Abstract
Skeletal muscle mitochondrial content varies extensively between human subjects. Biochemical measures of mitochondrial proteins, enzyme activities and lipids are often used as markers of mitochondrial content and muscle oxidative capacity (OXPHOS). The purpose of this study was to determine how closely associated these commonly used biochemical measures are to muscle mitochondrial content and OXPHOS. Sixteen young healthy male subjects were recruited for this study. Subjects completed a graded exercise test to determine maximal oxygen uptake (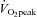 ) and muscle biopsies were obtained from the vastus lateralis. Mitochondrial content was determined using transmission electron microscopy imaging and OXPHOS was determined as the maximal coupled respiration in permeabilized fibres. Biomarkers of interest were citrate synthase (CS) activity, cardiolipin content, mitochondrial DNA content (mtDNA), complex I–V protein content, and complex I–IV activity. Spearman correlation coefficient tests and Lin's concordance tests were applied to assess the absolute and relative association between the markers and mitochondrial content or OXPHOS. Subjects had a large range of
) and muscle biopsies were obtained from the vastus lateralis. Mitochondrial content was determined using transmission electron microscopy imaging and OXPHOS was determined as the maximal coupled respiration in permeabilized fibres. Biomarkers of interest were citrate synthase (CS) activity, cardiolipin content, mitochondrial DNA content (mtDNA), complex I–V protein content, and complex I–IV activity. Spearman correlation coefficient tests and Lin's concordance tests were applied to assess the absolute and relative association between the markers and mitochondrial content or OXPHOS. Subjects had a large range of 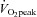 (range 29.9–71.6 ml min−1 kg−1) and mitochondrial content (4–15% of cell volume). Cardiolipin content showed the strongest association with mitochondrial content followed by CS and complex I activities. mtDNA was not related to mitochondrial content. Complex IV activity showed the strongest association with muscle oxidative capacity followed by complex II activity. We conclude that cardiolipin content, and CS and complex I activities are the biomarkers that exhibit the strongest association with mitochondrial content, while complex IV activity is strongly associated with OXPHOS capacity in human skeletal muscle.
(range 29.9–71.6 ml min−1 kg−1) and mitochondrial content (4–15% of cell volume). Cardiolipin content showed the strongest association with mitochondrial content followed by CS and complex I activities. mtDNA was not related to mitochondrial content. Complex IV activity showed the strongest association with muscle oxidative capacity followed by complex II activity. We conclude that cardiolipin content, and CS and complex I activities are the biomarkers that exhibit the strongest association with mitochondrial content, while complex IV activity is strongly associated with OXPHOS capacity in human skeletal muscle.
Key points
Several biochemical measures of mitochondrial components are used as biomarkers of mitochondrial content and muscle oxidative capacity. However, no studies have validated these surrogates against a morphological measure of mitochondrial content in human subjects.
The most commonly used markers (citrate synthase activity, cardiolipin content, mitochondrial DNA content (mtDNA), complex I–V protein, and complex I–IV activity) were correlated with a measure of mitochondrial content (transmission electron microscopy) and muscle oxidative capacity (respiration in permeabilized fibres).
Cardiolipin content followed by citrate synthase activity and complex I activity were the biomarkers showing the strongest association with mitochondrial content.
mtDNA was found to be a poor biomarker of mitochondrial content.
Complex IV activity was closely associated with mitochondrial oxidative phosphorylation capacity.
Introduction
A common experimental approach in bioenergetic research is the determination of mitochondrial content. Mitochondrial volume or content is an important quantitative indicator of oxidative capacity and is often used to normalize global measures of muscle bioenergetic capacity. Even though it is well established that the mitochondrial population exists in a three-dimensional network (Ogata & Yamasaki, 1997), two-dimensional imaging using transmission electron microscopy (TEM) is still regarded as the golden standard for measuring mitochondrial fractional area (mitochondrial content). Since the TEM technique is time consuming and may not be available for many laboratories, biochemical measures of mitochondrial proteins, lipids, enzyme activities and DNA have often been used as surrogate measures of mitochondrial content (biomarkers). However, it is not known which of these commonly used markers of mitochondrial content has the strongest association with a morphological measure of the actual mitochondrial content.
During the past two decades there has been an increased interest in determining the intrinsic functional properties of mitochondria. Studies on isolated mitochondria or permeabilized muscle fibres have shown that acute exercise (Fernström et al. 2004), high fat diet (Anderson et al. 2009), type 2 diabetes (Phielix et al. 2008), chronic obstructive pulmonary disease (Naimi et al. 2011) and obesity (Anderson et al. 2008) may change the intrinsic properties of the mitochondria. Intrinsic changes are most often determined by normalizing the functional measure of interest to a marker of mitochondrial content. The most commonly used markers of mitochondrial content are citrate synthase (CS) activity (Mogensen et al. 2006a; Boushel et al. 2007; Fernström et al. 2007; Rabol et al. 2009a, b), mitochondrial DNA (mtDNA) (Boushel et al. 2007; Phielix et al. 2008), cardiolipin (Ritov et al. 2006) and activity of cytochrome c oxidase (COX) (Picard et al. 2011). The array of diverse biomarkers currently used for normalization may explain some of the controversies that exist in some of the above-mentioned areas. Hence, studies have also shown that the intrinsic mitochondrial functionality is not altered with acute exercise (Molnar et al. 2006), type 2 diabetes (Boushel et al. 2007; Hey-Mogensen et al. 2010) and obesity (Ara et al. 2011). Thus, a determination of which biomarker of mitochondrial content is the most appropriate and valid to use is highly warranted.
The purpose of the present study was to evaluate the association and validity of CS activity, cardiolipin content, mtDNA, complex I–V protein content and complex I–IV activity as biomarkers of mitochondrial content. A secondary aim was to repeat these association analyses to investigate which of these biochemical measures could be used as a marker of muscle oxidative capacity (OXPHOS). OXPHOS was determined as the maximal coupled respiration (state 3) in permeabilized fibres.
Methods
Ethical approval
Subjects were informed orally and in writing about the purpose of the study, experimental procedures, and all its potential risks prior to providing written consent to participate. The study was approved by the local ethics committee of Frederiksberg and Copenhagen municipality (H-D-2007-0026) and was performed in accordance with the Declaration of Helsinki.
Subject characteristics
Sixteen subjects were recruited for this study. All subjects were healthy non-smoking males, who were not taking any medication nor had any known family history of type 2 diabetes, severe obesity, or cardiovascular diseases. In order to increase the sensitivity of the statistical analysis the recruited subjects represented a large range of physical training levels and accordingly were assumed to exhibit large variations in mitochondrial content.
Participants reported to the laboratory on two occasions. On the first occasion subjects completed an incremental cycle exercise test to determine maximal oxygen uptake (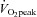 ) (Oxycon Pro, Jaeger, Würzburg, Germany) and a dual-energy X-ray absorptiometry scan (Lunar Prodigy Advance; Lunar, Madison, WI, USA) for determination of body composition. On the second occasion, subjects reported to the laboratory after a 12 h fast. After 30 min rest, a blood sample was taken for later analysis of plasma concentrations of glucose (Larsen et al. 2009), insulin and lipids (Larsen et al. 2011). A biopsy was taken from the vastus lateralis muscle under local anaesthesia (3–5 ml 5% lidocaine (lignocaine)) followed by 20 min rest. Thereafter subjects completed a graded cycle test to determine maximal whole body lipid oxidation (Achten et al. 2002).
) (Oxycon Pro, Jaeger, Würzburg, Germany) and a dual-energy X-ray absorptiometry scan (Lunar Prodigy Advance; Lunar, Madison, WI, USA) for determination of body composition. On the second occasion, subjects reported to the laboratory after a 12 h fast. After 30 min rest, a blood sample was taken for later analysis of plasma concentrations of glucose (Larsen et al. 2009), insulin and lipids (Larsen et al. 2011). A biopsy was taken from the vastus lateralis muscle under local anaesthesia (3–5 ml 5% lidocaine (lignocaine)) followed by 20 min rest. Thereafter subjects completed a graded cycle test to determine maximal whole body lipid oxidation (Achten et al. 2002).
Preparation of muscle biopsies
The muscle biopsy (approximately 200 mg) was divided into three parts. One part was prepared for respiratory measurements in saponin-permeabilized fibres, another part of the biopsy was prepared for TEM, and a third part was snap frozen in liquid nitrogen and stored at −80°C until further analysis of enzyme activities, protein content (western blotting), genomic and mitochondrial DNA content, and skeletal muscle lipids.
Mitochondrial respiratory protocols
Permeabilized fibres were prepared according to the previously described protocol (Kunz et al. 1993). Respiratory rates were measured using high resolution respirometers (Oxygraph-2k; Oroboros, Innsbruck, Austria) in MiR05 buffer (containing 110 mmol l−1 sucrose, 60 mmol l−1 potassium lactobionate, 0.5 mmol l−1 EGTA, 3 mmol l−1 MgCl2.6H2O, 20 mmol l−1 taurine, 10 mmol l−1 KH2PO4, 20 mmol l−1 Hepes, 1 g l−1 BSA, pH 7.1) at 37°C. All respiratory protocols were made in duplicates. Protocols 1–3, 4 and 5 were modified from Larsen et al. 2011, Larsen et al. 2012 and Rabol et al. 2010, respectively.
Protocol 1
l-Malate (2 mmol l−1), ADP-Mg (5 mmol l−1), glutamate (10 mmol l−1) (GM3) and cytochrome c (10 μm, for validation of the intactness of the outer mitochondrial membrane) and carbonyl cyanide p-trifluorome thoxyphenylhydrazone (FCCP titration in steps of 0.25 mmol l−1, to induce maximal uncoupled respiration) (GM3u).
Protocol 2
l-Malate (2 mmol l−1), ADP-Mg (5 mmol l−1), glutamate (10 mmol l−1) and succinate (10 mmol l−1) (GMS3), followed by cytochrome c (10 μm) and finally FCCP titration (GMS3u).
Protocol 3
l-Malate (2 mmol l−1), ADP-Mg (5 mmol l−1) and octanoyl-l-carnitine (1.5 mmol l−1) (Oct3), followed by cytochrome c (10 μm) and finally FCCP titration (Oct3u).
Protocol 4
ADP-Mg (5 mmol l−1), cytochrome c (10 μm), antimycin A (2.5 μm) followed by ascorbate (2 mmol l−1) and N,N,N′,N′-tetramethyl-1,4-benzenediamine dihydrochloride (TMPD, 500 μm) (TMPD + Asc).
Protocol 5
l-Malate (2 mmol l−1), glutamate (10 mmol l−1), pyruvate (5 mmol l−1) and ADP-Mg (5 mmol l−1) (PGM3). Succinate (10 mmol l−1) (PGMS3) and octanoyl-l-carnitine (1.5 mmol l−1) (PGMSOct3). Finally, cytochrome c (10 μm) followed by oligomycin (2 μg ml−1) to induce State 4 respiration (state 4o).
Tissue fixation and processing for TEM
Muscle biopsies were fixed as previously described (Nielsen et al. 2010). Briefly, biopsy specimens were fixed with 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.3) for 24 h and subsequently rinsed four times in 0.1 m sodium cacodylate buffer. Following rinsing, muscle specimens were post-fixed with 1% osmium tetroxide and 1.5% potassium ferrocyanide (K4Fe(CN)6) in 0.1 m sodium cacodylate buffer for 60 min at 4°C, and, after rinsing 3 times in H2O, with 1% uranyl acetate in H2O. After post-fixation, the muscle specimens were rinsed twice in H2O at 4°C, dehydrated through a graded series of alcohol at 4–20°C, infiltrated with graded mixtures of propylene oxide (substituted by acetone in 2 samples) and Epon at 20°C, and embedded in 100% Epon at 30°C. In order to obtain as many fibres as possible, the ultra-thin sections were cut (using a Leica Ultracut UCT ultramicrotome; Rowako AB, Vendelsö, Sweden) in three depths separated by 150 nm. The sections were contrasted with uranyl acetate and lead citrate, and examined and photographed in a pre-calibrated Philips EM 208 electron microscope and a Megaview III FW camera (both FEI Company, Eindhoven, the Netherlands).
Stereological methods
In the sections from the three depths of each biopsy, all the longitudinally oriented fibres were included obtaining a mean of seven fibres per biopsy (range: 5–9). Intermyofibrillar (IMF) mitochondrial area and subsarcolemmal (SS) mitochondrial area per fibre surface area were estimated by point counting and direct fibre length measurement (Weibel, 1980) using 24 images obtained at ×20,000 magnification in a randomized systematic order including 12 from the SS region and 6 from both the superficial and central region of the myofibrillar space. Total (IMF + SS) mitochondria was determined by recalculating SS mitochondria to a volume density by the formula: Volume beneath the surface area of a cylindrical-shaped fibre (Vb) =R× 0.5 ×A, where R is fibre radius and A is the fibre surface area. Fibre radius was assumed to be 40 μm. Two representative images of ×20,000 magnification are presented in Fig. 1A and B.
Figure 1. Representative electron microscopy images and western blots of complex I–V.
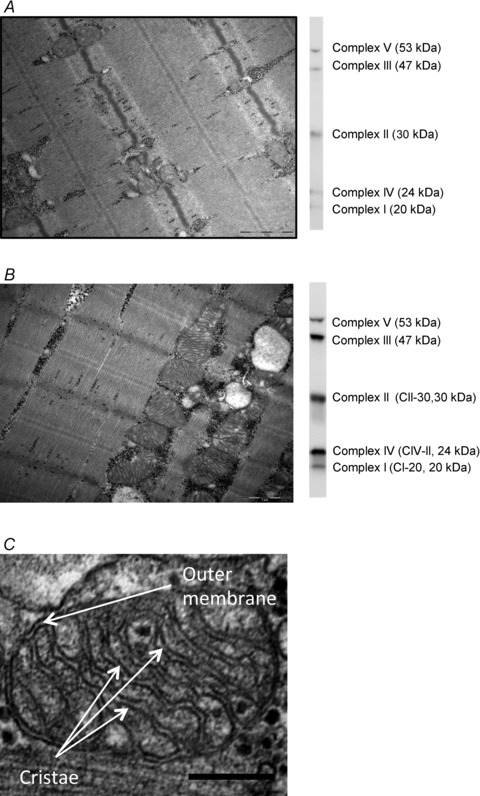
Images and blots are from the two subjects with the lowest mitochondrial content (A) and second highest mitochondrial content (B). C, representative image used for measuring cristae surface area. A and B are ×20,000 magnification; scale bars are 1 μm. C is ×80,000 magnification and scale bar is 200 nm. Arrows shows examples of outer membrane and cristae.
IMF and SS mitochondria cristae surface areas were estimated by intersection and point counting (Weibel, 1980; Gundersen et al. 1988) in an average of 31 (range: 12–63) mitochondria per biopsy photographed at ×80,000 magnification. One representative image of ×80,000 magnification is presented in Fig. 1C. Samples from three subjects failed quality control. Mitochondrial content and cristae surface area was therefore only determined in thirteen subjects.
CS activity
CS activity was measured as previously described (Andersen et al. 2003), with one minor change: a TissueLyser II (Qiagen, Hilden, Germany) was used to make the homogenate. Enzyme activity was determined per gram wet weight (ww).
Complex I–V protein content and myosin heavy chain distribution
The homogenates for CS analysis were also used for western blotting analysis of complex I–V (Mitoprofile Human Total OXPHOS Human WB Antibody Cocktail; Mitosciences, USA) and quantification of myosin heavy chain (MHC) composition as previously described (Andersen & Aagaard, 2000; Larsen et al. 2011). Two representative blots of complex I–V are presented in Fig. 1A and B.
Activity of electron transport chain complex I–IV
Preparation of muscle tissue homogenates and enzyme activity measures of electron transport chain complex I–IV was carried out as previously described (Wibrand et al. 2010). Enzyme activity was determined at 37°C in a Varian Cary 100 Bio spectrophotometer equipped with a thermostattable multicell holder.
Quantification of genomic and mitochondrial DNA content
Total DNA was precipitated from approximately 15 mg frozen tissue and copy numbers of both genomic and mitochondrial DNA per milligram wet tissue weight were determined as previously described (Kraunsoe et al. 2010).
Quantification of skeletal muscle cardiolipin content
Lipids were extracted from approximately 3 mg freeze-dried muscle tissue (dw) using chloroform/methanol (Bartels et al. 2002). Cardiolipin was quantified with a thin-layer chromatography-based method as previously described (Ruiz & Ochoa, 1997; Bartels et al. 2002; Nielsen et al. 2002).
Statistics
Pearson correlation coefficients were calculated to investigate the correlation between the absolute values. To investigate the association in the relative variation a Lin's concordance test was performed (Lin, 1989). Lin's concordance coefficient (Rc) defines how well the relationship between two variables is represented by a line through the origin at an angle of 45 deg (slope = 1). Lin's scale is between 0 and 1 where 0.21–0.40 shows a fair concordance, 0.41–0.60 a moderate concordance, 0.61–0.80 a substantial concordance and 0.81–1.00 an almost perfect concordance (Lin, 1989). This analysis was performed using relative data (individual values were related to one particular subject values in all measures. This particular subject had the median value in the mitochondrial content measures). Only the biomarkers that were significantly correlated (Pearson correlation coefficient, P < 0.05) to the mitochondrial content, cristae surface area or OXPHOS were included for calculating Lin's Rc. Only the biomarkers that had Rc above 0.61 (‘a substantial concordance’) were considered as valid biomarkers of mitochondrial content or OXPHOS. Comparison of different respiratory states was analysed using a one-way ANOVA with repeated measures. Data are presented as mean ± SEM. Significance was accepted at P < 0.05.
Results
Subject characteristics
In accordance with the predetermined recruitment criteria subjects showed large variation in 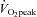 and maximal lipid oxidation, and had normal plasma glucose and insulin concentrations (Table 1). Mitochondrial fractional area ranged from 4 to 15% of the cellular volume (Table 2) and
and maximal lipid oxidation, and had normal plasma glucose and insulin concentrations (Table 1). Mitochondrial fractional area ranged from 4 to 15% of the cellular volume (Table 2) and 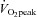 ranged from 29.9 to 71.6 ml min−1 kg−1 (Table 1).
ranged from 29.9 to 71.6 ml min−1 kg−1 (Table 1).
Table 1.
Subject characteristics
| Subjects characteristics (n= 16) | mean ± SEM | Range |
|---|---|---|
| Age (years) | 24.3 ± 0.9 | 20–32.6 |
| BMI | 23.4 ± 0.5 | 19.9–27.5 |
| Fat mass (%) | 14.0 ± 1.7 | 4.2–30.6 |
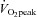 (ml min−1 kg−1) (ml min−1 kg−1) |
50.0 ± 2.4 | 29.9–71.6 |
| Maximal lipid oxidation (g min−1) | 0.34 ± 0.03 | 0.19–0.58 |
| Plasma glucose (mmol l−1) | 5.3 ± 0.1 | 4.6–5.8 |
| Insulin (pmol l−1) | 21.5 ± 2.3 | 11.0–37.7 |
| Cholesterol (mmol l−1) | 3.9 ± 0.2 | 2.0–5.6 |
| NEFA (μmol l−1) | 321 ± 37 | 94–580 |
| HDL (mmol l−1) | 1.3 ± 0.1 | 0.6–1.8 |
| LDL (mmol l−1) | 2.3 ± 0.2 | 1.1–4.0 |
| MHC I (%) | 49.9 ± 4.0 | 22.8–78.7 |
| MHC IIa (%) | 43.0 ± 2.8 | 21.3–62.3 |
| MHC IIx (%) | 7.2 ± 1.8 | 0–20.3 |
BMI: body mass index. 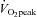 : maximal oxygen uptake per kg body weight. NEFA: plasma non-esterified free fatty acids. HDL: plasma high density lipoproteins. LDL: plasma low density lipoproteins. MHC: myosin heavy chain isotype I, IIa and IIx.
: maximal oxygen uptake per kg body weight. NEFA: plasma non-esterified free fatty acids. HDL: plasma high density lipoproteins. LDL: plasma low density lipoproteins. MHC: myosin heavy chain isotype I, IIa and IIx.
Table 2.
Correlation matrix showing the Pearson correlation coefficient (r) and the related P value
| Mitochondrial content | Total cristae area | GMS3 | PGMSOct3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | r | P | Rc | r | P | Rc | r | P | Rc | r | P | Rc | |
| Mitochondrial fractional area (μm2μm−2) | 0.04–0.15 | 0.93 | <0.001 | 0.91 | 0.81 | 0.001 | 0.58 | 0.65 | 0.03 | 0.56 | |||
| Total cristae surface area (m2 cm−2) | 1.3–6.1 | 0.75 | 0.003 | 0.49 | 0.66 | 0.03 | 0.50 | ||||||
| GMS3 (pmol s−1 mg−1) | 51–115 | 0.58 | 0.03 | 0.42 | |||||||||
| PGMSOct3 (pmol s−1 mg−1) | 46–93 | ||||||||||||
| Cardiolipin (μg (mg dw)−1) | 1.8–7.1 | 0.86 | <0.001 | 0.85 | 0.73 | 0.005 | 0.67 | 0.70 | 0.003 | 0.58 | 0.50 | 0.07 | |
| CS (μmol g−1 min−1) | 76–233 | 0.84 | <0.001 | 0.80 | 0.72 | 0.005 | 0.64 | 0.66 | 0.006 | 0.58 | 0.52 | 0.055 | |
| Complex I activity (U (g ww)−1) | 5.6–18.2 | 0.78 | 0.002 | 0.77 | 0.76 | 0.003 | 0.71 | 0.52 | 0.048 | 0.41 | 0.34 | 0.25 | |
| Complex II protein content (AU) | 0.59–2.60 | 0.73 | 0.005 | 0.72 | 0.72 | 0.006 | 0.71 | 0.48 | 0.06 | 0.55 | 0.04 | 0.49 | |
| Complex IV activity (U (g ww)−1) | 48–176 | 0.79 | 0.001 | 0.71 | 0.75 | 0.003 | 0.64 | 0.69 | 0.004 | 0.64 | 0.34 | 0.25 | |
| Complex V protein content (AU) | 0.40–2.24 | 0.74 | 0.004 | 0.69 | 0.75 | 0.003 | 0.70 | 0.65 | 0.006 | 0.54 | 0.59 | 0.03 | 0.48 |
| Complex II activity (U (g ww)−1) | 4.8–17.2 | 0.73 | 0.004 | 0.67 | 0.63 | 0.02 | 0.54 | 0.68 | 0.005 | 0.63 | 0.33 | 0.27 | |
| Complex III activity (U (g ww)−1) | 16–70 | 0.70 | 0.01 | 0.60 | 0.62 | 0.02 | 0.52 | 0.64 | 0.01 | 0.59 | 0.39 | 0.19 | |
| SCR act. (U (g ww)−1) | 6.8–25.9 | 0.69 | 0.01 | 0.60 | 0.59 | 0.03 | 0.56 | 0.55 | 0.03 | 0.43 | 0.30 | 0.31 | |
| Complex III protein content (AU) | 0.07–2.07 | 0.61 | 0.03 | 0.47 | 0.59 | 0.03 | 0.48 | 0.56 | 0.02 | 0.39 | 0.44 | 0.11 | |
| Complex IV protein content (AU) | 0.09–1.63 | 0.55 | 0.05 | 0.44 | 0.62 | 0.03 | 0.50 | 0.53 | 0.03 | 0.40 | 0.38 | 0.18 | |
| mtDNA (copies (mg ww)−1) | 8.32 × 107– | 0.35 | 0.23 | 0.31 | 0.30 | 0.46 | 0.07 | 0.50 | 0.07 | ||||
| 34.6 × 107 | |||||||||||||
| Complex I protein content (AU) | 0.1–1.24 | 0.19 | 0.54 | 0.30 | 0.32 | 0.22 | 0.42 | 0.27 | 0.36 | ||||
If the association was significantly correlated (P < 0.05) the Lin's concordance coefficient (Rc) is also shown. Correlations are between total mitochondrial volume, total cristae area, ADP-stimulated fibre respiration using glutamate + malate + succinate as substrate (GMS3) or ADP-stimulated fibre respiration using pyruvate + glutamate + malate + succinate + octanoyl-l-carnitine (PGMSOct3), and the potential biomarkers of mitochondrial volume. The Lin's scale is defined as follows: 0.21–0.40 shows a fair concordance, 0.41–0.60 a moderate concordance, 0.61–0.80 a substantial concordance and 0.81–1.00 an almost perfect concordance. AU, arbitrary units; ww, wet weight; dw, dry weight; SCR act., succinate-cytochrome c reductase activity.
Respiration in permeabilized fibres
The different respiratory rates can be seen in Fig. 2. As expected, respiration activated with TMPD + Asc (direct electron donor to complex IV) showed the highest respiratory rate followed by uncoupled respiration using both reduced form of nicotinamide-adenine dinucleotide (NADH)- and the reduced form of flavin adenine dinucleotide (FADH2)-generating substrates (GMS3u). There was no statistical difference between GMS3, PGMS3 and PGMSOct3, suggesting that GMS provides sufficient NADH and FADH2 to reach maximal coupled respiration. The integrity of the outer mitochondrial membrane was assessed by addition of cytochrome c in protocols 1–3 and 5. No effect of this addition was seen (data not shown).
Figure 2. Fibre respiration.
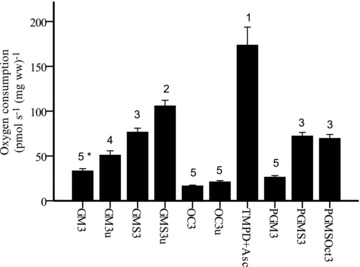
Oxygen consumption (pmol s−1 (mg ww)−1) in permeabilized fibres. GM3: glutamate + malate + ADP-Mg; GM3u: glutamate + malate + ADP-Mg + FCCP; GMS3: glutamate + malate + ADP-Mg + succinate; GMS3u: glutamate + malate + ADP-Mg + succinate + FCCP; Oct3: l-malate + octanoyl-l-carnitine + ADP-Mg; Oct3u: malate + octanoyl-l-carnitine + ADP-Mg + FCCP; TMPD: ADP-Mg + cytochrome c+ antimycin A + ascorbate +N,N,N′,N′-tetramethyl-1,4-benzenediamine dihydrochloride; PGM3: pyruvate + malate + glutamate + ADP-Mg; PGMS3: pyruvate + glutamate + malate + ADP-Mg + succinate; PGMSOct3: pyruvate + glutamate + malate + ADP-Mg + succinate + octanoyl-l-carnitine. Numbers denote the statistical rank using one-way ANOVA with repeated measures, i.e. values denoted with the number 3 are significantly lower than values denoted with the number 2 and significantly higher than the values denoted with the number 4. *P= 0.06 compared with GM3u. Values are mean ± SEM.
Correlation between the biomarkers and the mitochondrial fractional area
Several biomarkers showed a strong correlation and high concordance with mitochondrial fractional area. The correlation coefficients, P values and Lin concordance coefficients can be seen in Table 2 where the biomarkers are listed according to their Rc rank to the mitochondrial fractional area. Cardiolipin content had the strongest correlation and highest Rc value of 0.85 defined as an ‘almost perfect concordance’ (Lin, 1989; Fig. 3A). CS activity showed the second highest correlation and concordance (Fig. 3B). CS activity, complex I, II and IV activity and complex II and V protein content all had Rc values defined as a ‘substantial concordance’. Interestingly there was no correlation between mtDNA content and mitochondrial fractional area (r= 0.35, P= n.s.) (Fig. 3G). In contrast there was a very strong correlation between fibre respiration using TMPD + Asc and mitochondrial fractional area (r= 0.94, P < 0.001) (Fig. 3H). The Rc was 0.74 which is defined as a ‘substantial concordance’ (Rc= 0.74).
Figure 3. Correlation plots.
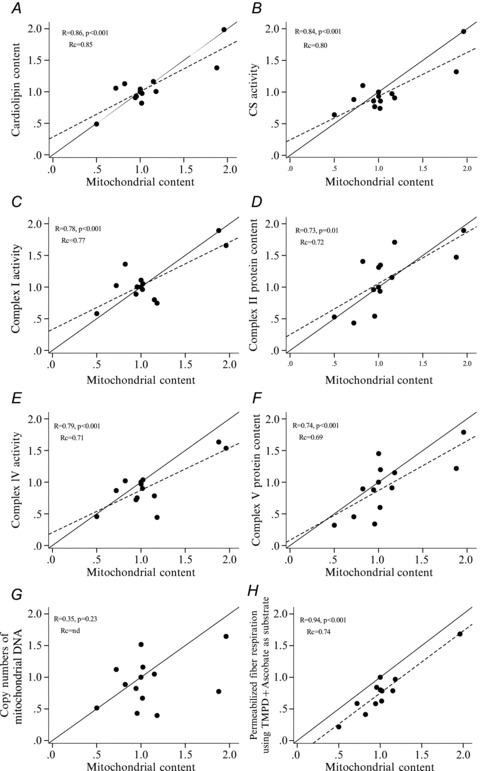
Correlation between the relative variation of the mitochondrial content and the six biomarkers (A–F) of mitochondrial content that showed the highest Lin's concordance coefficient (Rc) and two biomarkers of certain interest (G and H). TMPD + ascorbate are redox substrates feeding electrons directly to complex IV. Continuous lines represent the perfect linear fit (slope = 1) and dashed lines represent the actual linear fit. The linear fit was only shown when significance was present.
Correlation between the biomarkers and mitochondrial cristae surface area
There was a strong correlation between cristae surface area and mitochondrial fractional area (r= 0.93, P < 0.001). The Lin's concordance test showed an almost perfect fit (Rc= 0.91) suggesting that the relative variation in these two values, in this group of subjects, is closely associated.
The cristae surface area was significantly correlated with the same biomarkers as the mitochondrial content (Table 2). The biomarker that showed the strongest concordance with cristae surface area was complex I activity (Rc= 0.71) and complex II protein content (Rc= 0.71), followed by complex V protein content (Rc= 0.70), cardiolipin content (Rc= 0.67), CS activity (Rc= 0.67) and complex IV activity (Rc= 0.64). As can be seen in Table 2, the concordance was generally lower between the biomarkers and the cristae surface area when compared with mitochondrial surface area.
Correlation between the biomarkers and mitochondrial respiratory capacity
In order to investigate biomarkers of muscle OXPHOS, the highest coupled respiratory state (GMS3) and the respiratory rate with the maximal substrate supply (PGMSOct3) were chosen as measures of muscle OXPHOS (Table 2). GMS3 had a strong correlation with mitochondrial fractional area (r= 0.81). The correlation between PGMSOct3 and mitochondrial fractional area was also significant (r= 0.65) but not as strong as with GMS3. GMS3 was significantly correlated to several biomarkers showing the highest concordance to complex IV activity (Rc= 0.64), and complex II activity (Rc= 0.63) both defined as a ‘substantial concordance’. Only complex II protein content (Rc= 0.49) and complex V protein content (Rc= 0.48) were significantly correlated to PGMSOct3, both defined as having a ‘moderate concordance’.
Discussion
The main finding of the present study was that several biomarkers showed a strong correlation and high concordance with the mitochondrial fractional area. The biomarker with the strongest correlation and highest relative concordance was cardiolipin content, followed by CS and complex I activities. The cristae surface area was strongly correlated and showed a strong concordance with mitochondrial fractional area, suggesting that cristae surface area per mitochondrial fractional area does not vary much in skeletal muscle between subjects. Finally, we found that complex IV activity was the biomarker that had the highest concordance with muscle OXPHOS determined as state 3 respiration using glutamate, malate and succinate as substrates, followed by complex II activity, cardiolipin content and CS activity.
Mitochondrial fractional area and cristae surface area
In the present study we determined mitochondrial fractional area and cristae surface area using two-dimensional TEM as representative measures of mitochondrial content. We reasoned that cristae surface area may be a more robust measure of mitochondrial content than mitochondrial fractional area since many of the proteins and enzymes involved in substrate oxidation, electron transport and phosphorylation are attached to or imbedded in the inner mitochondrial membrane. Furthermore, the measure of cristae area is not affected by mitochondrial shrinking or swelling. In accordance with a previous study using cat skeletal muscle (Hoppeler et al. 1987), we found a very strong correlation and concordance between mitochondrial fractional area and cristae surface area (Table 2). However, in contrast to our hypothesis we found that the biomarkers and respiratory measures showed a stronger correlation and higher concordances with mitochondrial fractional area than cristae surface area (Table 2). This could be due to the fact that the estimate of mitochondrial fractional area is based on a greater diversity of mitochondrial morphologies and therefore more representative of the muscle biopsy compared to the estimate of total cristae surface area. Indeed, cristae surface area was only estimated in a subset of mitochondria (12–63 of several hundreds of mitochondrial profiles) of which the cristae could be clearly identified.
Markers of mitochondrial content
In the present study we have shown that several biochemical measures have a strong correlation and high concordance with mitochondrial fractional area (mitochondrial content). This includes cardiolipin content (Rc= 0.85), CS activity (Rc= 0.80), complex I activity (Rc= 0.77), complex II protein content (Rc= 0.72), complex IV activity (Rc= 0.71), complex V protein content (Rc= 0.69) and complex II activity (Rc= 0.67). These results validate the use of these biochemical measures as markers of mitochondrial content. However, depending on the experimental setup, the use of some markers may have advantages over others. If the purpose of the study is to use the biomarker as a denominator for calculating intrinsic mitochondrial functionality (i.e. respiration per mitochondrial content) it seems more appropriate to use non-enzymatic markers that are not involved in mitochondrial substrate oxidation and phosphorylation. In the present study two such measures were determined, cardiolipin and mtDNA content. Cardiolipin, which is a phospholipid located in the inner mitochondrial membrane, is not directly involved in substrate oxidation, electron transport or phosphorylation. However, it has been suggested to affect inner membrane proton leak (Hoch, 1998) and stabilizing the complexes in the electron transport chain (Pfeiffer et al. 2003). Previous studies have shown that cardiolipin content increases in human vastus lateralis after exercise training along with an increase in other markers of mitochondrial content (Menshikova et al. 2005, 2007). Thus, the present and previous studies show that cardiolipin is a very appropriate and valid biomarker of mitochondrial content.
In contrast, mtDNA content was not significantly correlated with mitochondrial content (r= 0.35, P= 0.23). This result was somewhat unexpected since mtDNA is used in various studies as a marker of mitochondrial content. Moreover, studies have shown a significant positive correlation between CS activity and mtDNA content in human vastus lateralis (Wang et al. 1999) and an increase in mtDNA content in response to training (Puntschart et al. 1995; Menshikova et al. 2006). However, studies have also shown that training does not increase mtDNA content in human (Menshikova et al. 2005; Pesta et al. 2011) and rodent skeletal muscle (Schultz & Wiesner, 2000) despite an increase in other markers of mitochondrial content. The inconsistency of these results is most likely due to the relatively low power used in these studies and the fact that mitochondrial DNA copy number can vary considerably (4–6 copies per cell) between individuals. Nevertheless, this further supports our finding that mtDNA content is a poor biomarker of mitochondrial content in skeletal muscle.
CS activity is a frequently used biomarker of mitochondrial content. Previous studies have validated its use as a marker of mitochondrial content showing a close association between the change in its activity and morphological change in mitochondrial content in response to electrically stimulated training in rabbit tibialis anterior muscle (Reichmann et al. 1985). Even though our data suggest that cardiolipin had a stronger correlation (cardiolipin content r = 0.86 vs. CS activity r = 0.84) and higher relative concordance (cardiolipin content Rc= 0.85 vs. CS activity Rc= 0.80) with mitochondrial content, taking into account the relatively low number of subjects and the variability in the measures, the small difference between these two biomarkers is not enough to claim that cardiolipin is superior to CS activity as a biomarker of mitochondrial content. However, if cardiolipin content and CS activity are to be used as a nominator for calculating intrinsic mitochondrial functionality, the use of cardiolipin content has several advantages over CS activity. In contrast to cardiolipin, CS activity can be acutely regulated by exercise (Tonkonogi et al. 1997). Furthermore, CS activity may contribute to the oxidation of substrates in some respiratory protocols. The advantage, however, is that CS activity is easy to measure and it can be determined in both whole tissue, in isolated mitochondrial-rich solution, and in the same solution that has been used for measuring mitochondrial respiration (Mogensen et al. 2006b).
An interesting finding was that there was a very strong correlation between mitochondrial content and the respiratory rate in permeabilized fibres when using TMPD + ascorbate as substrate (r= 0.94, P < 0.001, Fig. 3H). The advantage of using TMPD + ascorbate respiration in permeabilized fibres as a marker of mitochondrial content is that it can be performed on the same day as the other respiratory measures, avoiding the potential effects of long-term storage of the muscle sample (Pache & Reichmann, 1990). But most importantly, unless inhibitors of COX activity have been used, TMPD + ascorbate respiration can always be determined as the final step in a respiratory protocol. In this way, the functional measure and the measure of the mitochondrial content can be determined in the same tissue preparation. However, the disadvantage is that regardless of which protocol is being used, complex IV is always part of the mitochondrial substrate oxidation. Furthermore, when using TMPD + ascorbate as substrate the respiratory rate does not reach a steady state but rather it displays a spiked response, which can be difficult to interpret.
Biomarkers of mitochondrial oxidative capacity
A secondary purpose of the present study was to investigate which biochemical measure could be used as a biomarker of muscle OXPHOS. Muscle OXPHOS was defined as the maximal coupled respiration in permeabilized fibres. This was accomplished when using glutamate, malate and succinate as substrates. Several biochemical measures showed a strong correlation with GMS3 but only complex IV activity (Rc= 0.64) and complex II activity (Rc= 0.63) had Rc values above 0.61 (Table 2). From our point of view complex IV activity is the most appropriate marker of muscle OXPHOS capacity, since the correlation between GMS3 and complex II activity could be due to the experimental conditions where succinate exerts a high control over the GMS3 respiration and is the substrate for the complex II protein. Furthermore, in contrast to complex II activity, complex IV activity was also significantly correlated with the respiratory states where succinate was not used as substrate (GM3 r = 0.53, P= 0.04; Oct3 r= 0.54, P= 0.04).
Conclusion
The purpose of the present study was to investigate which of the most commonly used markers of mitochondrial content had the strongest correlation with a morphological determination of the actual mitochondrial content in skeletal muscle of healthy young human subjects. We conclude the following: (1) Cardiolipin has the strongest association with the mitochondrial content and is the most valid biomarker for investigating changes in mitochondrial content and for calculating intrinsic mitochondrial functionality. (2) CS activity is also strongly associated with the mitochondrial content. The advantage of CS activity is that it the most commonly used marker of mitochondrial content and it is easy to measure. The disadvantage is that in some functional measures the CS enzyme is involved in mitochondrial substrate oxidation. (3) mtDNA was not associated with the mitochondrial content and is therefore regarded as a poor biomarker of mitochondrial content. (4) Complex II activity and complex IV activity were strongly associated with the muscle OXPHOS determined as the maximal coupled respiratory capacity in permeabilized fibres. Complex IV activity is the most valid biomarker of muscle OXPHOS due to its consistent correlation with many of the respiratory measures in diverse protocols.
Acknowledgments
The authors wish to express their gratitude to Regitze Kraunsøe, Jeppe Bach, Anne Sylvest Olsen, Kirsten Hansen, Karin Trampedach and Karen Rasmussen for excellent technical assistance. Rasmus Rabøl is thanked for helping with the ethical approval. Jesper L. Andersen is thanked for conducting the fibre type analysis. The work was supported by grants from the Nordea Foundation and the Danish Medical Research Council. None of the authors have any conflicts of interest to disclose.
Glossary
- CS
citrate synthase
- dw
dry weight
- IMF
intramyofibrillar
- MHC
myocin heavy chain
- mtDNA
mitochondrial DNA
- OXPHOS
oxidative capacity
- SS
subsarcolemmal
- TEM
transmission electron microscopy
- ww
wet weight
Author contributions
The sample collection, whole body analysis, respiratory analysis in permeabilized fibres, and western blots were performed at the Center for Healthy Aging–Department of Biomedical Science, Copenhagen University, Denmark. Measures of cardiolipin content were performed in the Department of Clinical Biochemistry, Copenhagen University Hospital, Denmark. Measures of complex I–IV activity were performed at the Metabolic Laboratory, Department of Clinical Genetics, Copenhagen University Hospital, Denmark. Measures and analysis of transmission electron microscopy imaging were performed at the Department of Clinical Pathology, Odense University Hospital, Denmark and Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Denmark. M.H.-M. contributed to the design of the experiments, the data collection, analysis and interpretation of the data, and article composition. S.L., N.S., F.D. and J.N., contributed to the design of the experiments, collection of data, interpretation of data and manuscript revision. R.B., J.W.H. and H.D.S. contributed to the design of the study, interpretation of data and manuscript revision. C.N.H., L.B.N. and F.W. contributed to the data collection, analysis and manuscript revision for important intellectual content. All authors approved the final version of this manuscript.
References
- Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Schjerling P, Andersen LL, Dela F. Resistance training and insulin action in humans: effects of de-training. J Physiol. 2003;551:1049–1058. doi: 10.1113/jphysiol.2003.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Boyle KE, Houmard JA, Neufer PD. Obesity is associated with reduced glutathione content, increased mitochondrial H2O2 emitting potential and a more oxidized redox environment in human skeletal muscle. Diabetes. 2008;57:A430–A430. [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara I, Larsen S, Stallknecht B, Guerra B, Morales-Alamo D, Andersen JL, Ponce-González JG, Guadalupe-Grau A, Galbo H, Calbet JAL, Helge JW. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int J Obes (Lond) 2011;35:99–108. doi: 10.1038/ijo.2010.123. [DOI] [PubMed] [Google Scholar]
- Bartels ED, Lauritsen M, Nielsen LB. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes. 2002;51:1233–1239. doi: 10.2337/diabetes.51.4.1233. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernström M, Bakkman L, Tonkonogi M, Shabalina IG, Rozhdestvenskaya Z, Mattsson CM, Enqvist JK, Ekblom B, Sahlin K. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol. 2007;102:1844–1849. doi: 10.1152/japplphysiol.01173.2006. [DOI] [PubMed] [Google Scholar]
- Fernström M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hey-Mogensen M, Hojlund K, Vind BF, Wang L, Dela F, Beck-Nielsen H, Fernström M, Sahlin K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia. 2010;53:1976–1985. doi: 10.1007/s00125-010-1813-x. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and mitochondrial proton-selective leakage. J Bioenerg Biomembr. 1998;30:511–532. doi: 10.1023/a:1020576315771. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol. 1987;385:661–675. doi: 10.1113/jphysiol.1987.sp016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraunsoe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Stockel M, Mikines KJ, Dela F. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol. 2010;588:2023–2032. doi: 10.1113/jphysiol.2009.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz WS, Kuznetsov AV, Schulze W, Eichhorn K, Schild L, Striggow F, Bohnensack R, Neuhof S, Grasshoff H, Neumann HW, Gellerich FN. Functional characterization of mitochondrial oxidative phosphorylation in saponin-skinned human muscle fibers. Biochim Biophys Acta. 1993;1144:46–53. doi: 10.1016/0005-2728(93)90029-f. [DOI] [PubMed] [Google Scholar]
- Larsen S, Ara I, Rabol R, Andersen JL, Boushel R, Dela F, Helge JW. Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia. 2009;52:1400–1408. doi: 10.1007/s00125-009-1353-4. [DOI] [PubMed] [Google Scholar]
- Larsen S, Hey-Mogensen M, Rabøl R, Stride N, Helge JW, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol (Oxf) 2012 doi: 10.1111/j.1748-1716.2012.02408.x. DOI: 10.1111/j.1748-1716.2012.02408.x. [DOI] [PubMed] [Google Scholar]
- Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Andersen JL, Madsbad S, Worm D, Helge JW, Dela F. Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia. 2011;54:1427–1436. doi: 10.1007/s00125-011-2098-4. [DOI] [PubMed] [Google Scholar]
- Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol. 2007;103:21–27. doi: 10.1152/japplphysiol.01228.2006. [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Toledo FGS, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:E818–E825. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- Mogensen M, Bagger M, Pedersen PK, Fernström M, Sahlin K. Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol. 2006a;571:669–681. doi: 10.1113/jphysiol.2005.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M, Sahlin K, Femström M, Glintborg D, Beek-Nielsen H, Hojlund K. Mitochondrial respiratory function is decreased in skeletal muscles of patients with type 2 diabetes. Diabetologia. 2006b;49:121–122. [Google Scholar]
- Molnar AM, Servais S, Guichardant M, Lagarde M, Macedo DV, Pereira-Da-Silva L, Sibille B, Favier R. Mitochondrial H2O2 production is reduced with acute and chronic eccentric exercise in rat skeletal muscle. Antioxid Redox Signal. 2006;8:548–558. doi: 10.1089/ars.2006.8.548. [DOI] [PubMed] [Google Scholar]
- Naimi AI, Bourbeau J, Perrault H, Baril J, Wright-Paradis C, Rossi A, Taivassalo T, Sheel AW, Rabol R, Dela F, Boushel R. Altered mitochondrial regulation in quadriceps muscles of patients with COPD. Clin Physiol Funct Imaging. 2011;31:124–131. doi: 10.1111/j.1475-097X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Mogensen M, Vind BF, Sahlin K, Hojlund K, Schroder HD, Ortenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E706–E713. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem. 2002;277:27014–27020. doi: 10.1074/jbc.M203458200. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pache T, Reichmann H. On the stability of key enzymes of energy metabolism in muscle biopsies. Enzyme. 1990;43:183–187. doi: 10.1159/000468729. [DOI] [PubMed] [Google Scholar]
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1078–R1087. doi: 10.1152/ajpregu.00285.2011. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard MP, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R. Messenger RNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol Cell Physiol. 1995;38:C619–C625. doi: 10.1152/ajpcell.1995.269.3.C619. [DOI] [PubMed] [Google Scholar]
- Rabøl R, Højberg PMV, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. Improved glycaemic control decreases inner mitochondrial membrane leak in type 2 diabetes. Diabetes Obes Metab. 2009a;11:355–360. doi: 10.1111/j.1463-1326.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- Rabol R, Larsen S, Hojberg PM, Almdal T, Boushel R, Haugaard SB, Andersen JL, Madsbad S, Dela F. Regional anatomic differences in skeletal muscle mitochondrial respiration in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2010;95:857–863. doi: 10.1210/jc.2009-1844. [DOI] [PubMed] [Google Scholar]
- Rabol R, Svendsen PF, Skovbro M, Boushel R, Haugaard SB, Schjerling P, Schrauwen P, Hesselink MK, Nilas L, Madsbad S, Dela F. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism. 2009b;58:1145–1152. doi: 10.1016/j.metabol.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Reichmann H, Hoppeler H, Mathieucostello O, Vonbergen F, Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch. 1985;404:1–9. doi: 10.1007/BF00581484. [DOI] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, Kelley DE. Analysis of cardiolipin in human muscle biopsy. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:63–71. doi: 10.1016/j.jchromb.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Ruiz JI, Ochoa B. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J Lipid Res. 1997;38:1482–1489. [PubMed] [Google Scholar]
- Schultz J, Wiesner RJ. Proliferation of mitochondria in chronically stimulated rabbit skeletal muscle – Transcription of mitochondrial genes and copy number of mitochondrial DNA. J Bioenerg Biomembr. 2000;32:627–634. doi: 10.1023/a:1005630813227. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Harris B, Sahlin K. Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand. 1997;161:435–436. doi: 10.1046/j.1365-201X.1997.00233.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hiatt WR, Barstow TJ, Brass EP. Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur J Appl Physiol Occup Physiol. 1999;80:22–27. doi: 10.1007/s004210050553. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods. London: Academic Press; 1980. [Google Scholar]
- Wibrand F, Jeppesen TD, Frederiksen AL, Olsen DB, Duno M, Schwartz M, Vissing J. Limited diagnostic value of enzyme analysis in patients with mitochondrial tRNA mutations. Muscle Nerve. 2010;41:607–613. doi: 10.1002/mus.21541. [DOI] [PubMed] [Google Scholar]


