Abstract
Mouse embryonic stem cells (ESCs) and induced pluripotent stem (iPS) cells can be used as models of neuronal differentiation for the investigation of mammalian neurogenesis, pharmacological testing, and development of cell-based therapies. Recently, mouse iPS cell lines have been generated by Sleeping Beauty (SB) transposon-mediated transgenesis (SB-iPS). In this study, we determined for the first time the differentiation potential of mouse SB-iPS cells to form neuronal progenitor cells (NPCs) and neurons. Undifferentiated SB-iPS and ES cells were aggregated into embryoid bodies (EBs) and cultured in neuronal differentiation medium supplemented with 5 μM all-trans retinoic acid. Thereafter, EBs were dissociated and plated to observe further neuronal differentiation. Samples were fixed on days 10 and 14 for immunocytochemistry staining using the NPC markers Pax6 and Nestin and the neuron marker βIII-tubulin/Tuj1. Nestin-labeled cells were analyzed further by flow cytometry. Our results demonstrated that SB-iPS cells can generate NPCs and differentiate further into neurons in culture, although SB-iPS cells produced less nestin-positive cells than ESCs (6.12±1.61 vs. 74.36±1.65, respectively). In conclusion, the efficiency of generating SB-iPS cells–derived NPCs needs to be improved. However, given the considerable potential of SB-iPS cells for drug testing and as therapeutic models in neurological disorders, continuing investigation of their neuronal differentiation ability is required.
Introduction
Pluripotent stem cell research holds great promise for revolutionizing the future of medicine, especially for the regeneration of damaged and diseased organs. Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass of preimplantation blastocyst-stage embryos that can differentiate in vivo and in vitro into all cell types of an adult animal (Evans and Kaufman, 1981). Mouse ESCs are used as model systems for neurological diseases and investigation of mammalian neurogenesis. In particular, they can be used in the generation of neurons for pharmacological testing and development of models for cell therapy applications, which may help to overcome incurable neurological diseases, such as stroke, spinal cord injuries, Alzheimer's disease, and Parkinson's disease (Langston, 2005; Taupin, 2006).
Recently, a novel alternative method has been developed to establish induced pluripotent stem (iPS) cells. Mouse and human iPS cells have been directly reprogrammed from adult cells (e.g., fibroblasts) by the introduction of pluripotency transcription, initially by Oct3/4, Sox2, c-Myc, and Klf4, known as Yamanaka factors (Takahashi and Yamanaka, 2006), or complementing/replacing them partially with other factors, such as Nanog and Lin28 (Liao et al., 2008). These iPS cells resemble ESCs with respect to morphology, proliferation, gene expression, teratoma formation, their ability to differentiate into all three germ layers, and, in the case of mouse iPS cells, to also form mouse chimeras (Maherali et al., 2007; Takahashi and Yamanaka et al., 2006). The iPS cells might also be useful for patient- and disease-specific cell transplantation through their differentiation potential into several different cell lineages, including cardiac cells (Zwi et al., 2009), hepatic cells (Iwamaru et al., 2010), hematopoietic cells (Tolar et al., 2011), and neurons (Hu et al., 2010).
To date, iPS cells have been established by several methods, such as viral transduction (Takahashi et al., 2007), recombinant cell-penetrating proteins (Zhou et al., 2009), administration of synthetic modified mRNA (Warren et al., 2010), and recently by transposon-transposase–mediated transgenics using the piggyBac transposon (Kaji et al., 2009; Nagy et al., 2011; Woltjen et al., 2009). Transposons are sequences of DNA that have the capability to change their positions within the genome by use of a transposition mechanism. Besides PiggyBack, another transposon, Sleeping Beauty (SB), was engineered by the molecular reconstruction of the inactive Tc1/mariner element found in the salmonid fish genome (Ivics et al., 1997). This transposon has been used as a powerful tool to introduce genes into various cell types (Essner et al., 2005; Izsvák et al., 2009). Very recently, SB has been reported by us to be a suitable tool for mouse iPS cell line generation (Muenthaisong et al., 2012) and as offering an alternative method for the efficient generation of iPS cells.
Neuroepithelial (NEP) cells are multipotent cells in the neural tube that have the capability to self-renew and give rise to neurons in the central nervous system (CNS) and in the peripheral nervous system (PNS), such as glial cells and ependymal cells (Pevny and Rao, 2003). Differentiation of NEP cells occurs via the generation of two major types of progenitor cells, including neuroblasts or neuronal progenitor cells (NPCs) that can generate into multiple kinds of neurons (Kalyani et al., 1998; Mayer-Proschel et al., 1997). The developmental conversion of the undifferentiated inner cell mass in the early embryo into committed neurons has been partially emulated by in vitro differentiation of ESCs (Okabe et al., 1996). It has been reported that ESCs are able to form neurons, astrocytes, and oligodendrocytes (Bain et al., 1995). Early development and neuronal differentiation of mouse ESCs has been extensively studied in vitro. For the initial steps of neuronal induction, most strategies include use of aggregates of a few hundred stem cells, so called embryoid bodies (EBs). Although EBs consist of several cell types of many lineages, it has been shown that supplementation with retinoic acid can induce the formation of a relatively uniform glutamatergic neuronal population (Bibel et al., 2007).
Retinoic acid (RA) is a biologically active form of retinol (vitamin A) and has been demonstrated to have a significant role during embryogenesis and CNS development (Maden, 2001; Ross et al., 2000). RA first appears in the mouse embryo at the mid-primitive streak to the late allantoic bud stage (E7.5) (Ulven et al., 2000). In vitro, RA applied to mouse ESCs induced concentration- and time-dependent differentiation toward neuronal, cardiac, myogenic, adipogenic, and vascular smooth muscle cell types (Rohwedel et al., 1999). Furthermore, RA has been considered to be an important inductive signal for neuronal differentiation of mouse ES cells in vitro (Lu et al., 2009). Induction of neuronal differentiation has been achieved by the application of RA (10−6 to 10−7 M) at early stages of development (Fraichard et al., 1995; Strübing et al., 1995). Recently, a study has reported that the addition of RA, when used at the concentration of 5 μM added to 4-day-old EBs in suspension for a further 4 days, induced mouse ESCs to form high yields of NPCs that exhibit the characteristics of Pax6-positive radial glial cells (Bibel et al., 2004).
In this study, our goal was to demonstrate the differentiation potential of iPS cells to form NPCs using a mouse model. We compared the differentiation capacity of mouse ESCs and SB-iPS cells into NPCs and neurons via EB formation.
Materials and Methods
Materials and cell culture condition
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA), and culture media were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA), unless specified otherwise. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Medium was changed daily for mouse ESC and iPS cell cultures and on every second day during differentiation.
Mouse pluripotent cell cultures
Mouse (129SV/Ola) HM1 ESCs (Selfridge et al., 1992) (kindly provided by Dr. Jim McWhir, Roslin Institute, Roslin, UK) and the mouse B5 iPS cell line derived by Sleeping Beauty-transposition from the C57BL/6 mouse background (Muenthaisong et al., 2012) were cultured on mitomycin C–inactivated mouse embryonic fibroblasts (MEFs) as described (Magin et al., 1992). The pluripotent cells were maintained in ES medium: Dulbecco's modified Eagle's medium (DMEM) containing 15% (vol/vol) fetal bovine serum (FBS; Sera Laboratories International, West Sussex, RH17 5PB, UK), 0.1 mM nonessential amino acids (NEAA), 0.1 mM β-mercaptoethanol (β-ME), 50 U penicillin/mL 50 μg streptomycin/mL, and 1000 U/mL mouse leukemia inhibitory factor (LIF; ESGRO, Chemicon International, Temecula, CA). The cells were passaged prior to reaching 70% confluency (approximately every 1–2 days). ESCs at passage 25 and SB-iPS cells at passage 22 were cultured on gelatin-coated dishes in the presence of LIF (2000 U/mL) in ES medium for at least one passage prior to differentiation to deplete potentially present MEF cells from the system.
In vitro neuronal differentiation
Mouse pluripotent cells were induced to differentiate into the neuronal lineage as previously described, with some modifications (Bibel et al., 2007). Mouse ESCs and iPS cell colonies were harvested into single cells using 0.05% (wt/vol) Trypsin, then seeded at a density of 3×105 cells/mL in differentiation medium (ES medium without LIF) onto bacteriological dishes precoated with poly(2-hydroxyethyl methacrylate) (poly-HEMA) to prevent cell attachment. Pluripotent cells were allowed to aggregate in suspension and form EBs for 4 days. Then 5 μM all-trans RA was then added to the medium and EBs were cultured for a further 4 days. Thereafter, 8-day-old EBs were dissociated and plated onto poly-l-ornithine- and laminin- (Roche, CA, USA) coated dishes at a density of 2×105 cells/cm2 in Dulbecco's modified Eagle medium Nutrient Mixture F-12 (DMEM/F-12) containing 3 mg/mL of d-(+)-glucose, 3 mg/mL AlbuMaxI, 50 U/mL penicillin, 50 μg/mL streptomycin, 1% (vol/vol) N2 supplement, and 10 ng/mL recombinant human basic fibroblast growth factor (bFGF). Two days later, the medium was changed to DMEM/F12:neurobasal medium (1:1), 1 mM glutamax, 3 mg/mL AlbuMaxI, 50 U/mL penicillin, 50 μg/mL streptomycin, 0.5% (vol/vol) N2 Supplement, and 1% (vol/vol) B27 supplement. The medium was renewed every second day until day 14. The cells were harvested for further analyses on days 10 and 14 after the start of the EB formation. The experiments were repeated three times.
Immunocytochemistry
ESCs and SB-iPS cells were prepared for characterization by 2-day culture (until reaching 70% confluency) on gelatin-coated coverslips. Differentiating cells, following dissociation of EBs, were plated onto poly-l-ornithine/laminin–coated coverslips for 2 and 6 days (referred as day 10 and 14, respectively). Cells were washed with phosphate-buffered saline (PBS) and fixed with 4% (vol/vol) paraformaldehyde (PFA) for 15 min at room temperature (RT). Fixed cells were washed and stored in PBS at 4°C until analysis. Permeabilization was performed using 0.2% (vol/vol) Triton X-100 (for intracellular staining) for 30 min at RT. Cells were then blocked with 3% (wt/vol) bovine serum albumin containing 0.5% (vol/vol) Tween 20 in PBS for 30 min. The cells were then incubated sequentially with the following primary antibodies diluted in blocking solution overnight at 4°C: pluripotent marker Oct4 (sc9081, dilution 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA; mouse), Sox2 (SC-20088, dilution 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA; rabbit), Nanog (AF-2729, dilution 1:20, R&D Systems, Minneapolis, MN, USA; mouse), neuroectodermal marker Pax6 (Pax6, dilution 1:200, DSHB; mouse), neuronal progenitor marker Nestin (Rat-401, dilution 1:200, DSHB; mouse), and neuron marker βIII-tubulin (Tuj1, dilution 1:2,000; Covance, PRB-435P; rabbit). The cultures were washed three times with PBS and then incubated with fluorescently labeled secondary antibodies [Alexa Fluor® 488, Alexa Fluor® 594, and Alexa Fluor® 647-labeled goat immunoglobulin G (IgG); dilution 1:2000; Gibco] for 1 h at RT. After three washes with PBS, the cells were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) in Vectashield mounting medium (Vector Laboratory, Temecula, CA, USA). The images were taken on a Zeiss AxioImager fluorescent microscope using Digital Image Processing Software (AxioVision 4.8.1, Carl Zeiss MicroImaging GmbH, Germany).
Flow cytometric analysis
On day10 of the neuronal differentiation process, cells were trypsinized into single cells, washed with PBS, and centrifuged at 1000 rpm for 5 min. Cells were fixed in 4% PFA for 15 min at RT. Following washing with PBS, cells were stained with the primary antibody Nestin (Rat-401, dilution: 1:50, DSHB; mouse) in 0.1% (vol/vol) Triton X-100 in PBS for 1 h at RT. Cells were washed once with 0.05% Tween-20 in PBS, then incubated with Alexa Fluor® 647–labeled secondary antibody (goat IgG; dilution 1:500; Gibco) for 1 h at RT. The cells were washed and resuspended in PBS. Flow cytometry was performed using a Becton-Dickson (Palo Alto, Temecula, CA, USA) FACSCalibur flow cytometer.
Statistical analysis
Data concerning flow cytometry analysis are expressed as mean±standard error of the mean (SEM) and include at least three independent experiments. Statistical analyses for comparison between nestin-positive cells derived from ESCs and iPS cells were conducted using the Student's t-test. A p<0.05 was considered statistically significant.
Results
Immunocytochemistry analysis of pluripotent cells
The SB-iPS cell lines exhibited characteristics typical for pluripotent stem cells, including ESC-like morphology, strong alkaline phosphatase (ALP) positivity, and pluripotency marker gene expression patterns (verified by quantitative real-time PCR) as recently described by us (Muenthaisong et al., 2012). In this study, iPS cells were examined for immunofluorescent staining patterns as shown in Figure 1. The results showed that SB-iPS cell lines expressed the pluripotency markers Oct4, Sox2, and Nanog when cultured in an undifferentiated state.
FIG. 1.
Immunocytochemistry analysis of iPS cell line. Mouse SB-mediated iPS cells were maintained in an undifferentiated stage before neuronal differentiation. The cells were stained with the pluripotent markers Oct4, Sox2, and Nanog. Scale bars, 100 μm.
Characterization of neuronal phenotype
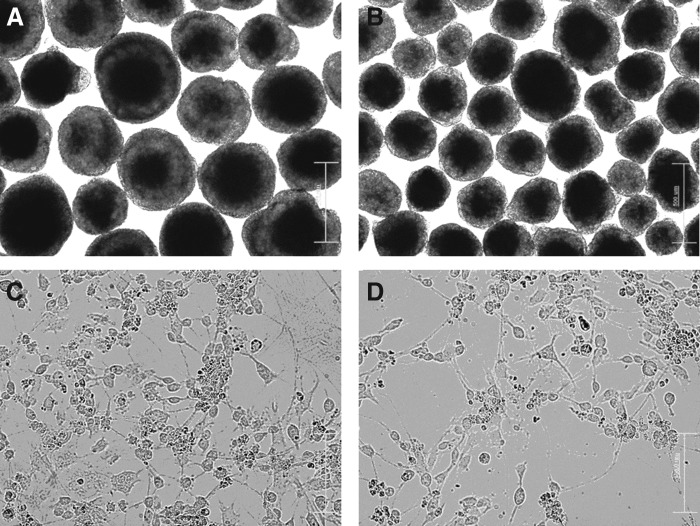
To investigate the ability of neuronal differentiation of mouse ESCs and SB-iPS cells, these pluripotent cells were induced to differentiate into NPCs and neurons through EB formation and supplementation of RA (Rungarunlert et al., 2011). The morphology of 8-day-old EBs derived from ESCs and SB-iPS cells are shown in Figure 2, A and B. We found that the 8-day-old EBs derived from ESCs and SB-iPS cells show the spherical structures with various sizes. SB-iPS cells–derived EBs formed smaller aggregates when compared with those from the ESC line.
FIG. 2.
The morphology of mouse ESCs and iPS cells upon neuronal differentiation. Phase-contrast images demonstrate 8-day-old EBs derived from mouse HM1 ES (A) and SB-mediated iPS cells (B). The cells were induced to differentiate into the neuronal lineage using RA. A neuron-like phenotype, such as neurite processes, can be observed 2 days after EB dissociation in both ESCs (C) and iPS (D) cells. Scale bars, 500 μm (A, B) and 100 μm (C, D).
Two days after plating the cells onto culture dishes (day 10 of differentiation), the cells exhibited a neuron-like appearance with neurite processes organized in a network (Fig. 2C, D).
Immunocytochemistry analysis of differentiating cells
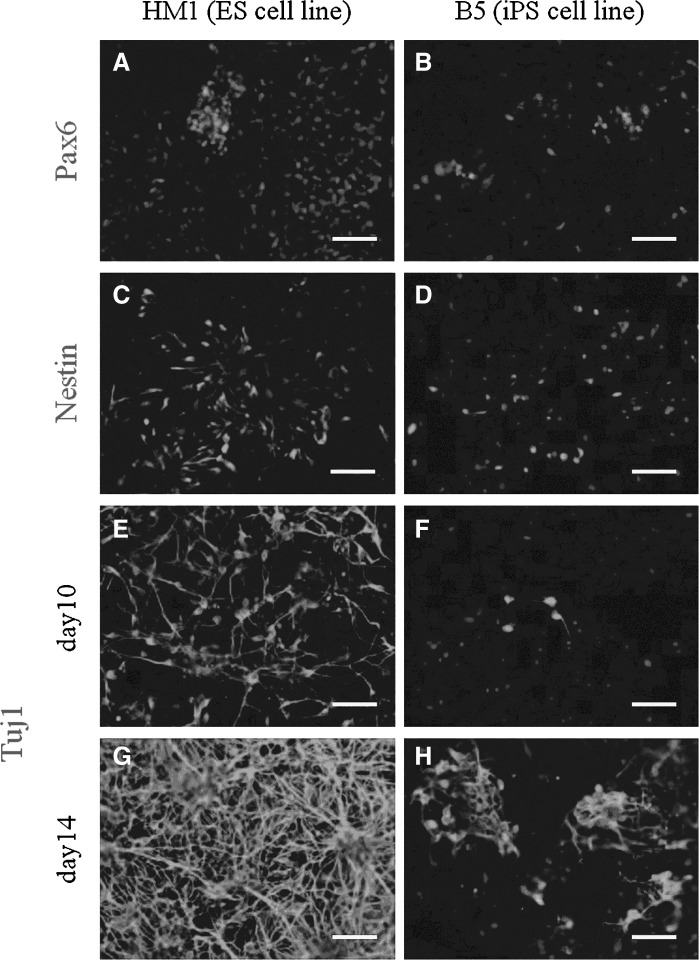
The results of the immunostaining showed that undifferentiated ESCs and SB-iPS cells expressed the pluripotency markers Oct4, Sox2, and Nanog (Fig. 1). After performing the neuronal differentiation procedure (see Materials and Methods), ESCs and SB-iPS cells subsequently expressed neuronal markers as well. Within 2 weeks in culture, ESCs and SB-iPS cells were able to differentiate into cells expressing Pax6, a neuroectodermal marker (Suter et al., 2009) (Fig. 3A, B), and nestin, a specific antibody against the intermediate filament protein of NPCs (Lin et al., 1995) (Fig. 3C, D). In particular, SB-iPS cells started to generate few neurons indicated by the early postmitotic neuronal marker Tuj-1 (Lee and Pixley, 1994) (Fig. 3F). Then the neuronal population was gradually increased by day 14 (Fig. 3H), although approximately 2–3 times lower in number when compared to ESCs (Fig. 3G, H). Our results demonstrated that mouse ESCs and SB-iPS cells have the ability to generate NPCs and differentiate further into neurons through EB formation in culture.
FIG. 3.
Differentiation potential of HM1 ESC and B5 iPS cell lines into neuronal lineage. The neuroectoderm (radial glia) marker Pax6, the neuronal progenitor cell marker Nestin, and the neuronal tubulin marker βIII-tubulin (Tuj1) are expressed in differentiated cells on day 10 (A–F) and day 14 (G–H). Scale bars, 200 μm.
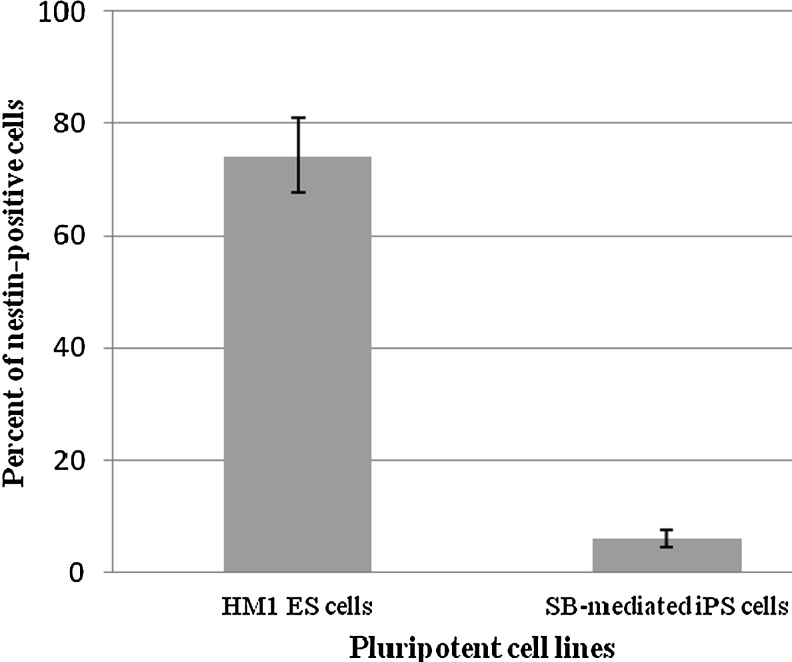
A quantitative analysis was performed by flow cytometry on both ESC- and SB-iPS–derived NPC populations. Two days after EB dissociation and plating, 76.68% of the ESC-derived NPCs were labeled by the nestin-specific antibody, whereas the amount of nestin-positive SB-iPS cells–derived NPCs was only 6.2% (Fig. 4). However, we showed that SB-iPS cells have the potential to differentiate further into neurons, although SB-iPS cells exhibited the unfavorable potential to generate a NPC population.
FIG. 4.
Quantitative analysis of nestin expression in ESC- and iPS-differentiated cells by flow cytometry. Representative histogram of flow-activated cell sorting (FACS) analysis showing the percent of the NPC marker (nestin) expression in differentiated cells derived from SB-mediated iPS cells compared with mouse ESCs following 10 days of neuronal differentiation. Data presented as mean±SEM. The difference was considered significant for p<0.05.
Discussion
We have investigated neuronal differentiation of mouse ESCs and SB-iPS cells using EB formation to initially induce the cells in the presence of RA followed by plating and culture in defined media. Our method allowed SB-iPS cells to aggregate and differentiate in suspension culture and form EBs. EBs recapitulate many aspects of cell differentiation during early mammalian embryogenesis, and the cells can be terminally differentiated into cell types belonging to the three germ layers (Keller, 1995). The lack of structural organization and positional information within EBs during pluripotent cell differentiation results in heterogeneity both within and between EBs. However, a high yield of neuronal population can be generated from ESC-derived EBs by using RA to commit cell fate to the neuronal lineage (Bain et al., 1995).
This is the first report describing the capability of mouse SB-iPS cells, reprogrammed by the Sleeping Beauty transposon, to differentiate into NPCs and neurons. This is also the first in vitro study where the efficiency of neuronal induction of SB-iPS cells was compared with the efficiency of that in ESC lines. The neuronal phenotypes were observed through phase-contrast microscope (Fig. 1C–D) and by immunofluorescence staining (Fig. 3). SB-iPS–derived neuronal cells expressed Pax6, an essential transcription factor in neurogenesis involved in controlling neural stem cell proliferation and multipotency (Sansom et al., 2009). Nestin is a type IV intermediate neurofilament expressed specifically in NEP stem cells or NPCs. In the developing embryos, nestin is expressed in both the ventricular and subventricular zones of the CNS and is also expressed in radial glial cells (Hockfield and McKay, 1985; Lendahl et al., 1990).
We found that differentiated cells derived from SB-iPS cells had a significantly lower level of Pax6- and nestin-positive cells than those originating from ESCs. However, SB-iPS cells did show a potential to differentiate further into neurons expressing Tuj-1, which is a marker of early postmitotic neural cell types. There are many specific-neuronal markers, apart from Pax6 and nestin, that have been used for determination of NPC fate, including SOX1 (Aubert et al., 2003), SOX2 (Ellis et al., 2004), Musashi-1 (MacNicol et al., 2008), and Cx43 (Duval et al., 2002).
To determine a more specific cell fate for our NPCs, further investigation is required. In the future, by applying these markers (Sox1, Cx43, or Musashi), the cell types will be classified further, and perhaps differences between ESC- and SB-iPS–derived NPCs can be revealed. It has been reported that mouse iPS cells possess morphological, molecular and developmental features closely resembling those of ESCs (Takahashi and Yamanaka, 2006). A recent study showed that different pluripotent cell lines or even subclones of the same cell line can display different potentials to form EBs or to generate NPCs. The reasons behind these differences are not yet clear. We can only speculate whether these difference are related to different epigenetic modifications or perhaps cell cycle–related gene expression differences (Martinez et al., 2011). One of probable reasons is that the reprogramming cassette remains in our SB-iPS cells. The transposase system is now addressing this and is being developed further to render practical and more promising iPS cell lines for efficient and safety application issues. However, these SB-iPS cells have been differentiated into the neuronal lineage even when containing the transposon construct.
We hypothesized that perhaps the silencing of transgenes in SB-iPS cells occurred during the neuronal differentiation process. A previous report revealed that repression of the exogeneous pluripotent factors is necessary for allowing efficient cell differentiation toward lineages (Chamberlain et al., 2008). Moreover, incomplete promoter DNA methylation has been reported, which results in the retention of transcriptional memory and may predispose somatic cell–derived iPS cells to differentiate more readily into the particular lineage of their starting cell types (Bar-Nur et al., 2011; Ohi et al., 2011). Also, differences in the cell response toward neuronal inducers have been detected during iPS cell differentiation (Hu et al., 2010). This might explain the different response of the iPS cells to neuronal differentiation stimuli, which reportedly have more variability than ESC lines. Consequently, co-culturing of pluripotent cells with stromal cells/conditioned medium (Kawasaki et al., 2000; Yamazoe and Iwata, 2006) or as a monolayer culture in defined medium (Ying et al., 2003) may have different effects on ESCs and iPS cells.
Conclusions
In the future, human iPS cells may become a valuable source of neural cells for the regeneration and repair of tissue for traumatic injuries of the spinal cord and for potential treatment of neurodegenerative disorders, including Parkinson's disease and Huntington's disease (Salewski et al., 2010; Schwarz and Schwarz, 2010). However, there are many basic biological and technical issues, such as epigenetic modification, an efficient system of transposon and transposase construct, genotoxic risk, and optimal differentiation strategies that still need to be resolved (VandenDriessche et al., 2009).
Our study demonstrated that by using the same differentiation procedure, ESCs and SB-iPS cells show a difference in their capacity to differentiate toward the neuronal lineage. Even though the neuronal differentiation rates of iPS cells need to be improved, our results are encouraging and show that SB-iPS cells are capable of forming neurons. Thus, the Sleeping Beauty transposon–mediated reprogramming approach may be a suitable tool for obtaining these much sought after iPS cell lines.
Acknowledgments
This study was financially supported by a CHE-TRF senior scholarship, No. RTA 5080010. N. Klincumhom and S. Rungarunlert were supported by a grant under the Strategic Scholarships for Frontier Research Network program for the Joint Ph.D., the Thai Doctoral degree program from the Office of the Higher Education Commission, Thailand, No. CHE-PhD-SW-2007-115 and CHE-PhD-SW-2005-100, respectively, and by the Thailand Research Fund (MRG-4980108), the Chulalongkorn University Centenary Academic Development Project, and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (HR1166I). Support from grants from the EU FP7 (“PartnErS”, PIAP-GA-2008-218205; “InduHeart”, EU FP7-PEOPLE-IRG-2008-234390; “InduStem”, PIAP-GA-2008-230675; “Plurisys”, HEALTH-F4-2009-223485; AniStem, PIAP-GA-2011-286264; InduVir, PEOPLE-IRG-2009-245808, STEMCAM PIAP-GA-2009-251186) and NKTH/KPI (NKTH-OTKA FP7 “Mobility” HUMAN-MB08C-80205; BONUS HU_08/2-2009-0008) provided further resources.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Aubert J. Stavridis M.P. Tweedie S. O'Reilly M. Vierlinger K. Li M. Ghazal P. Pratt T. Mason J.O. Roy D. Smith A. Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl 1):11836–11841. doi: 10.1073/pnas.1734197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G. Kitchens D. Yao M. Huettner J.E. Gottlieb D.I. Embryonic stem cell express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bar-Nur O. Russ H.A. Efrat S. Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Bibel M. Richter J. Schrenk K. Tucker K.L. Staiger V. Korte M. Goetz M. Barde Y.A. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat. Neurosci. 2004;7:1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- Bibel M. Richter J. Lacroix E. Barde Y.A. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat. Protoc. 2007;2:1034–1043. doi: 10.1038/nprot.2007.147. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.J. Li X.J. Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–235. doi: 10.1007/s10048-008-0147-z. [DOI] [PubMed] [Google Scholar]
- Duval N. Gomès D. Calaora V. Calabrese A. Meda P. Bruzzone R. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J. Cell Sci. 2002;115(Pt 16):3241–3251. doi: 10.1242/jcs.115.16.3241. [DOI] [PubMed] [Google Scholar]
- Ellis P. Fagan B.M. Magness S.T. Hutton S. Taranova O. Hayashi S. McMahon A. Rao M. Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Essner J.J. McIvor R.S. Hackett P.B. Awakening gene therapy with Sleeping Beauty transposons. Curr. Opin. Pharmacol. 2005;5:513–519. doi: 10.1016/j.coph.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Evans M.J. Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fraichard A. Chassande O. Bilbaut G. Dehay C. Savatier P. Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell Sci. 1995;108(Pt 10):3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Hockfield S. McKay R.D. Identification of major cell classes in the developing mammalian nervous system. J. Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.Y. Zhang S.C. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.Y. Weick J.P. Yu J. Ma L.X. Zhang X.Q. Thomson J.A. Zhang S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z. Hackett P.B. Plasterk R.H. Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Iwamuro M. Komaki T. Kubota Y. Seita M. Kawamoto H. Yuasa T. Shahid J.M. Hassan R.A. Hassan W.A. Nakaji S. Nishikawa Y. Kondo E. Yamamoto K. Fox I.J. Kobayashi N. Hepatic differentiation of mouse iPS cells in vitro. Cell Transplant. 2010;19:841–847. doi: 10.3727/096368910X508960. [DOI] [PubMed] [Google Scholar]
- Izsvák Z. Chuah M.K. Vandendriessche T. Ivics Z. Efficient stable gene transfer into human cells by the Sleeping Beauty transposon vectors. Methods. 2009;49:287–297. doi: 10.1016/j.ymeth.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Kaji K. Norrby K. Paca A. Mileikovsky M. Mohseni P. Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani A.J. Piper D. Mujtaba T. Lucero M.T. Rao M.S. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H. Mizuseki K. Nishikawa S. Kaneko S. Induction of midbrain dopaminergic neurons from ESC by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Keller G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Langston J.W. The promise of stem cells in Parkinson disease. J. Clin. Invest. 2005;115:23–25. doi: 10.1172/JCI24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V.M. Pixley S.K. Age and differentiation-related differences in neuron-specific tubulin immunostaining of olfactory sensory neurons. Brain Res. Dev. Brain Res. 1994;83:209–215. doi: 10.1016/0165-3806(94)00139-1. [DOI] [PubMed] [Google Scholar]
- Lendahl U. Zimmerman L.B. McKay R.D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Liao J. Wu Z. Wang Y. Cheng L. Cui C. Gao Y. Chen T. Rao L. Chen S. Jia N. Dai H. Xin S. Kang J. Pei G. Xiao L. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Res. 2008;18:600–603. doi: 10.1038/cr.2008.51. [DOI] [PubMed] [Google Scholar]
- Lin R.C. Matesic D.F. Marvin M. McKay R.D. Brüstle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol. Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- Lu J. Tan L. Li P. Gao H. Fang B. Ye S. Geng Z. Zheng P. Song H. All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol. 2009;10:57. doi: 10.1186/1471-2121-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol A.M. Wilczynska A. MacNicol M.C. Function and regulation of the mammalian Musashi mRNA translational regulator. Biochem. Soc. Trans. 2008;36(Pt 3):528–530. doi: 10.1042/BST0360528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Role and distribution of retinoic acid during CNS development. Int. Rev. Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- Magin T.M. McWhir J. Melton D.W. A new mouse embryonic stem cell line with good germ line contribution and gene targeting frequency. Nucleic Acids Res. 1992;20:3795–3796. doi: 10.1093/nar/20.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N. Sridharan R. Xie W. Utikal J. Eminli S. Arnold K. Stadtfeld M. Yachechko R. Tchieu J. Jaenisch R. Plath K. Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Martinez Y. Béna F. Gimelli S. Tirefort D. Dubois-Dauphin M. Krause K.H. Preynat-Seauve O. Cellular diversity within embryonic stem cells: pluripotent clonal sublines show distinct differentiation potential. J. Cell. Mol. Med. 2011 doi: 10.1111/j.1582-4934.2011.01334.x. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M. Kalyani A.J. Mujtaba T. Rao M.S. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Muenthaisong S. Ujhelly O. Polgar Z. Varga E. Ivics Z. Pirity M.K. Dinnyes A. Generation and Characterization of mouse induced pluripotent stem (iPS) cell lines by Sleeping Beauty transposon. Exp Cell. Res. 2012 doi: 10.1016/j.yexcr.2012.07.014. (accepted). [DOI] [PubMed] [Google Scholar]
- Nagy K. Sung H.K. Zhang P. Laflamme S. Vincent P. Agha-Mohammadi S. Woltjen K. Monetti C. Michael I.P. Smith L.C. Nagy A. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 2011;7:693–702. doi: 10.1007/s12015-011-9239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y. Qin H. Hong C. Blouin L. Polo J.M. Guo T. Qi Z. Downey S.L. Manos P.D. Rossi D.J. Yu J. Hebrok M. Hochedlinger K. Costello J.F. Song J.S. Ramalho-Santos M. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 2011;13:541–549. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S. Forsberg-Nilsson K. Spiro A. Segal M. McKay R. Development of neuronal precursor cells and functional postmitotic neurons form embryonic stem cells in vitro. Mech. Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- Pevny L. Rao M.S. The stem-cell menagerie. Trends Neurosci. 2003;26:351–359. doi: 10.1016/S0166-2236(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Rohwedel J. Guan K. Wobus A.M. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs. 1999;165:190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Ross S.A. McCaffery P.J. Drager U.C. De Luca L.M. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Rungarunlert S. Techakumphu M. Pirity M.K. Dinnyes A. Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors. World J. Stem Cells. 2009;1:11–21. doi: 10.4252/wjsc.v1.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungarunlert S. Klincumhom N. Bock I. Nemes C. Techakumphu M. Pirity M.K. Dinnyes A. Enhanced cardiac differentiation of mouse embryonic stem cells by use of the slow-turning, lateral vessel (STLV) bioreactor. Biotechnol. Lett. 2011;33:1565–1573. doi: 10.1007/s10529-011-0614-8. [DOI] [PubMed] [Google Scholar]
- Salewski R.P. Eftekharpour E. Fehlings M.G. Are induced pluripotent stem cells the future of cell-based regenerative therapies for spinal cord injury? J. Cell Physiol. 2010;222:515–521. doi: 10.1002/jcp.21995. [DOI] [PubMed] [Google Scholar]
- Sansom S.N. Griffiths D.S. Faedo A. Kleinjan D.J. Ruan Y. Smith J. van Heyningen V. Rubenstein J.L. Livesey F.J. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 2009;5:e1000511. doi: 10.1371/journal.pgen.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S.C. Schwarz J. Translation of stem cell therapy for neurological diseases. Transl. Res. 2010;156:155–160. doi: 10.1016/j.trsl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Selfridge J. Pow A.M. McWhir J. Magin T.M. Melton D.W. Gene targeting using a mouse HPRT minigene/HPRT-deficient embryonic stem cell system: Inactivation of the mouse ERCC-1 gene. Somat. Cell Mol. Genet. 1992;18:325–336. doi: 10.1007/BF01235756. [DOI] [PubMed] [Google Scholar]
- Strübing C. Ahnert-Hilger G. Shan J. Wiedenmann B. Hescheler J. Wobus A. M. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech. Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- Suter D.M. Tirefort D. Julien S. Krause K.H. A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells. 2009;27:49–58. doi: 10.1634/stemcells.2008-0319. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Okita K. Nakagawa M. Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Taupin P. Neurogenesis in the adult central nervous system. C. R. Biol. 2006;329:465–475. doi: 10.1016/j.crvi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Tolar J. Park I.H. Xia L. Lees C.J. Peacock B. Webber B. McElmurry R.T. Eide C.R. Orchard P.J. Kyba M. Osborn M.J. Lund T.C. Wagner J.E. Daley G.Q. Blazar B.R. Hematopoietic differentiation of induced pluripotent stem cells from patients with mucopolysaccharidosis type I (Hurler syndrome) Blood. 2011;117:839–847. doi: 10.1182/blood-2010-05-287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven S.M. Gundersen T.E. Weedon M.S. Landaas V.O. Sakhi A.K. Fromm S.H. Geronimo B.A. Moskaug J.O. Blomhoff R. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev. Biol. 2000;220:379–391. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- VandenDriessche T. Ivics Z. Izsvák Z. Chuah M.K. Emerging potential of transposons for gene therapy and generation of induced pluripotent stem cells. Blood. 2009;114:1461–1468. doi: 10.1182/blood-2009-04-210427. [DOI] [PubMed] [Google Scholar]
- Warren L. Manos P.D. Ahfeldt T. Loh Y.H. Li H. Lau F. Ebina W. Mandal P.K. Smith Z.D. Meissner A. Daley G.Q. Brack A.S. Collins J.J. Cowan C. Schlaeger T.M. Rossi D.J. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K. Michael I.P. Mohseni P. Desai R. Mileikovsky M. Hämäläinen R. Cowling R. Wang W. Liu P. Gertsenstein M. Kaji K. Sung H.K. Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe H. Iwata H. Efficient generation of dopaminergic neurons from mouse embryonic stem cells enclosed in hollow fibers. Biomaterials. 2006;27:4871–4880. doi: 10.1016/j.biomaterials.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Ying Q.L. Stavridis M. Griffiths D. Li M. Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Zhou H. Wu S. Joo J.Y. Zhu S. Han D.W. Lin T. Trauger S. Bien G. Yao S. Zhu Y. Siuzdak G. Schöler H.R. Duan L. Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi L. Caspi O. Arbel G. Huber I. Gepstein A. Park I.H. Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]