Research Center at the Medical College of Wisconsin and allowed access to low salt chow (Dyets 0.4% NaCl) and water ad libitum, and maintained on a 12:12 h. light–dark cycle. All experimental protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee and conform to principles of UK regulations.
Experimental design
All animals were initially instrumented with short indwelling catheters implanted into a femoral artery and vein. After ≥2 days of recovery from surgical implantation of catheters, pre-CBD (control) measurements were obtained using whole-body, flow-through plethysmography. Breathing and blood pressure were measured, while at rest breathing room air (RA; 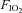 = 0.21, bal. N2) for 10–20 min, followed by a 10 min hypercapnic challenge (
= 0.21, bal. N2) for 10–20 min, followed by a 10 min hypercapnic challenge (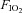 = 0.21,
= 0.21,  , = 0.07, bal. N2) or a poikilocapnic hypoxic challenge (
, = 0.07, bal. N2) or a poikilocapnic hypoxic challenge (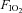 = 0.12, bal. N2). Arterial blood samples (0.3–0.4 ml) were obtained during the last 3 min of RA breathing or during the respiratory challenge in nearly all experiments. Each animal underwent a total of four control experiments (2 with hypercapnic and 2 with hypoxic challenges). One group of BN (n= 7), SS (n= 8) and SD (n= 6) rats then underwent bilateral CBD, and a second group of BN (n= 8), SS (n= 6) and SD (n= 6) rats underwent sham CBD surgery. Ventilation was studied every other day for the first 8 days post-CBD beginning on post-op day 1 (Day 1, 3, 5, and 7) or 2 (Day 2, 4, 6 and 8), and then studied once between 10–15 and 16–21 days post-surgery. To verify denervation, ventilation was measured during injections (0.1 ml) of NaCN (3 mg ml−1) were made intravenously before, 2–4 days, and ≥2 weeks after CBD or sham surgery.
= 0.12, bal. N2). Arterial blood samples (0.3–0.4 ml) were obtained during the last 3 min of RA breathing or during the respiratory challenge in nearly all experiments. Each animal underwent a total of four control experiments (2 with hypercapnic and 2 with hypoxic challenges). One group of BN (n= 7), SS (n= 8) and SD (n= 6) rats then underwent bilateral CBD, and a second group of BN (n= 8), SS (n= 6) and SD (n= 6) rats underwent sham CBD surgery. Ventilation was studied every other day for the first 8 days post-CBD beginning on post-op day 1 (Day 1, 3, 5, and 7) or 2 (Day 2, 4, 6 and 8), and then studied once between 10–15 and 16–21 days post-surgery. To verify denervation, ventilation was measured during injections (0.1 ml) of NaCN (3 mg ml−1) were made intravenously before, 2–4 days, and ≥2 weeks after CBD or sham surgery.
Surgical protocols
All surgeries were performed using aseptic techniques. Anaesthesia was induced by placing the animal into a clear 10 litre chamber, which contained a secondary container filled with gauze soaked with 20% isoflurane in propylene glycol. Upon loss of the righting reflex, animals were quickly transferred to a warm surgical table and placed on a nose cone to maintain surgical levels of anaesthesia (2.5% isoflurane in 100% O2 at a flow rate of 1.0 L min−1). All animals received intraoperative injections of Carprofan (Rimadyl; 5 mg kg−1 i.p.) for analgesia and the antibiotic enrofloxacin (Baytril; 1 mg (100 g)−1) to prevent infection. The surgical field was prepared by shaving the skin and alternating 70% alcohol and surgical scrub (Betadine), and covered with a sterile drape.
Catheterization surgery
Catheters were prepared by connecting Tygon tubing (venous line: 0.51 mm ID, 1.52 mm OD; VWR) or RenaPulse tubing (arterial line: 1.02 mm ID, 0.64 mm OD; Braintree Scientific) to a ∼3 cm section of Micro-Renathane tubing (0.025 inches ID, 0.012 inches OD; Braintree Scientific, MA, USA). Through a lateral skin incision, the femoral artery and vein were isolated and lifted before a small incision was made on the vessels permitting complete advancement of the 3 cm segment of each catheter, which was then anchored with suture. Sterilized catheters were then subcutaneously tunneled and externalized between the scapulae and anchored to the muscle. The catheters were trimmed to length (∼3 cm), and the incisions closed before application of antibiotic ointment. The rats were then maintained on medicated water (Baytril (1 mg/100 ml)) for the remainder of the protocol.
Carotid body denervation (CBD)
After anaesthesia induction (20% isoflurane in propylene glycol) and maintenance (2.5% isoflurane in O2), the sterile surgical fields were prepared. Bilateral neck incisions, rather than a single midline incision, were made to minimize disruption of the upper airway musculature and nerves (Serra et al. 2001). In both Sham operated and bilateral CBD animals, blunt dissection was used to visualize the bifurcation of the common carotid artery. In CBD animals, the vagus nerve was gently separated, and the innervation from the carotid sinus nerve was identified exiting the glossopharyngeal nerve and innervating the internal carotid artery near the bifurcation and beneath the occipital artery. The nerve was then stripped from the artery and the skin incisions closed.
Physiological measurements
Plethysmography
Ventilatory measurements were made using a custom-built 10 litre Plexiglas flow-through plethysmograph using methods similar to those described previously (Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005). Air inflow (10 L min−1) was measured and maintained using a flow meter (Dwyer) and needle valve, and (vacuum) outflow measured and maintained at the same flow rate to provide rapid gas exchange, avoid CO2 accumulation, and to maintain the absolute chamber pressure at or slightly above atmospheric pressure. Oxygen and CO2 levels in the chamber outflow were measured using an O2 Capnograph (07-0193; Oxigraf, Mountain View, CA, USA), which was calibrated weekly with certified standard (known) gas concentrations of 7% CO2, 21% O2, bal. N2, and 12% O2, bal. N2. Chamber pressure (Validyne, Northridge, CA, USA differential pressure transducer), chamber temperature (∼23°C) and relative humidity ((∼0–30%) HX93A; Omega, Standford, CT, USA), arterial blood pressure (MAP) and heart rate (HR) were measured continuously. Volume calibrations (0.3 ml at 1.5–2 Hz) were used to calibrate the ventilatory signal after each study. All analog signals were connected to a 16-channel A/DAYS converter and digitally recorded using data acquisition software (Windaq, Akron, OH, USA) sampled at 200 Hz/channel. Animal temperature was obtained following each experimental period using a J-type rectal thermocouple probe and reader (BAT-12, Life Science Instruments, Woodland Hills, CA, USA). Arterial blood samples (∼0.4 ml) were drawn into heparinized (<0.05 ml) 1.0 ml syringes for analysis with a Rapid Lab Model 248 blood gas analyzer (Bayer Healthcare; serviced yearly). The blood gas analyzer was calibrated hourly, and a two point calibration performed prior to the analysis of all blood samples. All blood gas data were corrected for barometric pressure and animal temperature.
Ventilatory response to intravenous NaCN
Ventilation during RA breathing was measured via plethysmography as described above. After more than 5 min of quiet resting breathing, 2–3 bolus injections of 0.1 ml NaCN (0.3 mg ml−1) in 0.9% NaCl (volume/concentration) were made intravenously, at 5 min intervals.
Data analysis
All data collected were analysed offline using a waveform browser (Windaq). Breathing frequency (breaths per minute), tidal volume (VT), minute ventilation (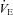 ), and MAP and HR were analysed. Stretches of raw data from the 10 min of RA breathing prior to and during the last 5 min of the respiratory challenge longer than 15 s and free of animal movements, sniffing, or other behaviours were selected for analysis. Peaks and valleys in both the ventilatory (end inspiration and expiration, respectively) and blood pressure (systolic (SP) and diastolic (DP) pressures, respectively) were exported as a text file, and the ventilatory, temperature and RH and calibration data used to calculate the estimated VT per breath similar to previous methods (Drorbaugh & Fenn 1955; Hodges et al. 2002). VT was multiplied by breathing frequency to obtain
), and MAP and HR were analysed. Stretches of raw data from the 10 min of RA breathing prior to and during the last 5 min of the respiratory challenge longer than 15 s and free of animal movements, sniffing, or other behaviours were selected for analysis. Peaks and valleys in both the ventilatory (end inspiration and expiration, respectively) and blood pressure (systolic (SP) and diastolic (DP) pressures, respectively) were exported as a text file, and the ventilatory, temperature and RH and calibration data used to calculate the estimated VT per breath similar to previous methods (Drorbaugh & Fenn 1955; Hodges et al. 2002). VT was multiplied by breathing frequency to obtain 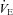 , which is expressed as both weight-normalized and un-normalized values. Bicarbonate (HCO3−) levels were calculated by the Henderson–Hasselbalch equation and using measured (BTPS corrected) pH and
, which is expressed as both weight-normalized and un-normalized values. Bicarbonate (HCO3−) levels were calculated by the Henderson–Hasselbalch equation and using measured (BTPS corrected) pH and  values, where: [HCO3−]= 0.03 ×
values, where: [HCO3−]= 0.03 × × 10(pH−6.1). Similarly, ventilatory responses to NaCN injections were calculated by dividing 5 s of ventilation at the peak NaCN response (3–7 s following the injection) by the 5 s
× 10(pH−6.1). Similarly, ventilatory responses to NaCN injections were calculated by dividing 5 s of ventilation at the peak NaCN response (3–7 s following the injection) by the 5 s  prior to NaCN injection, giving rise to the response ratio. Mean arterial blood pressure (MAP, mmHg) was calculated as MAP = 0.333(SP – DP) + DP, and heart rate (HR) as beats min−1.
prior to NaCN injection, giving rise to the response ratio. Mean arterial blood pressure (MAP, mmHg) was calculated as MAP = 0.333(SP – DP) + DP, and heart rate (HR) as beats min−1.
Statistics
Due to technical problems with catheters, a few rats did not contribute complete sets of data (i.e. some only contributed  and not blood gases) and therefore statistical calculations used different n values. Statistical analyses were performed using SigmaPlot 12.0 software (Systat Software Inc., San Jose, CA, USA). A one-way ANOVA was employed to determine intra-strain variation among pre-Sham and pre-CBD data. In all cases, there were no differences in group data pre-surgery, and thus all data were pooled for inter-strain comparisons (unless otherwise noted as in Table 1). A simple natural log transformation was employed to obtain proper normality for eupnoeic
and not blood gases) and therefore statistical calculations used different n values. Statistical analyses were performed using SigmaPlot 12.0 software (Systat Software Inc., San Jose, CA, USA). A one-way ANOVA was employed to determine intra-strain variation among pre-Sham and pre-CBD data. In all cases, there were no differences in group data pre-surgery, and thus all data were pooled for inter-strain comparisons (unless otherwise noted as in Table 1). A simple natural log transformation was employed to obtain proper normality for eupnoeic 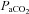 data. Two-way ANOVAs with repeated measures were employed using the factors Group (Sham or CBD) × Time (days pre- or post-CBD) for within strain comparisons, or Strain (BN, SS, or SD) × Time for within Group comparisons. A Bonferroni or other appropriate post hoc analysis was used to determine significance among multiple pairwise comparisons, and significant interaction terms between factors noted (see also Results). Significance thresholds were P < 0.05.
data. Two-way ANOVAs with repeated measures were employed using the factors Group (Sham or CBD) × Time (days pre- or post-CBD) for within strain comparisons, or Strain (BN, SS, or SD) × Time for within Group comparisons. A Bonferroni or other appropriate post hoc analysis was used to determine significance among multiple pairwise comparisons, and significant interaction terms between factors noted (see also Results). Significance thresholds were P < 0.05.
Table 1.
Acute and chronic effects of CBD on arterial blood gases
| Strain | Condition | pH | HCO3− | 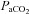 |
 |
|---|---|---|---|---|---|
| Pre-CBD | |||||
| BN (n= 7) | RA | 7.484 ± 0.006 | 23.2 ± 0.3 | 32.0 ± 0.8 | 84.1 ± 1.6 |
| 7% CO2 | 7.355 ± 0.008 | 24.9 ± 1.0 | 46.2 ± 1.9 | 113.0 ± 2.4 | |
| SS (n= 7) | RA | 7.498 ± 0.007 # | 23.8 ± 0.2 | 31.7 ± 0.4 | 86.7 ± 1.9 |
| 7% CO2 | 7.379 ± 0.006 # | 25.3 ± 0.4 | 44.3 ± 0.8 | 114.2 ± 2.4 | |
| SD (n= 6) | RA | 7.474 ± 0.005 | 23.7 ± 1.0 | 33.4 ± 1.5 | 87.1 ± 1.8 |
| 7% CO2 | 7.352 ± 0.010 | 26.5 ± 1.6 | 49.6 ± 3.1 # | 128.3 ± 3.9 # | |
| 1–4 days post-CBD | |||||
| BN (n= 7) | RA | 7.484 ± 0.007 | 28.5 ± 0.7* | 40.7 ± 0.8* | 71.9 ± 2.0* |
| 7% CO2 | 7.376 ± 0.010* | 28.9 ± 0.5* | 51.0 ± 0.6* | 109.3 ± 1.5 | |
| SS (n= 7) | RA | 7.478 ± 0.010* | 30.4 ± 0.9* | 42.4 ± 1.0* | 68.4 ± 3.5* |
| 7% CO2 | 7.401 ± 0.007*# | 30.5 ± 0.4* | 50.9 ± 0.6* | 110.9 ± 2.1 | |
| SD (n= 6) | RA | 7.454 ± 0.008 | 27.9 ± 0.9 | 41.1 ± 1.2* | 69.6 ± 3.0* |
| 7% CO2 | 7.367 ± 0.010* | 28.5 ± 0.7 | 51.2 ± 0.8 | 113.3 ± 3.5* | |
| >10 days post-CBD | |||||
| BN (n= 7) | RA | 7.500 ± 0.007 | 22.9 ± 0.4 | 31.1 ± 0.9 | 85.8 ± 3.2 |
| 7% CO2 | 7.350 ± 0.007 | 25.2 ± 0.3 | 47.4 ± 1.2 | 115.1 ± 4.7 | |
| SS (n= 7) | RA | 7.479 ± 0.010* | 24.8 ± 1.0 | 34.5 ± 1.3* | 76.9 ± 2.4* |
| 7% CO2 | 7.391 ± 0.007 # | 25.1 ± 0.6 | 42.9 ± 0.9 | 108.5 ± 1.2 | |
| SD (n= 6) | RA | 7.471 ± 0.009 | 24.3 ± 0.7 | 34.6 ± 1.6 | 79.3 ± 3.0* |
| 7% CO2 | 7.351 ± 0.003 | 26.5 ± 0.7 | 49.6 ± 1.2 | 116.5 ± 3.7* | |
Significantly different from pre-CBD (P < 0.05)
significant difference among strains within time point (P < 0.05) by 2-way RM ANOVA.
Results
Eupnoeic breathing before and after sham or bilateral CBD
Prior to surgery, we found no differences in all ventilatory parameters while breathing room air (P > 0.05). Therefore all pre-surgery data were pooled to determine potential intra-strain differences. There were no significant differences before CBD among BN (n= 14), SS (n= 12), and SD (n= 12) rat strains in eupnoeic  (32.3 ± 0.5 mmHg, 31.7 ± 0.4 mmHg, 32.8 ± 0.9, mmHg; P > 0.05),
(32.3 ± 0.5 mmHg, 31.7 ± 0.4 mmHg, 32.8 ± 0.9, mmHg; P > 0.05), 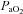 (83.6 ± 1.1 mmHg, 87.0 ± 1.5 mmHg, 86.4 ± 0.1.2 mmHg; P > 0.05), and arterial pH (7.481 ± 0.003, 7.490 ± 0.005, and 7.478 ± 0.004; P > 0.05) levels, respectively. Consistent with the blood gases, eupnoeic
(83.6 ± 1.1 mmHg, 87.0 ± 1.5 mmHg, 86.4 ± 0.1.2 mmHg; P > 0.05), and arterial pH (7.481 ± 0.003, 7.490 ± 0.005, and 7.478 ± 0.004; P > 0.05) levels, respectively. Consistent with the blood gases, eupnoeic 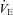 was not different among BN (107.4 ± 5.5 ml min−1), SS (115.7 ± 3.1 ml min−1) and SD (109.9 ± 4.8 ml min−1) rats (P > 0.05). Likewise, breathing frequency and VT did not differ among all rat strains (P > 0.05). In line with previous reports (Strohl et al. 1997; Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005), age-matched BN rats weighed less (211.1 ± 12.0 g) than SS (280.2 ± 13.9 g; P= 0.010) and SD (314.5 ± 22.9 g; P < 0.001) rats, and thus we also calculated weight-normalized VT and
was not different among BN (107.4 ± 5.5 ml min−1), SS (115.7 ± 3.1 ml min−1) and SD (109.9 ± 4.8 ml min−1) rats (P > 0.05). Likewise, breathing frequency and VT did not differ among all rat strains (P > 0.05). In line with previous reports (Strohl et al. 1997; Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005), age-matched BN rats weighed less (211.1 ± 12.0 g) than SS (280.2 ± 13.9 g; P= 0.010) and SD (314.5 ± 22.9 g; P < 0.001) rats, and thus we also calculated weight-normalized VT and 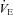 . In contrast to previous reports, we found that weight-normalized eupnoeic
. In contrast to previous reports, we found that weight-normalized eupnoeic 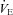 in BN rats (50.8 ± 2.3 ml min−1 100 g−1; n= 14) was greater than both SS (42.5 ± 2.6 ml min−1 100 g−1; P < 0.001; n= 13), and SD (38.2 ± 2.5 ml min−1 100 g−1; P= 0.002; n= 12) rats, due to a greater eupnoeic VT measured in BN rats (0.5 ± 0.0 ml breath−1 100 g−1) compared to SS (0.4 ± 0.0 ml breath−1 100 g−1; P= 0.016) and SD rats (0.4 ± 0.0 ml breath−1 100 g−1; P= 0.008). It is unclear which (un-normalized vs. weight-normalized) is appropriate for expressing VT or
in BN rats (50.8 ± 2.3 ml min−1 100 g−1; n= 14) was greater than both SS (42.5 ± 2.6 ml min−1 100 g−1; P < 0.001; n= 13), and SD (38.2 ± 2.5 ml min−1 100 g−1; P= 0.002; n= 12) rats, due to a greater eupnoeic VT measured in BN rats (0.5 ± 0.0 ml breath−1 100 g−1) compared to SS (0.4 ± 0.0 ml breath−1 100 g−1; P= 0.016) and SD rats (0.4 ± 0.0 ml breath−1 100 g−1; P= 0.008). It is unclear which (un-normalized vs. weight-normalized) is appropriate for expressing VT or 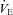 , and thus we present both along with arterial blood gases. We also noted that the SD rats had a higher rectal temperature (TR; 37.8 ± 0.2°C) compared to BN (37.0 ± 0.1°C; P= 0.018) rats, but not SS (37.1 ± 0.2°C; P > 0.05) rats.
, and thus we present both along with arterial blood gases. We also noted that the SD rats had a higher rectal temperature (TR; 37.8 ± 0.2°C) compared to BN (37.0 ± 0.1°C; P= 0.018) rats, but not SS (37.1 ± 0.2°C; P > 0.05) rats.
Bilateral CBD led to significant effects on eupnoeic ventilation and blood gases. BN, SS, and SD rats significantly hypoventilated at rest 1–2 days after CBD (P < 0.001; Fig. 1A), indicated by elevated (relative to pre-CBD values) eupnoeic  levels of 41.2 ± 1.0 mmHg (P < 0.001), 42.6 ± 1.3 mmHg (P < 0.001), 41.3 ± 1.4 mmHg (P < 0.001), respectively.
levels of 41.2 ± 1.0 mmHg (P < 0.001), 42.6 ± 1.3 mmHg (P < 0.001), 41.3 ± 1.4 mmHg (P < 0.001), respectively.  ,
, 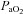 , pH and HCO3− were not different between BN, SS and SD rats from 1–4 days following CBD (Table 1; P > 0.05). Thereafter, eupnoeic
, pH and HCO3− were not different between BN, SS and SD rats from 1–4 days following CBD (Table 1; P > 0.05). Thereafter, eupnoeic 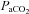 steadily returned to near pre-CBD levels in all strains, where 15–23 days after CBD
steadily returned to near pre-CBD levels in all strains, where 15–23 days after CBD 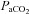 no longer differed from control (P > 0.05; Fig. 1A). However, there were strain differences in the time required for
no longer differed from control (P > 0.05; Fig. 1A). However, there were strain differences in the time required for 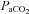 to return to control levels, as SD and BN rats returned 7–8 days and SS rats 15–23 days after CBD (Fig. 1A). Sham denervation had no effect on eupnoeic
to return to control levels, as SD and BN rats returned 7–8 days and SS rats 15–23 days after CBD (Fig. 1A). Sham denervation had no effect on eupnoeic 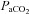 in all strains throughout the 3 weeks following surgery (P > 0.05; Fig. 1B). Likewise, eupnoeic
in all strains throughout the 3 weeks following surgery (P > 0.05; Fig. 1B). Likewise, eupnoeic  significantly decreased (relative to pre-CBD values) in all strains 1–2 days after CBD (P < 0.001; Fig. 1C), but was no longer different from pre-CBD levels by 3–4 days after CBD in BN (P > 0.05) and SS (P > 0.05) rats and by 7–8 days after CBD in SD rats (P > 0.05; Fig. 1C). Sham denervation had no effect on eupnoeic
significantly decreased (relative to pre-CBD values) in all strains 1–2 days after CBD (P < 0.001; Fig. 1C), but was no longer different from pre-CBD levels by 3–4 days after CBD in BN (P > 0.05) and SS (P > 0.05) rats and by 7–8 days after CBD in SD rats (P > 0.05; Fig. 1C). Sham denervation had no effect on eupnoeic  in all strains (P > 0.05; Fig. 1D). At rest, pH values were not different (P > 0.05) before (7.481 ± 0.003, 7.490 ± 0.005, 7.478 ± 0.004) or after (1–2 days and after 14 days) CBD in BN, SS, and SD rats, respectively.
in all strains (P > 0.05; Fig. 1D). At rest, pH values were not different (P > 0.05) before (7.481 ± 0.003, 7.490 ± 0.005, 7.478 ± 0.004) or after (1–2 days and after 14 days) CBD in BN, SS, and SD rats, respectively.
Figure 1. Resting 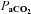 and
and 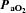 (mmHg) in CBD (A and C, respectively) and Sham (B and D, respectively) animals before and at multiple time periods after sham or CBD surgery in BN, SS and SD rats.
(mmHg) in CBD (A and C, respectively) and Sham (B and D, respectively) animals before and at multiple time periods after sham or CBD surgery in BN, SS and SD rats.
Red, blue and green asterisks, significantly (P < 0.05) different from pre-CBD values for BN, SS and SD rats, respectively.
While eupnoeic 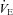 in BN rats was greater than SS and SD rats before CBD surgery, BN and SS decreased
in BN rats was greater than SS and SD rats before CBD surgery, BN and SS decreased  to 76% (P= 0.002) and 60% (P < 0.001) of pre-CBD values 1–2 days after CBD, respectively. SD rats significantly decreased
to 76% (P= 0.002) and 60% (P < 0.001) of pre-CBD values 1–2 days after CBD, respectively. SD rats significantly decreased 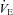 to 76% (P= 0.018) by 3–4 days after CBD (Fig. 2A). The decrease in
to 76% (P= 0.018) by 3–4 days after CBD (Fig. 2A). The decrease in 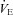 post-CBD was due to a significant decrease in VT (P= 0.006) in SS rats or non-significant tendencies of reduced breathing frequency and VT in BN and SD rats 1–2 days post-CBD (Fig. 2B and C). By 10 or more days after CBD,
post-CBD was due to a significant decrease in VT (P= 0.006) in SS rats or non-significant tendencies of reduced breathing frequency and VT in BN and SD rats 1–2 days post-CBD (Fig. 2B and C). By 10 or more days after CBD, 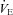 returned to pre-CBD values in all strains.
returned to pre-CBD values in all strains.  was not altered after Sham denervation in BN, SS and SD rats (P > 0.05; data not shown).
was not altered after Sham denervation in BN, SS and SD rats (P > 0.05; data not shown).
Figure 2. Resting minute ventilation (A; 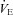 ), tidal volume (B; VT) and breathing frequency (C; f) as a percentage of pre-CBD values for BN, SS, and SD CBD groups.
), tidal volume (B; VT) and breathing frequency (C; f) as a percentage of pre-CBD values for BN, SS, and SD CBD groups.
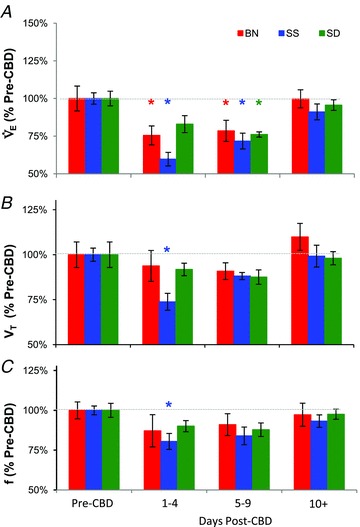
Pre-CBD values include ventilatory data collected during room air breathing. Red, blue and green asterisks, significantly (P < 0.05) different from pre-CBD values for BN, SS and SD rats, respectively.
Prior to Sham or CBD surgery, mean arterial blood pressure (MAP; mmHg) was greater than other strains in the SS rats (128.6 ± 5.4 mmHg; P < 0.001; n= 12), a well-known phenotype of this salt-sensitive rat strain even when fed a low salt diet (De Miguel et al. 2010). In contrast, MAP in BN (102.0 ± 0.9 mmHg; n= 12) and SD (101.8 ± 2.8 mmHg; n= 14) rats did not differ prior to Sham or CBD surgery (P > 0.05). Sham or CBD surgery did not affect resting MAP measured 1–6 days after surgery in all strains (P > 0.05). HR (beats min−1) was greater in SD (461.6 ± 32.1) and SS (447.5 ± 29.5) compared to BN (410.5 ± 20.6; P= 0.005) prior to Sham or CBD surgery. CBD had no significant effect on resting HR 1–6 days post-denervation in all three strains (P > 0.05).
Hypercapnia and CO2 sensitivity (Δ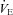 /Δ
/Δ )
)
Expressing 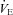 relative to room air breathing (% control), we noted that BN rats had a lower HCVR (147.4. ± 8.9%) compared to both SS (255.1 ± 8.4%; P < 0.001) and SD (252.4 ± 10.6%; P= 0.001) rats, consistent with previous reports (Dwinell et al. 2005; Forster et al. 2003; Hodges et al. 2002). Bilateral CBD and sham surgery in BN, SS and SD rats had no effects (P > 0.05) on the HCVR throughout all time points following CBD (Fig. 3A) or Sham (data not shown) surgery. Likewise, hypercapnic breathing frequency (% control) and VT (% control) were not different from pre-CBD values in all strains following CBD or Sham surgery (Fig. 3B and C; Sham data not shown), with one exception. We noted that after CBD, absolute breathing frequency differed from pre-CBD values ≥10 days post-CBD in SS rats (P= 0.002). Hypercapnia had no significant effect on MAP or HR in BN and SD strains, but significantly increased MAP (P= 0.005) but not HR in SS rats prior to Sham or CBD surgery. CBD had no effect on MAP or HR during the hypercapnic exposure 1–6 days post-CBD in BN and SD rats (P > 0.05), and MAP was unaffected (P > 0.05) but HR was greater (P= 0.002) in SS rats 1–6 days after CBD during hypercapnia.
relative to room air breathing (% control), we noted that BN rats had a lower HCVR (147.4. ± 8.9%) compared to both SS (255.1 ± 8.4%; P < 0.001) and SD (252.4 ± 10.6%; P= 0.001) rats, consistent with previous reports (Dwinell et al. 2005; Forster et al. 2003; Hodges et al. 2002). Bilateral CBD and sham surgery in BN, SS and SD rats had no effects (P > 0.05) on the HCVR throughout all time points following CBD (Fig. 3A) or Sham (data not shown) surgery. Likewise, hypercapnic breathing frequency (% control) and VT (% control) were not different from pre-CBD values in all strains following CBD or Sham surgery (Fig. 3B and C; Sham data not shown), with one exception. We noted that after CBD, absolute breathing frequency differed from pre-CBD values ≥10 days post-CBD in SS rats (P= 0.002). Hypercapnia had no significant effect on MAP or HR in BN and SD strains, but significantly increased MAP (P= 0.005) but not HR in SS rats prior to Sham or CBD surgery. CBD had no effect on MAP or HR during the hypercapnic exposure 1–6 days post-CBD in BN and SD rats (P > 0.05), and MAP was unaffected (P > 0.05) but HR was greater (P= 0.002) in SS rats 1–6 days after CBD during hypercapnia.
Figure 3. Minute ventilation (A; 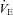 ), tidal volume (B; VT), and breathing frequency (C; f) during hypercapnia before and at multiple time periods after CBD surgery in BN, SS and SD rats.
), tidal volume (B; VT), and breathing frequency (C; f) during hypercapnia before and at multiple time periods after CBD surgery in BN, SS and SD rats.
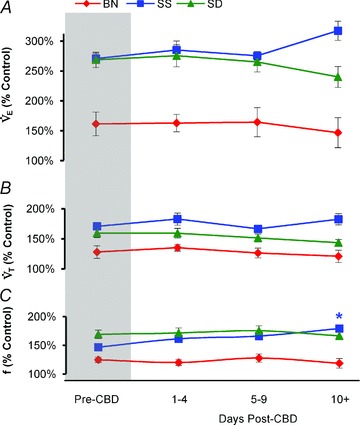
Data are expressed as a percentage of resting values (% Control). *Significantly (P < 0.05) different from pre-CBD values for SS rats.
The response to CO2 was also expressed as the slope of the relationship between ventilation and  (Δ
(Δ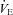 /Δ
/Δ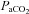 , or CO2 sensitivity) from breathing room air to 7% CO2. Prior to Sham or CBD surgery, CO2 sensitivity in BN (1.6 ± 0.4 ml min−1 mmHg−1; n= 12) rats was less than SD (3.9 ± 0.6 ml min−1 mmHg−1; P= 0.010; n= 11) and SS (6.0 ± 0.6 ml min−1 mmHg−1; P < 0.001; n= 12) rats and CO2 sensitivity in SD rats was less than SS rats (P= 0.009; Fig. 4). CO2 sensitivity was unaffected by CBD in all three strains (Fig. 4A–C), although there was an obvious rightward shift in
, or CO2 sensitivity) from breathing room air to 7% CO2. Prior to Sham or CBD surgery, CO2 sensitivity in BN (1.6 ± 0.4 ml min−1 mmHg−1; n= 12) rats was less than SD (3.9 ± 0.6 ml min−1 mmHg−1; P= 0.010; n= 11) and SS (6.0 ± 0.6 ml min−1 mmHg−1; P < 0.001; n= 12) rats and CO2 sensitivity in SD rats was less than SS rats (P= 0.009; Fig. 4). CO2 sensitivity was unaffected by CBD in all three strains (Fig. 4A–C), although there was an obvious rightward shift in 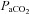 for a given
for a given  , reflecting hypoventilation at rest and during hypercapnic challenges 1–4 days post-CBD. CO2 sensitivity ≥10 days after CBD was not different from pre-CBD values within all three strains and remained different between strains.
, reflecting hypoventilation at rest and during hypercapnic challenges 1–4 days post-CBD. CO2 sensitivity ≥10 days after CBD was not different from pre-CBD values within all three strains and remained different between strains.
Figure 4. Ventilation (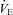 ; ml min−1) and
; ml min−1) and  (mmHg) during room air and CO2 breathing before, 1–4 and 10 or more days following CBD in BN (A), SS (B) and SD (C) rats.
(mmHg) during room air and CO2 breathing before, 1–4 and 10 or more days following CBD in BN (A), SS (B) and SD (C) rats.
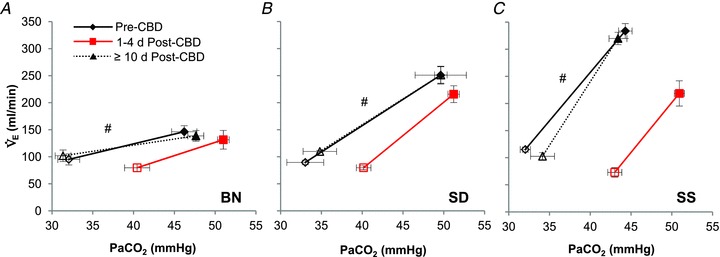
#Slope of relationship (Δ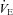 /Δ
/Δ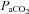 ) different (P < 0.05) from other strains.
) different (P < 0.05) from other strains.
We also plotted pre-CBD CO2 sensitivity for each animal from all three strains against the increase in eupnoeic  after CBD to determine if there is a relationship between CO2 sensitivity and the degree of hypoventilation following CBD (Fig. 5). We then derived a linear regression of the data to determine the slope and R2. The degree of hypoventilation 1–2 days following CBD was not positively correlated with pre-CBD CO2 sensitivities with a Pearson r correlation coefficient of 0.1691, which was near but did not reach statistical significance (P= 0.08). These and data in the preceding paragraphs suggest that bilateral CBD had no effect on CO2 sensitivity in all strains studied, and that the inherent differences in CO2 sensitivity were not a determinant of the degree of hypoventilation 1–2 days after CBD.
after CBD to determine if there is a relationship between CO2 sensitivity and the degree of hypoventilation following CBD (Fig. 5). We then derived a linear regression of the data to determine the slope and R2. The degree of hypoventilation 1–2 days following CBD was not positively correlated with pre-CBD CO2 sensitivities with a Pearson r correlation coefficient of 0.1691, which was near but did not reach statistical significance (P= 0.08). These and data in the preceding paragraphs suggest that bilateral CBD had no effect on CO2 sensitivity in all strains studied, and that the inherent differences in CO2 sensitivity were not a determinant of the degree of hypoventilation 1–2 days after CBD.
Figure 5.
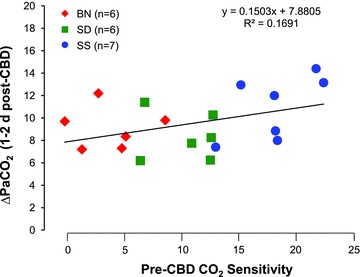
Correlation between pre-CBD CO2 sensitivities (Δ 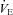 /Δ
/Δ ) and the change in
) and the change in 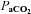 (Δ
(Δ ) breathing RA pre-CBD to 1–2 days post-CBD in BN, SS and SD rats P= 0.08.
) breathing RA pre-CBD to 1–2 days post-CBD in BN, SS and SD rats P= 0.08.
Verification of denervation: attenuation of the responses to hypoxia and venous NaCN
The hypoxic ventilatory response (HVR) when expressed as a percentage change from eupnoeic 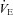 was not different between CBD or Sham groups within strains prior to surgery (P > 0.05), and thus were pooled to assess potential inter-strain variation. The HVRs amongst SS (121.1 ± 4.5%), BN (128.1 ± 4.8%) and SD (147.7 ± 8.5%) rats were not significantly (P > 0.05) different from one another. The level of hypoxaemia (
was not different between CBD or Sham groups within strains prior to surgery (P > 0.05), and thus were pooled to assess potential inter-strain variation. The HVRs amongst SS (121.1 ± 4.5%), BN (128.1 ± 4.8%) and SD (147.7 ± 8.5%) rats were not significantly (P > 0.05) different from one another. The level of hypoxaemia ( ) reached was only different between BN (38.8 ± 0.4 mmHg) and SD (36.3 ± 0.6 mmHg) rats (P= 0.042), and the
) reached was only different between BN (38.8 ± 0.4 mmHg) and SD (36.3 ± 0.6 mmHg) rats (P= 0.042), and the 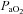 in SS rats was 37.2 ± 0.9 mmHg. Despite small differences in the absolute
in SS rats was 37.2 ± 0.9 mmHg. Despite small differences in the absolute  during hypoxia, each strain hyperventilated equally (P > 0.05), where
during hypoxia, each strain hyperventilated equally (P > 0.05), where  during the hypoxic challenge was 24.3 ± 0.4 mmHg (SS), 24.0 ± 0.4 mmHg (BN), and 24.3 ± 0.6 mmHg (SD). CBD attenuated the HVR 1–4 days following denervation in BN (P < 0.001) and SD (P < 0.001), but not SS (P > 0.05) rats as compared to pre-CBD values. We observed no effects of Sham surgery on the HVR in all strains (P > 0.05).
during the hypoxic challenge was 24.3 ± 0.4 mmHg (SS), 24.0 ± 0.4 mmHg (BN), and 24.3 ± 0.6 mmHg (SD). CBD attenuated the HVR 1–4 days following denervation in BN (P < 0.001) and SD (P < 0.001), but not SS (P > 0.05) rats as compared to pre-CBD values. We observed no effects of Sham surgery on the HVR in all strains (P > 0.05).
The ventilatory response to hypoxia was also expressed as the slope of the relationship between 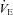 (ml min−1) and arterial
(ml min−1) and arterial 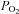 (Fig. 6). CBD and Sham groups did not significantly differ prior to surgery within each strain (P < 0.05), and so the data were pooled. We found no differences among the strains prior to Sham or CBD surgery in the HVR when expressed this way (P < 0.05). However, CBD attenuated the slope of the relationship between absolute
(Fig. 6). CBD and Sham groups did not significantly differ prior to surgery within each strain (P < 0.05), and so the data were pooled. We found no differences among the strains prior to Sham or CBD surgery in the HVR when expressed this way (P < 0.05). However, CBD attenuated the slope of the relationship between absolute 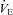 and
and 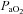 1–6 days after CBD in BN (P < 0.001; Fig. 6A), SS (P= 0.013; Fig. 6B), and SD (P= 0.011; Fig. 6C) rats.
1–6 days after CBD in BN (P < 0.001; Fig. 6A), SS (P= 0.013; Fig. 6B), and SD (P= 0.011; Fig. 6C) rats.
Figure 6. Ventilation ( ; ml min−1) and
; ml min−1) and 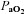 (mmHg) during room air and O2 breathing before and 1–4 days following CBD in BN (A), SS (B) and SD (C) rats.
(mmHg) during room air and O2 breathing before and 1–4 days following CBD in BN (A), SS (B) and SD (C) rats.
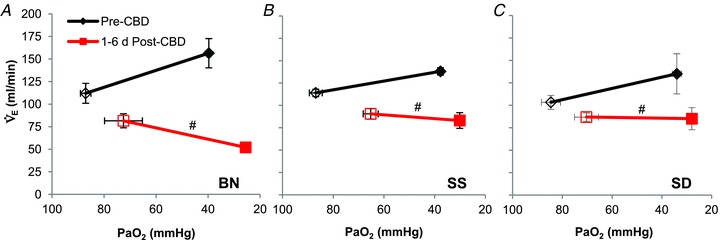
#Slope of relationship (Δ /Δ
/Δ ) different (P < 0.05) from pre-CBD values.
) different (P < 0.05) from pre-CBD values.
The NaCN ventilatory response ratio (VRR, see also Methods), was significantly attenuated 2–4 days post-CBD in BN and SS rats (P < 0.05), as well as SD rats (P < 0.001; Fig. 7A), but was unchanged in sham denervated rats (P > 0.05; Fig. 7B). Note also that the VRR was never completely eliminated following CBD in any strain, suggesting functional residual peripheral chemosensitivity elsewhere (Martin-Body et al. 1985, 1986; Serra et al. 2002).
Figure 7. NaCN response ratio (5 s of 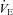 of breathing during the response divided by 5 s prior to injection) in CBD (A) and Sham (B) groups before and between 2–4 days post-surgery.
of breathing during the response divided by 5 s prior to injection) in CBD (A) and Sham (B) groups before and between 2–4 days post-surgery.
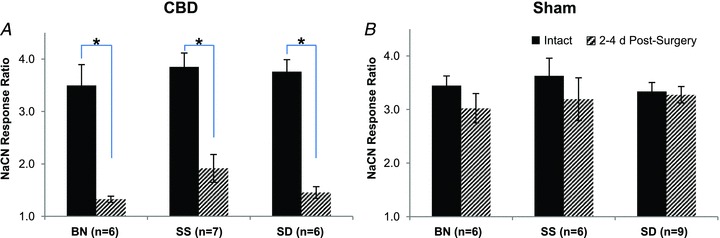
*Significantly different from pre-CBD values (P < 0.05).
Hypoxia had no effect on MAP or HR compared to room air breathing in BN and SS rats (P > 0.05), and had no effect on MAP (P > 0.05) but significantly decreased HR in SD rats (428.6 ± 11.1; P= 0.04) prior to surgery. CBD had no effect on HR 1–6 days post-denervation during hypoxia compared to control, but MAP decreased during hypoxia significantly in BN (78.2 ± 5.3 mmHg; P= 0.008), SS (63.9 ± 6.5 mmHg; P < 0.001), and SD (75.6 ± 3.1 mmHg; P < 0.001). The significant decrease in MAP after CBD during a hypoxic challenge is an effect consistent with carotid sinus (baroreceptor) denervation (Franchini et al. 1994).
Discussion
Here we characterized the acute and chronic effects of CBD on eupnoeic breathing and the ventilatory responses to hypoxia and hypercapnia in three rat strains with inherently different ventilatory CO2 sensitivities. The major findings of this comprehensive study were that in all three rats strains tested, CBD (1) led to an equal hypoventilation 1–2 days post CBD, and (2) had no effect on CO2 sensitivity.
Effects of CBD on eupnoeic ventilation and CO2 sensitivity in rats
CBD nearly uniformly leads to eupnoeic hypoventilation and attenuation of the hypoxic ventilatory response in multiple species, including rats (Favier & Lacaisse 1978; Martin-Body et al. 1985, 1986; Olson et al. 1988). We noted eupnoeic hypoventilation of +9.2 (BN), +11.0 (SS), and +8.7 (SD) mmHg  within 1–2 days following CBD, similar to the increase previously reported following CBD in SD rats (Olson et al. 1988), but in contrast to data from others showing no changes in resting
within 1–2 days following CBD, similar to the increase previously reported following CBD in SD rats (Olson et al. 1988), but in contrast to data from others showing no changes in resting 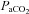 following CBD in Wistar rats (da Silva et al. 2011).
following CBD in Wistar rats (da Silva et al. 2011).  reached values of 41.3–42.6 mmHg in BN, SS and SD rats within 1–2 days post-CBD, which are comparatively lower than previous reports in SD rats (49.7 ± 1.6 mmHg) (Olson et al. 1988). In fact, the measurements of
reached values of 41.3–42.6 mmHg in BN, SS and SD rats within 1–2 days post-CBD, which are comparatively lower than previous reports in SD rats (49.7 ± 1.6 mmHg) (Olson et al. 1988). In fact, the measurements of 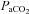 in this study were lower overall relative to those reported by Olson et al. (1988), for which we have no explanation as to the cause. The
in this study were lower overall relative to those reported by Olson et al. (1988), for which we have no explanation as to the cause. The  values presented here are, however, consistent with several other studies measuring
values presented here are, however, consistent with several other studies measuring  in these three strains (Serra et al. 2001; Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005).
in these three strains (Serra et al. 2001; Hodges et al. 2002; Forster et al. 2003; Dwinell et al. 2005).
There is a paucity of data documenting the effect of CBD on CO2 sensitivity in the unanaesthetized rat. In a series of experiments where they performed CBD in SD rats at various ages, Serra et al. (2001) noted that CO2 sensitivity (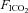 = 0.07) in the adult groups tended to be lower in rats 24 days post-CBD compared to controls, but they were unable to test this statistically due to having only a few observations (n= 3). In one other study it was reported that CO2 sensitivity (
= 0.07) in the adult groups tended to be lower in rats 24 days post-CBD compared to controls, but they were unable to test this statistically due to having only a few observations (n= 3). In one other study it was reported that CO2 sensitivity (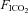 = 0.07) was unaltered following CBD in Wistar rats (da Silva et al. 2011). Thus, in the few experiments in which CO2 sensitivity was tested after CBD in rats, there was no effect. Similarly, BN, SS and SD rats also showed no change in the HCVR following CBD. Instead, these strains demonstrate a rightward-shift in the relationship between
= 0.07) was unaltered following CBD in Wistar rats (da Silva et al. 2011). Thus, in the few experiments in which CO2 sensitivity was tested after CBD in rats, there was no effect. Similarly, BN, SS and SD rats also showed no change in the HCVR following CBD. Instead, these strains demonstrate a rightward-shift in the relationship between 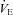 and
and 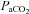 without a change in the slope. Overall, the data further support the conclusion that unlike other species, CBD does not affect CO2 sensitivity in unanaesthetized rats.
without a change in the slope. Overall, the data further support the conclusion that unlike other species, CBD does not affect CO2 sensitivity in unanaesthetized rats.
The peripheral chemoreceptors and ventilatory CO2 sensitivity
The postulated contribution of the carotid chemoreceptors to eupnoeic breathing and CO2 sensitivity has evolved over the last few decades. In 1938 (Comroe & Schmidt 1938) and 1966 (Fencl et al. 1966), it was concluded that the carotid chemoreceptors contribute minimally to eupnoeic breathing and CO2 sensitivity. Those conclusions were consistent with the relatively small increase in sinus nerve activity as 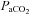 was increased in anaesthetized animals. However, it has been shown in the awake state in several mammals, including humans, that CBD causes significant hypoventilation and attenuation of CO2 sensitivity (Bisgard et al. 1976; Pan et al. 1998; Dahan et al. 2007, 2008). These data and additional data from other preparations have led to the hypothesis that the carotid chemoreceptors contribute about one-third of the stimulus for the CO2 hyperpnoea. However, these preparations may not provide a valid assessment of the carotid chemoreceptors’ contribution to CO2 responsiveness. For example, experiments using behaving awake dogs or goats in which a single carotid body is isolated (the other is denervated) and extracorporeally perfused (allowing for separate manipulation of the carotid and brain environments), carotid body perfusion with hyperoxic and hypocapnic blood led to hypoventilation and decreased CO2 sensitivity (Blain et al. 2009, 2010; Daristotle & Bisgard 1989). In contrast, stimulating the carotid body with hypoxia in this preparation accentuates the sensitivity to increasing arterial (brain)
was increased in anaesthetized animals. However, it has been shown in the awake state in several mammals, including humans, that CBD causes significant hypoventilation and attenuation of CO2 sensitivity (Bisgard et al. 1976; Pan et al. 1998; Dahan et al. 2007, 2008). These data and additional data from other preparations have led to the hypothesis that the carotid chemoreceptors contribute about one-third of the stimulus for the CO2 hyperpnoea. However, these preparations may not provide a valid assessment of the carotid chemoreceptors’ contribution to CO2 responsiveness. For example, experiments using behaving awake dogs or goats in which a single carotid body is isolated (the other is denervated) and extracorporeally perfused (allowing for separate manipulation of the carotid and brain environments), carotid body perfusion with hyperoxic and hypocapnic blood led to hypoventilation and decreased CO2 sensitivity (Blain et al. 2009, 2010; Daristotle & Bisgard 1989). In contrast, stimulating the carotid body with hypoxia in this preparation accentuates the sensitivity to increasing arterial (brain)  (Blain et al. 2009, 2010). These findings are in contrast to Day & Wilson (2007, 2009), who demonstrated in an in situ rat preparation that the carotid body responsiveness to hypoxia and hypercapnia was greater when the brainstem was held hypocapnic, suggesting a negative interaction (Day & Wilson 2007, 2009). Thus, irrespective of differences between awake and reduced preparations, the estimate of 33% contribution of the carotid bodies to CO2 sensitivity is not valid as it appears that there is no straightforward mathematical relationship describing the activity levels of the carotid body and how they alter the central response to hypercapnia, and how central chemoreceptor activity changes affect sensitivity of the carotid body.
(Blain et al. 2009, 2010). These findings are in contrast to Day & Wilson (2007, 2009), who demonstrated in an in situ rat preparation that the carotid body responsiveness to hypoxia and hypercapnia was greater when the brainstem was held hypocapnic, suggesting a negative interaction (Day & Wilson 2007, 2009). Thus, irrespective of differences between awake and reduced preparations, the estimate of 33% contribution of the carotid bodies to CO2 sensitivity is not valid as it appears that there is no straightforward mathematical relationship describing the activity levels of the carotid body and how they alter the central response to hypercapnia, and how central chemoreceptor activity changes affect sensitivity of the carotid body.
We reasoned that if in the awake state carotid body activity was a determinant of the responsiveness of central chemoreceptors in the rat, then CBD would either decrease the ventilatory response to hypercapnia as in other species, or potentially increase the ventilatory response to hypercapnia if there indeed is a negative interaction among the peripheral and central chemoreceptors in rats (Day & Wilson 2007, 2009). We further reasoned that CBD would have the least effect on CO2 sensitivity in the strain with the lowest inherent CO2 sensitivity, which may result from a dysfunctional interaction among the peripheral and central chemoreceptors. While CBD led to hypoventilation during eupnoeic breathing, we found no effect of CBD on CO2 sensitivity in all rat strains studied. It is possible that the combination of hypoventilation at rest and no change in the CO2 sensitivity could result from an increase in the ‘threshold’ of activation of CO2/H+ chemoreceptors after CBD, but no changes in the sensitivity of the system. In contrast, in other mammals such as goats or dogs, CBD might both increase this postulated threshold for activation of CO2/H+ chemoreceptors (eupnoeic hypoventilation) and decrease the ‘gain’ or sensitivity of the system through a removal of peripheral/central chemoreceptor interaction. In other words, the differences in conclusions regarding the nature of the interaction among peripheral and central chemoreceptors, including awake dogs as compared to the in situ perfused rat preparation, may not be due to the preparation, but may reflect true species differences. However, the findings herein do not support the hypothesis of a hyper-additive interaction or interdependence among the peripheral and central chemoreceptors in the HCVR in rats.
Another potential explanation for the unchanged CO2 sensitivity in the BN, SS and SD rats is that there is a greater (and perhaps immediate) compensation for the loss of the carotid bodies by other CNS sites in rats relative to other species. Primary afferents arising from the carotid body target sub-nuclei of the NTS, which then project to multiple nuclei in the respiratory network, including the retrotrapezoid nucleus (RTN) (Stornetta et al. 2006; Takakura et al. 2006; Alheid et al. 2011). CBD would presumably dampen these predominantly excitatory projections from the NTS to the respiratory network/RTN and thereby attenuate CO2 sensitivity. It is possible that the CBD-induced attenuation of excitatory drive to the RTN could be compensated in rats but not other species by increased excitatory neuromodulation by raphé serotonergic (5-HT) neurons, which augments cellular CO2/H+ chemosensitivity of RTN neurons in vitro and in vivo (Mulkey et al. 2007). Regardless, the mechanisms that underlie the differences among species in the effects of CBD on CO2 sensitivity are unclear, but any explanation has to also account for the relatively uniform effect of CBD on eupnoeic ventilation.
Dissociation of eupnoeic ventilation and CO2 sensitivity
There are a growing number of observations that demonstrate dissociation between eupnoeic 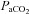 and CO2 sensitivity. Experimental perturbations, such as CBD and/or brainstem lesions, can often lead to decrease eupnoeic ventilation, increased
and CO2 sensitivity. Experimental perturbations, such as CBD and/or brainstem lesions, can often lead to decrease eupnoeic ventilation, increased 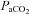 and decreased CO2 sensitivity. However, there are several instances that indicate disproportionate or completely separate effects on CO2 sensitivity and to resting ventilation. Killing 40–80% of medullary NK-1 receptor-expressing neurons led to a 50–60% reduction in CO2 sensitivity, but only a ∼10% decrease in eupnoeic ventilation (Nattie & Li 2002). Genetic deletion or acute silencing of most or all 5-HT neurons in mice can lead to large and selective effects on the HCVR without altering eupnoeic ventilation (Hodges et al. 2008; Ray et al. 2011). Moreover, recovery of eupnoeic
and decreased CO2 sensitivity. However, there are several instances that indicate disproportionate or completely separate effects on CO2 sensitivity and to resting ventilation. Killing 40–80% of medullary NK-1 receptor-expressing neurons led to a 50–60% reduction in CO2 sensitivity, but only a ∼10% decrease in eupnoeic ventilation (Nattie & Li 2002). Genetic deletion or acute silencing of most or all 5-HT neurons in mice can lead to large and selective effects on the HCVR without altering eupnoeic ventilation (Hodges et al. 2008; Ray et al. 2011). Moreover, recovery of eupnoeic 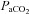 occurs at a slightly different rate and to a different degree compared to the recovery of the hypoxic ventilatory response and CO2 sensitivity following CBD in goats and ponies, perhaps suggesting separate mechanisms governing each type of plasticity. Here we show eupnoeic hypoventilation without an effect on CO2 sensitivity after CBD in rats. In addition, we found that CO2 sensitivity before CBD does not correlate with the degree of hypoventilation 1–2 days following CBD, further suggesting that the mechanisms governing eupnoeic blood gas regulation are distinguishable from those governing CO2 sensitivity. It remains to be determined if the mechanisms that control eupnoeic ventilation are indeed completely separable from the mechanisms of CO2/H+ chemoreception, or if this observation is unique to these specific experimental manipulations. Regardless, increasing our understanding of how these ventilatory control mechanisms can be unlinked could provide valuable insights into the fundamental organization of the respiratory network and the integration of its afferent inputs.
occurs at a slightly different rate and to a different degree compared to the recovery of the hypoxic ventilatory response and CO2 sensitivity following CBD in goats and ponies, perhaps suggesting separate mechanisms governing each type of plasticity. Here we show eupnoeic hypoventilation without an effect on CO2 sensitivity after CBD in rats. In addition, we found that CO2 sensitivity before CBD does not correlate with the degree of hypoventilation 1–2 days following CBD, further suggesting that the mechanisms governing eupnoeic blood gas regulation are distinguishable from those governing CO2 sensitivity. It remains to be determined if the mechanisms that control eupnoeic ventilation are indeed completely separable from the mechanisms of CO2/H+ chemoreception, or if this observation is unique to these specific experimental manipulations. Regardless, increasing our understanding of how these ventilatory control mechanisms can be unlinked could provide valuable insights into the fundamental organization of the respiratory network and the integration of its afferent inputs.
Respiratory plasticity following CBD
Our data also contrast to previous reports in the time needed for eupnoeic  values to return to control levels in rats, a form of respiratory plasticity (Forster, 2003). Olson and colleagues (1988) reported that hypoventilation elicited by CBD in adult SD rats does not return to control values until >70 days later (Olson et al. 1988). In contrast, the time required for recovery of eupnoeic breathing and blood gases in BN, SS and SD rats was relatively short (7–15 days post-CBD), perhaps due to different surgical methods (Serra et al. 2001) and/or the resulting airway trauma from a midline versus lateral incisions (Olson et al. 1988). The recovery period for
values to return to control levels in rats, a form of respiratory plasticity (Forster, 2003). Olson and colleagues (1988) reported that hypoventilation elicited by CBD in adult SD rats does not return to control values until >70 days later (Olson et al. 1988). In contrast, the time required for recovery of eupnoeic breathing and blood gases in BN, SS and SD rats was relatively short (7–15 days post-CBD), perhaps due to different surgical methods (Serra et al. 2001) and/or the resulting airway trauma from a midline versus lateral incisions (Olson et al. 1988). The recovery period for 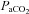 returning to control levels within this study is also more rapid relative to other species such as goats (Pan et al. 1998), ponies (Bisgard et al. 1980), and dogs (Rodman et al. 2001), and fairly uniform among these strains despite large phenotypic differences in CO2 sensitivity. Thus, the potential mechanisms governing the recovery of eupnoeic
returning to control levels within this study is also more rapid relative to other species such as goats (Pan et al. 1998), ponies (Bisgard et al. 1980), and dogs (Rodman et al. 2001), and fairly uniform among these strains despite large phenotypic differences in CO2 sensitivity. Thus, the potential mechanisms governing the recovery of eupnoeic 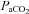 could be relatively uniform among these strains. These mechanisms may include the recruitment of other sets of peripheral chemoreceptors, such as aortic arch or cardiac chemoreceptors (Bisgard et al. 1976, 1980; Martin-Body et al. 1985; Pan et al. 1998; Serra et al. 2002), reorganization of one or several components of the central respiratory network (Hodges et al. 2005; Roux et al. 2000a,b), or a combination of peripheral and central mechanisms. Additional studies are needed to elucidate the mechanisms driving the respiratory neuroplasticity following CBD in mammals.
could be relatively uniform among these strains. These mechanisms may include the recruitment of other sets of peripheral chemoreceptors, such as aortic arch or cardiac chemoreceptors (Bisgard et al. 1976, 1980; Martin-Body et al. 1985; Pan et al. 1998; Serra et al. 2002), reorganization of one or several components of the central respiratory network (Hodges et al. 2005; Roux et al. 2000a,b), or a combination of peripheral and central mechanisms. Additional studies are needed to elucidate the mechanisms driving the respiratory neuroplasticity following CBD in mammals.
Summary and conclusions
Our hypothesis that CBD in BN, SS and SD rats will cause eupnoeic hypoventilation and attenuate ventilatory CO2 sensitivity was only partially validated, as CBD led to eupnoeic hypoventilation but did not attenuate CO2 sensitivity. We further hypothesized that the resulting hypoventilation following CBD would be greatest in the most CO2 sensitive strains and least in the CO2-insensitive BN rats, but the data did not support this hypothesis. Based on the effects of CBD in three strains of rats with large phenotypic variation in ventilatory sensitivity to CO2, we conclude that in the rat the carotid bodies (1) play an important role in the regulation of arterial blood gases during eupnoea independent of inherent differences in CO2 sensitivity, and (2) have little influence on the hypercapnic ventilatory response or CO2 sensitivity in the unanaesthetized rat.
Acknowledgments
Funding for this study was provided by NIH HL097033 (PI to M.R.H.).
Glossary
- BN
Brown Norway
- CB
carotid body
- CBD
carotid body denervation
- SD
Sprague–Dawley
- SS
Dahl Salt-Sensitive
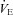
minute ventilation
- VT
tidal volume
Author contributions
G.C.M. performed surgeries and all experiments, analysed data and wrote the MS, H.V.F. contributed to intellectual discussions, and writing and editing the MS, and M.R.H. performed surgeries, analysed data and contributed to MS writing and editing. All experiments were performed at The Medical College of Wisconsin, Milwaukee, Wisconsin.
References
- Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970;208:121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J Appl Physiol. 1976;40:184–190. doi: 10.1152/jappl.1976.40.2.184. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupnoeic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comroe JH, Schmidt CM. The part played by reflexes from the carotid in the chemical regulation of the respiration in the dog. Am J Physiol. 1938;121:75–97. [Google Scholar]
- Cragg PA, Drysdale DB. Interaction of hypoxia and hypercapnia on ventilation, tidal volume and respiratory frequency in the anaesthetized rat. J Physiol. 1983;341:477–493. doi: 10.1113/jphysiol.1983.sp014818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Fisher JA, Duffin J. Central-peripheral respiratory chemoreflex interaction in humans. Respir Physiol Neurobiol. 2012;180:126–131. doi: 10.1016/j.resp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- da Silva GS, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LG, Glass ML. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflugers Arch. 2011;462:407–418. doi: 10.1007/s00424-011-0990-x. [DOI] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L. Plasticity in the brain: influence of bilateral carotid body resection (bCBR) on central CO2 sensitivity. Adv Exp Med Biol. 2008;605:312–316. doi: 10.1007/978-0-387-73693-8_54. [DOI] [PubMed] [Google Scholar]
- Daristotle L, Bisgard GE. Central-peripheral chemoreceptor ventilatory interaction in awake goats. Respir Physiol. 1989;76:383–391. doi: 10.1016/0034-5687(89)90078-9. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Specific carotid body chemostimulation is sufficient to elicit phrenic poststimulus frequency decline in a novel in situ dual-perfused rat preparation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R532–R544. doi: 10.1152/ajpregu.00812.2004. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Brainstem PCO2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol. 2007;578:843–857. doi: 10.1113/jphysiol.2006.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. A negative interaction between central and peripheral respiratory chemoreceptors may underlie sleep-induced respiratory instability: a novel hypothesis. Adv Exp Med Biol. 2008;605:447–451. doi: 10.1007/978-0-387-73693-8_78. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Dwinell MR, Forster HV, Petersen J, Rider A, Kunert MP, Cowley AW, Jr, Jacob HJ. Genetic determinants on rat chromosome 6 modulate variation in the hypercapnic ventilatory response using consomic strains. J Appl Physiol. 2005;98:1630–1638. doi: 10.1152/japplphysiol.01148.2004. [DOI] [PubMed] [Google Scholar]
- Engwall MJ, Vidruk EH, Nielsen AM, Bisgard GE. Response of the goat carotid body to acute and prolonged hypercapnia. Respir Physiol. 1988;74:335–344. doi: 10.1016/0034-5687(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Favier R, Lacaisse A. [O2 chemoreflex drive of ventilation in the awake rat (author's transl)] J Physiol (Paris) 1978;74:411–417. [PubMed] [Google Scholar]
- Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966;210:459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Forster HV, Dwinell MR, Hodges MR, Brozoski D, Hogan GE. Do genes on rat chromosomes 9, 13, 16, 18, and 20 contribute to regulation of breathing? Respir Physiol Neurobiol. 2003;135:247–261. doi: 10.1016/s1569-9048(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Forster HV, Martino P, Hodges MR, Krause K, Bonis J, Davis S, Pan LG. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupnoeic breathing. Adv Exp Med Biol. 2007;605:322–326. doi: 10.1007/978-0-387-73693-8_56. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Cestari IA, Krieger EM. Restoration of arterial blood oxygen tension increases arterial pressure in sinoaortic-denervated rats. Am J Physiol Heart Circ Physiol. 1994;266:H1055–1061. doi: 10.1152/ajpheart.1994.266.3.H1055. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol. 2002;93:974–983. doi: 10.1152/japplphysiol.00019.2002. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol. 2005;98:1234–1242. doi: 10.1152/japplphysiol.01011.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975;24:249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mokashi A, Mulligan E, Nishino T. Comparison of aortic and carotid chemoreceptor responses to hypercapnia and hypoxia. J Appl Physiol. 1981;51:55–61. doi: 10.1152/jappl.1981.51.1.55. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF, Konig A. Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centers. Ann N Y Acad Sci. 1963;109:651–660. doi: 10.1111/j.1749-6632.1963.tb13495.x. [DOI] [PubMed] [Google Scholar]
- Lowry TF, Forster HV, Pan LG, Serra A, Wenninger J, Nash R, Sheridan D, Franciosi RA. Effects on breathing of carotid body denervation in neonatal piglets. J Appl Physiol. 1999;87:2128–2135. doi: 10.1152/jappl.1999.87.6.2128. [DOI] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J Physiol. 1985;361:35–45. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J Physiol. 1986;380:61–73. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol. 1988;64:666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- Roux JC, Pequignot JM, Dumas S, Pascual O, Ghilini G, Pequignot J, Mallet J, Denavit-Saubie M. O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. Eur J Neurosci. 2000a;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000b;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol. 2001;91:1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R, Forster HV. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J Appl Physiol. 2002;92:893–900. doi: 10.1152/japplphysiol.00819.2001. [DOI] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ, St JP, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidruk EH, Olson EB, Jr, Ling L, Mitchell GS. Responses of single-unit carotid body chemoreceptors in adult rats. J Physiol. 2001;531:165–170. doi: 10.1111/j.1469-7793.2001.0165j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


