Abstract
Hormone sensitive lipase (HSL) regulates the hydrolysis of acylglycerols and cholesteryl esters (CE) in various cells and organs, including enterocytes of the small intestine. The physiological role of this enzyme in enterocytes, however, stayed elusive. In the present study we generated mice lacking HSL exclusively in the small intestine (HSLiKO) to investigate the impact of HSL deficiency on intestinal lipid metabolism and the consequences on whole body lipid homeostasis. Chow diet-fed HSLiKO mice showed unchanged plasma lipid concentrations. In addition, feeding with high fat/high cholesterol (HF/HC) diet led to unaltered triglyceride but increased plasma cholesterol concentrations and CE accumulation in the small intestine. The same effect was observed after an acute cholesterol load. Gavaging of radioactively labeled cholesterol resulted in increased abundance of radioactivity in plasma, liver and small intestine of HSLiKO mice 4 h post-gavaging. However, cholesterol absorption determined by the fecal dual-isotope ratio method revealed no significant difference, suggesting that HSLiKO mice take up the same amount of cholesterol but in an accelerated manner. mRNA expression levels of genes involved in intestinal cholesterol transport and esterification were unchanged but we observed downregulation of HMG-CoA reductase and synthase and consequently less intestinal cholesterol biosynthesis. Taken together our study demonstrates that the lack of intestinal HSL leads to CE accumulation in the small intestine, accelerated cholesterol absorption and decreased cholesterol biosynthesis, indicating that HSL plays an important role in intestinal cholesterol homeostasis.
Keywords: Hormone sensitive lipase, Cholesterol absorption, Triglyceride absorption, Intestine-specific HSL-deficient mice, Small intestine
Highlights
► HSLiKO mice accumulate cholesteryl esters in the small intestine. ► HSLiKO mice exhibit accelerated cholesterol absorption. ► HSLiKO mice show decreased intestinal cholesterol biosynthesis. ► Intestinal HSL deficiency does not affect triglyceride metabolism.
1. Introduction
Plasma cholesterol concentrations represent a balance between cholesterol absorption, cholesterol excretion and endogenous cholesterol biosynthesis [1]. The pathways involved in cholesterol biosynthesis have already been elucidated. The knowledge about regulatory processes and the mechanisms responsible for cholesterol absorption, however, is still incomplete.
Altmann et al. have recently identified Niemann–Pick C1 like 1 (NPC1L1) as target of ezetimibe, a potent cholesterol absorption inhibitor, thereby revealing NPC1L1 as the major cholesterol importer of the small intestine [2]. Once taken up into the enterocytes, cholesterol is esterified by acyl-CoA:cholesterol acyl transferase 2 (ACAT2), packed together with triglycerides (TG), phospholipids and apolipoproteins into chylomicrons and released into the lymph [3]. Dietary cholesterol can also be effluxed to high-density lipoprotein (HDL) via ATP-binding cassette, subfamily A, member 1 (ABCA1). This process contributes to approximately 30% of whole body plasma HDL cholesterol concentrations [4]. In addition, cholesterol is expelled into the intestinal lumen by the ABCG5/ABCG8 heterodimeric transporter for excretion via the feces [5]. The model of dietary cholesterol being esterified by ACAT2 and packed into chylomicrons is well established [3]. Whether the hydrolysis of cholesteryl esters (CE) plays a role in intestinal cholesterol absorption and metabolism has not been investigated so far.
Dietary TG is taken up by enterocytes after being hydrolyzed in the lumen of the small intestine. Once in the absorptive cells, fatty acids are re-esterified and packed into chylomicrons or stored within the enterocytes, thereby creating a TG storage pool [6]. This storage pool was shown to be surrounded by lipid-droplet binding proteins, to be flexible in size and to become smaller during absorption [7]. Which enzyme(s) mobilize fatty acids from intestinal stored TG, however, is rather unknown.
Hormone sensitive lipase (HSL) is one of the major neutral CE hydrolases in various tissues and cells like liver [8,9], adrenals [10], small intestine [11], and macrophages [12]. Moreover, HSL is known to efficiently hydrolyze a broad range of substrates like tri-, di, and monoacylglycerols as well as retinyl esters [13]. Mice lacking HSL have decreased nonesterified fatty acid (NEFA) and increased cholesterol concentrations in plasma [14]. Decreased NEFA levels were explained by less release of NEFAs from white adipose tissue (WAT) resulting in reduced plasma TG concentrations [14]. The reason for the elevation in plasma total cholesterol (TC) levels in HSL-deficient mice is not completely understood. It has been recently reported that a modification of hepatic cholesterol metabolism, especially an impairment of the scavenger receptor class B type 1 (SR-B1)-mediated uptake of HDL-derived CE, might be responsible for elevated plasma HDL cholesterol concentrations [8]. Previous findings suggested that HDL cholesterol levels are increased in fasted HSL-deficient mice due to enhanced lipoprotein lipase activity in muscle and WAT [14]. Furthermore, HSL-deficient mice are resistant to diet-induced obesity [15].
Grober et al. reported that HSL is expressed throughout the small intestine and that CE hydrolase activity is absent in HSL-deficient enterocytes. In addition, the authors observed a reduction of diglyceride (DG) hydrolase activity in the jejunum and ileum of the small intestine [11]. However, the role of HSL in the small intestine and the effect of its deficiency on intestinal and whole body lipid metabolism remained unclear. To address this question, we generated intestine-specific HSL-deficient (HSLiKO) mice. Our results demonstrate that HSL plays a pivotal role as CE hydrolase in the small intestine by promoting enterocyte CE hydrolysis. We provide evidence that deficiency of intestinal HSL modulates intestinal cholesterol absorption and biosynthesis and affects plasma cholesterol levels.
2. Theory
HSL-deficient mice have reduced CE and acylglycerol hydrolase activity in the small intestine, suggesting HSL to be involved in intestinal cholesterol and TG metabolism. Since HSL deficiency has severe consequences on lipid metabolism in several organs and cells, we hypothesize that the lack of HSL in enterocytes modulates intestinal lipid metabolism, thereby affecting overall lipid homeostasis.
3. Materials and methods
3.1. Animals and diets
The generation of HSLflox/flox mice is described elsewhere [14]. Mice with intestine-specific deletion of HSL were generated by crossing HSLflox/flox mice with Villin-Cre transgenic mice [16] (provided by Dr. S. Duncan, Medical College of Wisconsin, WI) to generate HSLflox/+ heterozygotes. These mice were then mated with HSLflox/flox mice to obtain HSLflox/flox/Villin-Cre (HSLiKO) and HSLflox/flox (control) mice. All experiments were performed using female HSLiKO mice and their corresponding control littermates aged between 12 and 16 weeks. Mice had free access to food and water under a 12-h light/12-h dark cycle in a temperature-controlled environment. HSLiKO and control mice were fed a normal chow diet (11.9% caloric intake from fat; Ssniff®, Soest, Germany) or switched to a high fat/high cholesterol (HF/HC) diet for 4 weeks at the age of 12 weeks. HF/HC diet contained 30% (wt/wt) crude fat and 1% cholesterol (Ssniff®, Soest, Germany). Mice fed a HF/HC diet were housed individually and the food intake was monitored over a period of 3 days. Food intake was calculated as g/day/mouse. All experiments were approved by the Division of Genetic Engineering and Animal Experiments, Austrian Federal Ministry of Science and Research (Vienna, Austria).
3.2. Plasma lipid analysis
Blood was collected from the retroorbital plexus and plasma was prepared. TG, TC and NEFA concentrations were assayed in plasma from overnight fasted mice using enzymatic kits according to manufacturer's protocol (DiaSys, Holzheim, Germany; Wako Chemicals GmbH, Neuss, Germany). Two hundred μl plasma (pool from 5 mice) were subjected to fast protein liquid chromatography (FPLC) (Pharmacia P-500) equipped with a Superose 6 column (Amersham Biosciences, Piscataway, NJ) to separate lipoproteins. TC concentrations in 500 μl lipoprotein fractions were measured enzymatically.
3.3. Lipid analyses in small intestines and livers
Lipid parameters in the small intestine and in the liver of HSLiKO and control mice fed chow or HF/HC diet for 4 weeks were analyzed after a 4 h fasting period. The middle 1/3rd part of the small intestine (jejunum) and livers from HSLiKO and control mice were collected and the lipids were isolated by Folch extraction. The lipid extract was dried under a stream of nitrogen. One hundred μl 1% Triton-X100 in chloroform was added and dried again under nitrogen gas. Thereafter, the samples were dissolved in 100 μl ddH2O, and TG, TC and free cholesterol (FC) (DiaSys, Holzheim, Germany; Wako Chemicals GmbH, Neuss, Germany) concentrations were measured enzymatically. CE concentrations were calculated by subtracting FC from TC. All values were normalized to protein concentrations. For measuring DG concentrations, lipid extracts were separated by thin layer chromatography (TLC) using n-hexane/diethylether/acetic acid (80/20/2, v/v/v). DG bands were scraped, lipids were extracted and dissolved in 100 μl ddH2O containing 1% Triton-X100. DG concentrations were measured using an aclyglycerol kit (DiaSys, Holzheim, Germany).
3.4. Measurement of intestinal cholesterol uptake and absorption
Cholesterol uptake and absorption studies were performed as previously described with minor modifications [17]. Briefly, mice fed chow diet were fasted for 4 h and gavaged with 100 μl corn oil containing 2 μCi [3H]cholesterol (ARC Inc., St. Louis, MO) and 500 μg cholesterol. Four h post-gavaging, mice were sacrificed and plasma, livers and intestines were collected, digested in 1 ml 1N NaOH overnight at 65 °C and radioactivity was determined by liquid scintillation counting. Plasma samples from each genotype were pooled, 200 μl were subjected to FPLC and the radioactivity in 500 μl fractions was determined by liquid scintillation counting.
For measurement of the cholesterol absorption rate, mice fasted for 4 h were gavaged with 100 μl corn oil containing 2 μCi [3H]cholesterol and 500 μg cholesterol. Four, 8, 12 and 24 h post-gavaging plasma were collected and radioactivity was determined by liquid scintillation counting.
For determination of FC and CE concentrations, lipids from 250 mg of 3 equal parts of the small intestine (duodenum, jejunum and ileum) were extracted and separated by TLC using n-hexane/diethylether/acetic acid (80/20/2, v/v/v). Radioactivity in FC- and CE-specific bands was measured by liquid scintillation counting. For calculation of the relative distribution the total amount of radioactivity in the small intestine was set to 100% and the percentage of CE and FC was calculated.
3.5. Fractional cholesterol absorption
Fractional cholesterol absorption was measured by using the fecal dual-isotope ratio method [17]. Briefly, mice fed chow diet were fasted for 4 h before they were given a single intragastric dose of 100 μl corn oil containing 0.2 μCi [3H]sitostanol (ARC Inc., St. Louis, MO) and 0.1 μCi [14C]cholesterol (ARC Inc., St. Louis, MO). Feces were collected for 48 h. Fecal lipids were isolated by Folch extraction and radioactivity was determined by liquid scintillation counting. Fractional cholesterol absorption was calculated by the following formula:
| % absorption = ((dose [14C]:[3H]-fecal [14C]:[3H])/dose [14C]:[3H]) ∗ 100. |
3.6. Measurement of intestinal TG absorption
We assessed dietary TG absorption using [3H]triolein (Perkin Elmer, Boston, MA) as previously described [18] with minor modifications. Briefly, mice fed chow diet were fasted for 4 h. Thereafter, they were intraperitoneally injected with the lipase inhibitor tyloxapol (500 mg/kg in PBS) to prevent peripheral lipolysis. Thirty min after injection, each mouse was gavaged with 100 μl corn oil containing 2 μCi [3H]triolein. Prior to tyloxapol injection and 2 as well as 4 h post-gavaging, blood was taken and radioactivity and TG concentration were measured in the plasma. Four h post-gavaging, mice were sacrificed, livers and intestines were collected, digested in 1 ml 1N NaOH overnight at 65 °C and radioactivity was determined by liquid scintillation counting. For determination of the distribution of lipid classes, lipids were extracted from 250 mg jejunum and separated by TLC using n-hexane/diethylether/acetic acid (80/20/2, v/v/v). Bands resembling TG, DG, NEFA and phospholipids were cut and radioactivity was measured by liquid scintillation counting.
3.7. RNA isolation and quantitative real-time PCR
Total RNA from tissues was extracted using TriFast according to the manufacturer's protocol (Peqlab, Erlangen, Germany). Two μg of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed on a Roche LightCycler 480 (Roche Diagnostics, Palo Alto, CA) using the QuantiFastTM SYBR® Green PCR Kit (Qiagen, Valencia, CA). Samples were analyzed in duplicate and normalized to the expression of cyclophilin A as a reference gene. Expression profiles and associated statistical parameters were determined using the public domain program Relative Expression Software Tool — REST 2008 (http://www.gene-quantification.com/download.html) [19]. Primer sequences are available upon request.
3.8. Western blotting analysis
Mucosal scrapings were sonicated (Labsonic B. Braun, Melsungen, Germany) in RIPA buffer and protein concentrations were determined by Bradford assay. Tissue homogenates (pools of 3 mice) were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. For detection of HSL protein, polyclonal anti-HSL antibody (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:800. The protein expression of ß-actin was determined as loading control using a monoclonal anti-mouse ß-actin antibody (1:5000) (Santa Cruz Biotechnology, Heidelberg, Germany).
3.9. CE, acylglycerol and retinyl ester hydrolase activity assay
Cytosolic fractions of mucosal scrapings were obtained by centrifugation (14.000 rpm for 1 h at 4 °C). Forty μg of protein from cytosolic fractions was used for each assay. Further steps were performed as previously described [20]. Briefly, 40 μg of protein emulsified in 100 μl of 100 mM potassium phosphate lysis buffer was incubated with 100 μl CE-substrate (20 nmol cholesteryl oleate/assay, cholesteryl [1-14C]oleate (50,000 cpm/nmol) (Amersham Biosciences, Piscataway, NJ)), TG-substrate (25 nmol triolein/assay and 40,000 cpm/nmol [9,10-3H]triolein (PerkinElmer, Waltham, MA)), or retinyl ester substrate (10 nmol retinyl palmitate and 50,000 cpm/nmol retinyl [9,10(n)-3H]palmitate), respectively. Each substrate contained 35.5 μg mixed micelles of phosphatidylcholine and phosphatidylinositol (3:1, w:w). After incubation at 37 °C for 1 h, the reaction was terminated by adding 3.25 ml of methanol/chloroform/heptane (10:9:7) and 1 ml 100 mM potassium carbonate (pH 10.5 with boric acid). After centrifugation (800 × g, 15 min, 4 °C), the radioactivity in 1 ml of the upper phase was determined by liquid scintillation counting.
3.10. Intestinal cholesterol synthesis
Intestinal cholesterol biosynthesis was performed as described previously [21] with minor modifications. Briefly, mice fed chow diet were orally gavaged with 200 μl PBS containing 4 μCi [3H]mevalonic acid (Perkin Elmer, Boston, MA). One h later the proximal part of the intestine (duodenum and jejunum) was collected and the lipids were extracted. FC and CE fractions were separated by TLC using n-hexane/diethylether/acetic acid (80/20/2, v/v/v). FC- and CE-specific bands were cut out and radioactivity was determined by liquid scintillation counting. TC data represent the sum of counts detected in CE- and FC-specific bands.
3.11. Gut transit
Overnight fasted mice were gavaged with 200 μl Evans blue suspension (5% Evans blue, 5% gum Arabic in PBS). Afterwards mice had free access to food and water and the duration until the detection of Evans blue in the feces was recorded.
3.12. Statistical analysis
Statistical differences between groups were analyzed using unpaired Student's t-test (GraphPad Prism 5.0, San Diego, CA). Data are reported as means ± SEM for the specified number of animals. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001 indicate significant difference between HSLiKO and control mice.
4. Results
4.1. CE hydrolase activity is almost abolished in small intestines of HSLiKO mice
First we compared HSL mRNA expression and activity in several tissues of control mice. Although less than in WAT, HSL mRNA was expressed in all three parts of the small intestine (duodenum, jejunum and ileum) (Supplemental Fig. S1A), in which we observed substantial CE hydrolase activities (Supplemental Fig. S1B).
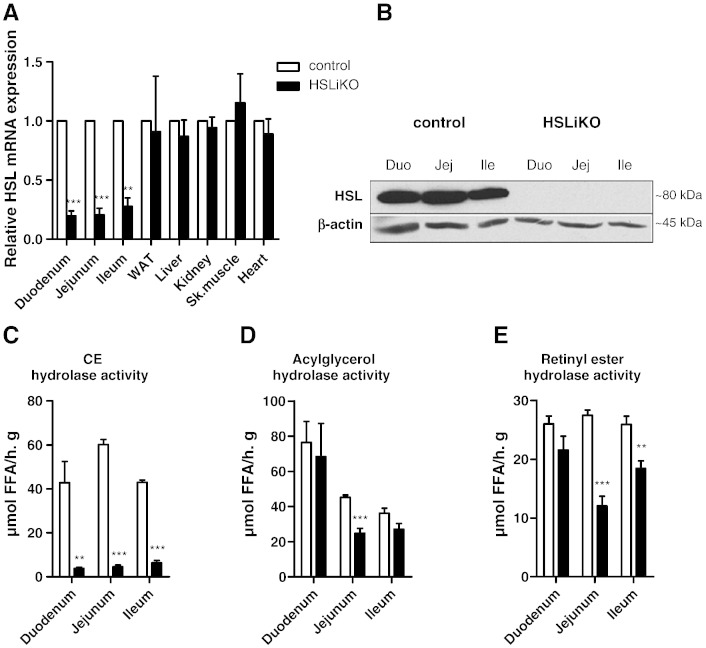
To elucidate the physiological function of intestinal HSL on whole body lipid homeostasis and to exclude systemic effects of a whole body knockout on intestinal lipid metabolism, we eliminated HSL from the intestinal epithelium. To confirm HSL deletion in adult HSLiKO intestine, we isolated duodenum, jejunum, and ileum and examined HSL mRNA and protein expression by real time PCR and Western blotting experiments, respectively. HSL mRNA was markedly downregulated throughout the small intestine of HSLiKO mice compared to control mice, whereas no differences were observed in WAT, liver, kidney, skeletal muscle and heart (Fig. 1A). HSL protein expression was undetectable in HSLiKO duodenum, jejunum and ileum (Fig. 1B). Consequently, we found strongly decreased CE hydrolase activity in all three parts of the small intestine (Fig. 1C). Acylglycerol hydrolase was significantly less (45%) in jejunum of HSLiKO compared to control mice, but unchanged in duodenum and ileum (Fig. 1D). Retinyl ester hydrolase activity was decreased in jejunum (56%) and ileum (29%) but unaltered in duodenum (Fig. 1E). These results suggest that HSL is a major neutral CE hydrolase in the small intestine and plays possible roles as acylglycerol or retinyl ester hydrolase.
Fig. 1.
HSL is deleted specifically in the small intestine of HSLiKO mice. (A) HSL mRNA levels were determined in all 3 parts of the small intestine (duodenum, jejunum, ileum) and in control tissues. mRNA levels were normalized to cyclophilin A as a reference gene. HSL mRNA levels in control mice were arbitrarily set to 1. Values represent means (n = 3) ± SEM. **p ≤ 0.01, ***p ≤ 0.001. (B) Protein lysates of pools from 3 mice of each genotype were separated by SDS-PAGE. HSL protein expression was analyzed by Western blotting. The expression of β-actin was determined as loading control. (C) CE, (D) acylglycerol and (E) retinyl ester hydrolase activities were measured in duodenum, jejunum and ileum. Data represent mean values (n = 4) ± SEM. **p ≤ 0.01, ***p ≤ 0.001.
4.2. Increased plasma cholesterol levels in HSLiKO mice fed a HF/HC diet
We fed control and HSLiKO mice chow diet or challenged the mice with HF/HC diet for 4 weeks. Food intake and body weight were comparable between HSLiKO and control mice (Supplemental Fig. S2A, B). No significant differences were found in lipid parameters of chow diet-fed mice and in TG and NEFA concentrations of HF/HC diet-fed mice (Table 1). In contrast, we observed a tendency toward increased plasma TC concentrations (1.3-fold, p = 0.07) in HSLiKO mice fed a HF/HC diet (Table 1).
Table 1.
Plasma lipid parameters of mice fed chow diet or HF/HC diet for 4 weeks. TG, TC and NEFA concentrations were determined enzymatically. Data are expressed as mean values ± SEM.
| Chow (n = 6–8) |
HF/HC (n = 5–8) |
|||||
|---|---|---|---|---|---|---|
| TC (mg/dl) | TG (mg/dl) | NEFA (mM) | TC (mg/dl) | TG (mg/dl) | NEFA (mM) | |
| Control | 118 ± 5.5 | 35.2 ± 4.5 | 1.21 ± 0.1 | 155 ± 15.1 | 43.6 ± 3.2 | 1.02 ± 0.07 |
| HSLiKO | 119 ± 5.8 | 30.2 ± 3.3 | 1.09 ± 0.1 | 202 ± 17.9 | 42.9 ± 4.3 | 0.98 ± 0.1 |
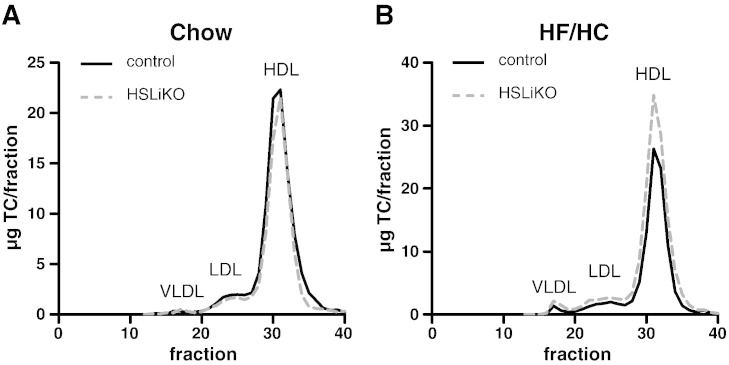
Lipoprotein profiles revealed unaltered cholesterol distribution in chow diet-fed HSLiKO mice (Fig. 2A) but increased cholesterol levels in the HDL fraction (1.3-fold) when fed a HF/HC diet (Fig. 2B).
Fig. 2.
Lipoprotein analyses of overnight fasted mice fed chow or HF/HC diet. Distribution of TC after fast performance liquid chromatography separation of pooled plasma from mice (n = 5) fed (A) chow or (B) HF/HC diet. TC concentrations in 0.5 ml fractions were determined spectrophotometrically.
4.3. HSLiKO mice fed HF/HC diet accumulate CE in the small intestine
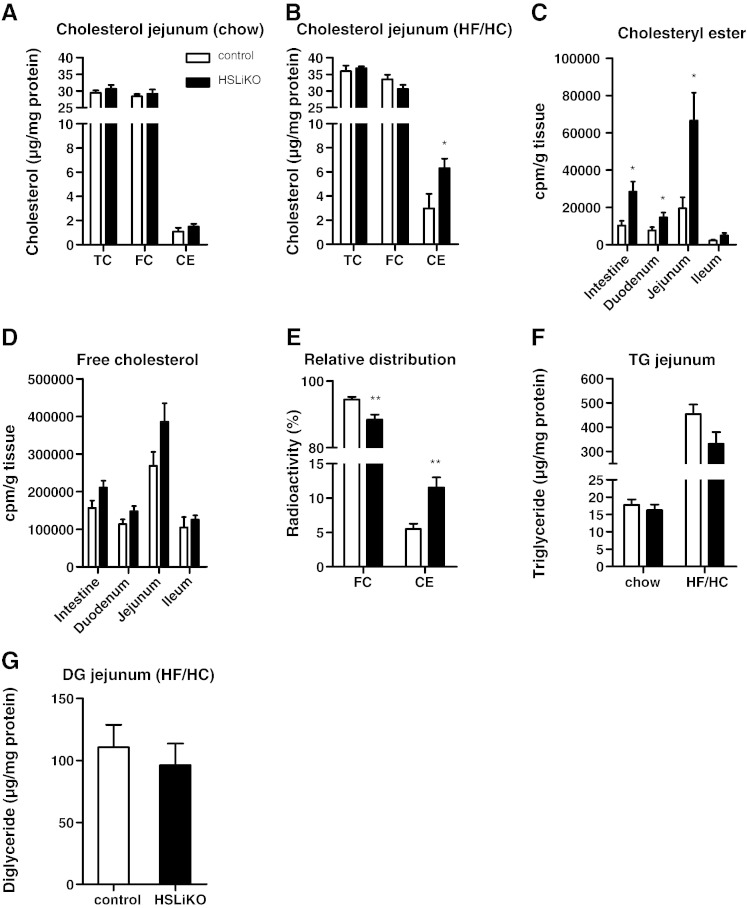
To investigate the impact of decreased CE hydrolase activity on intestinal cholesterol concentrations, we measured intestinal TC, FC and CE concentrations in mice fed either chow or HF/HC diets. HSLiKO and control mice fed chow diet had comparable cholesterol levels in the small intestine (Fig. 3A). When challenged with HF/HC diet, HSLiKO mice showed markedly elevated CE levels by 2.1-fold compared to control mice (Fig. 3B). To clarify if the accumulated CE in HSLiKO mice originates from dietary sources we gavaged mice with [3H]cholesterol. We observed a drastic increase by 2.8-fold of radioactivity in the CE fraction of HSLiKO compared to control small intestines with significant increases in the duodenum and jejunum (1.9- and 3.4-fold, respectively) (Fig. 3C). The amount of radioactivity in FC fractions was unaltered (Fig. 3D). Calculation of the relative distribution of the administered [3H]cholesterol revealed a switch from FC to CE in HSLiKO compared to control small intestines (Fig. 3E).
Fig. 3.
CE accumulation in small intestine of HSLiKO mice fed HF/HC diet. TC, FC and CE concentrations in jejunum of mice fed (A) chow or (B) HF/HC diet for 4 weeks. Data represent mean values (n = 4–5) ± SEM. *p ≤ 0.05. (C–E) Mice were gavaged with 100 μl corn oil containing 2 μCi [3H]cholesterol and 500 μg cholesterol. Radioactivity in (C) CE and (D) FC fractions per g tissue in the whole small intestine, duodenum, jejunum and ileum was determined by liquid scintillation counting. (E) Relative distribution of radioactive cholesterol in CE and FC fractions 4 h after gavaging. Data represent mean values (n = 6) ± SEM. *p ≤ 0.05, **p ≤ 0.01. (F, G) Mice were fed chow or HF/HC diet for 4 weeks and jejunal (F) TG and (G) DG were determined. Data represent mean values (n = 6–8) ± SEM.
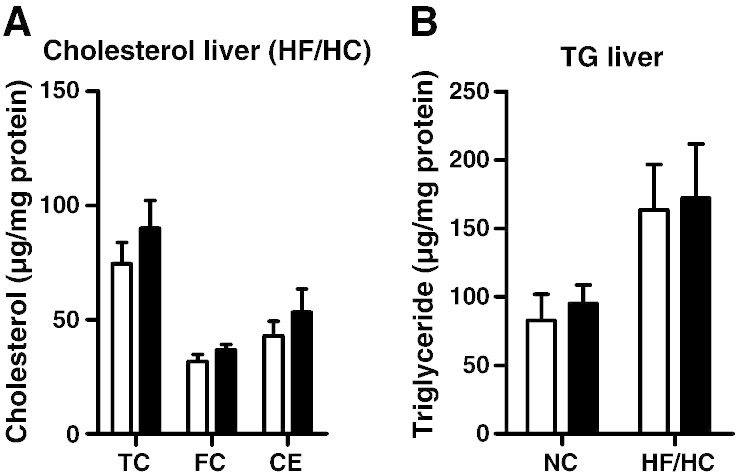
Since HSL is known to hydrolyze acylglycerols and we observed reduced acylglycerol hydrolase activity, we also measured TG and DG concentrations in the jejunum. No significant differences were observed between HSLiKO and control mice (Fig. 3F, G). To figure out if the lack of intestinal HSL modulates hepatic lipid content, we determined cholesterol and TG concentrations in the liver. We found unaltered hepatic lipid levels in HSLiKO compared to control mice (Fig. 4A, B).
Fig. 4.
Unchanged hepatic lipid parameters in HSLiKO mice. (A) Hepatic TC, FC and CE concentrations were measured in mice fed HF/HC diet for 4 weeks. (B) Hepatic TG levels were determined in mice fed chow or HF/HC diet for 4 weeks. Data represent mean values (n = 6–8) ± SEM.
4.4. Intestinal HSL deficiency modulates intestinal cholesterol uptake
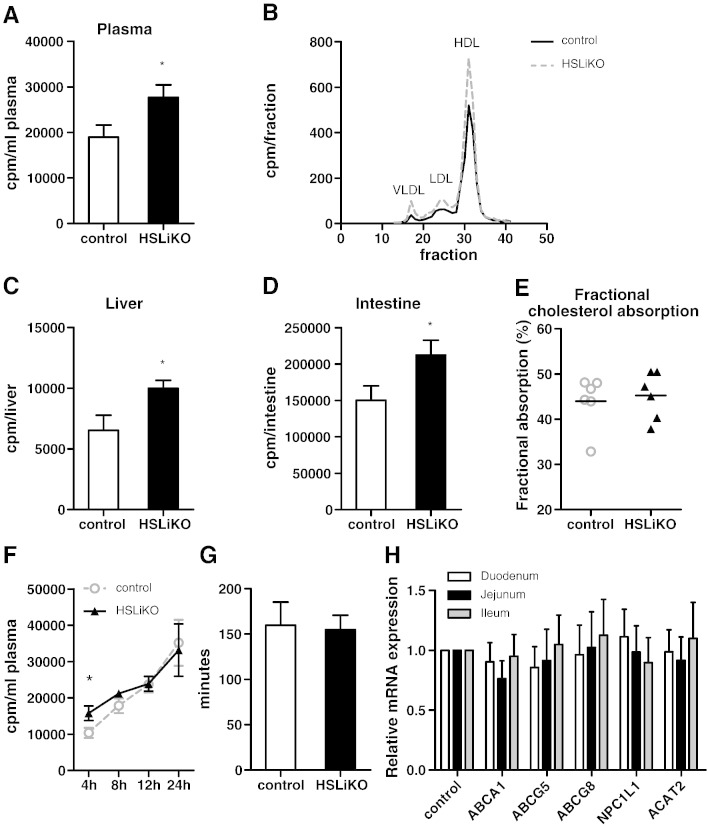
Grober et al. suggested a role of HSL in cholesterol absorption by influencing the efflux of FC by ABC transporters [11]. We therefore determined cholesterol absorption into liver, plasma and small intestine 4 h after an acute [3H]cholesterol load. We found 1.4-fold more radioactivity in plasma of HSLiKO compared to control mice (Fig. 5A). Lipoprotein profiling revealed that the increased amount of radioactivity in the plasma was found throughout all lipoprotein fractions (Fig. 5B). We also observed elevated amounts of radioactivity in livers (Fig. 5C) and small intestines of HSLiKO mice (Fig. 5D). In addition, we determined fractional cholesterol absorption by using the fecal dual-isotope ratio method but detected no differences in the two genotypes (Fig. 5E). Moreover, we measured cholesterol absorption rate over a period of 24 h and found differences in the plasma only 4 h post-gavaging (Fig. 5F). These results indicate that the same amount of cholesterol is taken up in HSLiKO mice but the uptake into the body is accelerated compared to control mice. We therefore investigated whether gut motility might be altered in HSLiKO mice. As shown in Fig. 5G, however, gut transit is identical in both genotypes. Small intestinal mRNA expression in 4 h fasted mice revealed that genes involved in cholesterol uptake and absorption (ABCA1, ABCG5, ABCG8, NPC1L1 and ACAT2) showed comparable mRNA abundances in both genotypes (Fig. 5H). These data suggest that the increased accumulation of radioactivity in liver and plasma of HSLiKO mice after an acute oral cholesterol load is independent on the intestinal expression of these genes.
Fig. 5.
Accelerated cholesterol absorption into plasma, liver and small intestine of HSLiKO mice. Mice fed chow diet were gavaged with 100 μl corn oil containing 2 μCi [3H]cholesterol and 500 μg cholesterol. Four h after gavaging, (A) plasma, (C) liver and (D) small intestine were collected and radioactivity was determined by liquid scintillation counting. Data represent mean values (n = 6) ± SEM. *p ≤ 0.05. (B) Plasma samples from each genotype were pooled and 200 μl was subjected to fast protein liquid chromatography using a Superose 6 column. [3H]Cholesterol in each fraction was determined by liquid scintillation counting. (E) Fractional cholesterol absorption was measured by the fecal dual-isotope ratio method. Data represent mean values (n = 6) ± SEM. (F) Cholesterol absorption rate was measured by gavaging 100 μl corn oil containing 2 μCi [3H]cholesterol and 500 μg cholesterol. Radioactivity in plasma was measured 4, 8, 12 and 24 h post-gavaging. (G) Mice were gavaged with 200 μl Evans blue and gut transit was determined by measuring the time until the first blue drop appeared in the feces. Data represent mean values (n = 3–4) ± SEM. (H) mRNA expression of genes involved in intestinal cholesterol uptake and absorption were analyzed by real time PCR and normalized to the expression of cyclophilin A as a reference gene. mRNA levels in control mice were arbitrarily set to 1. Values represent means (n = 3) ± SEM.
4.5. Intestinal cholesterol biosynthesis is decreased in HSLiKO mice
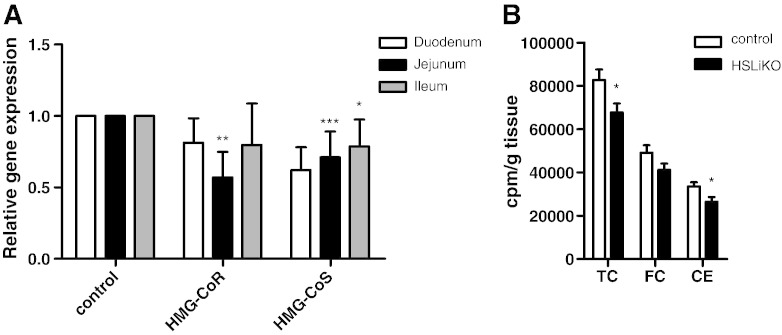
Previous studies reported that HSL deficiency is associated with a decrease in hepatic mRNA expression of genes involved in cholesterol biosynthesis [8,9]. We therefore measured mRNA expression of intestinal 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMG-CoR) and HMG-CoA synthase (HMG-CoS), two key enzymes of cholesterol biosynthesis. HMG-CoR mRNA was downregulated in jejunum but unchanged in duodenum and ileum of HSLiKO mice (Fig. 6A). HMG-CoS mRNA was downregulated in duodenum and jejunum of HSLiKO compared to control mice. TC and CE biosynthesis in the proximal intestine measured from orally administered [3H]mevalonic acid was decreased by 18% and 21%, respectively, in HSLiKO compared to control small intestines (Fig. 6B).
Fig. 6.
Reduced intestinal cholesterol biosynthesis in HSLiKO mice. (A) mRNA expression of HMG-CoR and HMG-CoS were determined by real time PCR and normalized to cyclophilin A as a reference gene. mRNA levels in control mice were arbitrarily set to 1. Values represent means (n = 3) ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. (B) Cholesterol biosynthesis of TC, FC and CE in the proximal part of the small intestine was measured using [3H]mevalonic acid as substrate. Data represent mean values (n = 5) ± SEM. *p ≤ 0.05.
4.6. TG absorption is unchanged in HSLiKO mice
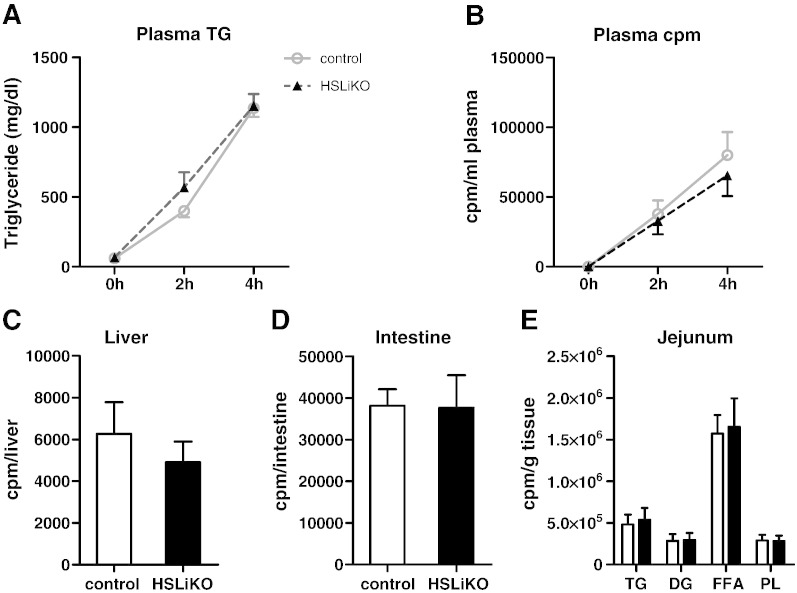
To investigate if the lack of intestinal HSL has a consequence on intestinal TG absorption we injected tyloxapol to inhibit peripheral lipolysis in HSLiKO and control mice and then gavaged [3H]trioleate provided in corn oil. Plasma TG levels were unchanged between HSLiKO and control mice during absorption (Fig. 7A). Furthermore, counts in plasma, liver and small intestine were unaltered (Fig. 7B, C and D), suggesting no difference in TG absorption. To clarify whether HSL deficiency modulates lipid composition, we analyzed the distribution of radioactivity in various lipid classes in the jejunum after gavaging [3H]trioleate. HSL deficiency had no effect on the incorporation of radioactivity into any lipid species indicating that HSL lacks an important role in intestinal acylglycerol metabolism (Fig. 7E).
Fig. 7.
TG absorption is not affected in HSLiKO mice. Mice fed chow diet were injected with tyloxapol to inhibit peripheral lipolysis and then gavaged with 100 μl corn oil containing 2 μCi [3H]trioleate. Plasma was collected prior to injection with tyloxapol and 2 and 4 h after gavaging to measure (A) TG concentrations and (B) radioactivity. Four h post-gavaging mice were sacrificed and radioactivity in (C) liver and (D) small intestine were determined by liquid scintillation counting. (E) Distribution of radioactivity in lipid species of jejunum 4 h post-gavaging. Data represent mean values (n = 5–6) ± SEM.
5. Discussion
For decades HSL was thought to be the major TG hydrolase in many cells and organs [22]. Recent data have shown that adipose triglyceride lipase (ATGL) is the main cytosolic TG hydrolase in WAT and also plays an important role in other tissues and cells like skeletal muscle, heart, liver, and macrophages [20,23–27]. We detected decreased acylglycerol hydrolase activity in the jejunum of HSLiKO mice, which is in agreement with previous data reporting that DG hydrolase activity was significantly reduced in jejunum of HSL-deficient mice [11]. The decreased acylglycerol hydrolase activity, however, failed to result in accumulation of intestinal TG or DG, respectively. Zhu et al. have recently reported that lipid droplets are formed in enterocytes during TG absorption, indicating that an intracellular lipase might play a role in this process [7]. Our findings argue against an important role of HSL in TG absorption and suggest that other lipases are involved in the hydrolysis of acylglycerols in enterocytes. One candidate is intestinal pancreatic triglyceride lipase (iPTL), which was shown to be an acylglycerol hydrolase in enterocytes of the small intestine [28]. Although this enzyme is regulated by dietary fat it is currently unknown, whether iPTL is involved in TG absorption. Another possible candidate is ATGL. ATGL-deficient mice accumulate TG in the ileum [23], suggesting a role of ATGL also in intestinal TG metabolism. In addition, one further candidate is lysosomal acid lipase (LAL). LAL-deficient mice accumulate TG and TC in the small intestine [29]. Moreover, the knockdown of magro, a homolog of LAL in flies, results in CE accumulation in the gut [30]. Compared to HSL, ATGL and iPTL, however, LAL is not a cytosolic enzyme and exerts its maximum activity at low pH conditions.
HSL was shown to be a CE hydrolase in various tissues and cells like liver, macrophages, adrenal glands, pancreas, and in the small intestine [8–12,31]. The accumulation of CE, however, was observed only under special circumstances. Two recent publications showed that CE accumulated in livers of HSL-deficient mice solely if they were fed HF or HC diets [8,9].
Compared to liver HSL mRNA is more abundant in the small intestine. Although less than WAT, the small intestine exhibits increased CE hydrolase activity levels compared to liver, kidney, cardiac and skeletal muscle. In the present study we show that the disruption of intestinal HSL results in a modulation of intestinal cholesterol metabolism. In the small intestine we observed CE accumulation under two different conditions: firstly, after 4 weeks of HF/HC diet feeding and secondly, after an intragastric gavage with high amounts of cholesterol. These results suggest that HSL not only has in vitro CE hydrolase activity in the small intestine but also plays a crucial role in CE hydrolysis in enterocytes in vivo. Since CE accumulation was observed after HF/HC feeding and after intragastric cholesterol load, it is very likely that HSL hydrolyzes CE, from which FC is absorbed by the enterocytes.
HSL modulates the transcription of genes involved in cholesterol biosynthesis [8,9,32]. Data obtained from livers of HSL-deficient mice revealed that in the absence of HSL several cholesterogenic genes are downregulated [8,9]. Accordingly, cells overexpressing HSL showed increased HMG-CoR activity and increased cholesterol biosynthesis [32]. In agreement with these data, we found that the lack of HSL in the small intestine resulted in a downregulation of HMG-CoR and HMG-CoS mRNA and consequently in a decrease of intestinal cholesterol biosynthesis. Although the relationship between cholesterol biosynthesis and HSL is still unknown, we speculate that decreased cholesterol biosynthesis in HSLiKO mice might result in unchanged CE concentrations as observed in mice fed chow diet. Cholesterol-enriched diet caused a strong reduction of HMG-CoR activity [33] and almost undetectable levels of HMG-CoR and HMG-CoS mRNA in small intestines of control mice ([21] and Supplemental Fig. S3). HSLiKO mice fed a HF/HC diet might no longer be able to counteract the increasing availability of CE by reducing cholesterol biosynthesis, which consequently leads to CE accumulation in the small intestine of HF/HC diet-fed HSLiKO mice.
Despite the fact that the small intestine is a highly cholesterogenic organ, which under certain physiological circumstances has a higher cholesterol biosynthesis rate than the liver, the gut is mainly known to absorb cholesterol and regulate its uptake [33]. HSL could play a role in cholesterol absorption for several reasons: (i) HSL might provide FC to be effluxed back into the intestinal lumen. In this case, HSL deficiency results in increased or at least accelerated cholesterol absorption. (ii) HSL might supply FC to lipidate HDL. In that case, reduced HDL cholesterol is the consequence of intestinal lack of HSL. (iii) The lack of HSL could modulate the expression of genes involved in intestinal cholesterol metabolism, thereby influencing cholesterol absorption. In the present study, we observed an earlier appearance of [3H]cholesterol in plasma and liver of HSLiKO mice 4 h post-gavaging but fractional cholesterol absorption was unaltered. These results implicate that the same amount of cholesterol is taken up in HSLiKO mice but in an accelerated manner, possibly due to less efflux back into the intestinal lumen by ABCG5/G8. We speculate that in the case of intestinal HSL deficiency less FC is pumped back out into the intestinal lumen and consequently more cholesterol can be found in the circulation 4 h post-gavaging. Since HSL deficiency did not modulate mRNA levels of genes involved in cholesterol absorption, we conclude that accelerated absorption in HSLiKO mice is independent on expressions of these genes. Since we found more radioactive tracer in the HDL fraction after gavaging [3H]cholesterol, we conclude that HSL is not responsible for providing ABCA1 with FC, at least not in the small intestine. A faster gut transit could be an explanation for the observed accelerated cholesterol absorption in HSLiKO mice. Determination of gut transit time, however, revealed no difference between control and HSLiKO mice. It is important to mention that gut transit was evaluated under different conditions compared with the cholesterol uptake study. Thus the transit of corn oil in the small intestine is not directly reflected by this experiment.
Since ACAT2 catalyzes the opposite reaction to that of HSL one would expect opposing effects in ACAT2-deficient compared to HSLiKO mice. This is indeed the case because (compared to control mice) ACAT2-deficient mice have decreased intestinal CE concentrations when fed a cholesterol-enriched diet [34]. In addition, thoracic lymph duct cannulation revealed a delayed appearance of radioactive cholesterol in lymph [3]. The lack of ACAT2, however, results in a decrease in fractional cholesterol absorption. This is very likely due to a modulation of liver X receptor (LXR) target genes, like upregulation of ABCG5 and downregulation of NPC1L1 by elevated FC levels [3,35]. Thus a downregulation of LXR targets in HSL-deficient conditions (due to less availability of FC) might be expected. Nevertheless, we observed unaltered FC concentrations and consequently no downregulation of LXR target genes in the intestines of HSLiKO mice.
Although intestinal HSL deficiency markedly affected cholesterol homeostasis in the small intestine, the consequences on whole body lipid metabolism were only marginal. Hepatic and plasma lipid parameters were comparable between HSLiKO and control mice fed chow diet. When fed a HF/HC diet, plasma cholesterol concentrations showed a tendency to elevated levels with enhancement of HDL cholesterol. These data demonstrate that beside changes in liver gene expressions and lipoprotein lipase activity the small intestine also contributes to increased cholesterol levels in HSL-deficient mice, at least when mice are challenged with high amounts of fat and cholesterol. Increased lipoprotein lipase expression in muscle and WAT [14] as well as reduced HDL-CE uptake by hepatic SR-B1 (despite unchanged SR-B1 expression) [8] are possible reasons for elevated HDL-cholesterol levels in whole body HSL-deficient mice. Enterocytes also take up HDL cholesterol [36]. As discussed in the livers of HSL-deficient mice [8], less HDL-CE clearance despite unaltered SR-B1 expression (data not shown) is conceivable in small intestines of HF/HC diet-fed HSLiKO mice.
In conclusion, our study demonstrates that disruption of intestinal HSL modulates intestinal cholesterol but not TG metabolism. Accumulation of CE in the intestine, accelerated cholesterol absorption and reduced intestinal cholesterol biosynthesis highlight the role of HSL as an important player in intestinal cholesterol metabolism in vivo.
Acknowledgements
This work was supported by the Austrian Science Fund (SFB-LIPOTOX F30, DK-MCD W1226, P19186 and P22832). S.O. is a fellow of and P.G.C., J.V.P. and T.P. were supported by the PhD Program Molecular Medicine of the Medical University of Graz. The authors thank A. Ibovnik for excellent technical assistance, I. Hindler for mice care, Branislav Radovic for critical discussion of the manuscript and Rudolf Schicho for helping with the gut transit experiment.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbalip.2012.07.013.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Dietschy J.M., Turley S.D., Spady D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 2.Altmann S.W., Davis H.R., Jr., Zhu L.J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P., Maguire M., Golovko A., Zeng M., Wang L., Murgolo N., Graziano M.P. Niemann–Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T.M., Sawyer J.K., Kelley K.L., Davis M.A., Rudel L.L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012;53:95–104. doi: 10.1194/jlr.M018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham L.R., Kruit J.K., Iqbal J., Fievet C., Timmins J.M., Pape T.D., Coburn B.A., Bissada N., Staels B., Groen A.K., Hussain M.M., Parks J.S., Kuipers F., Hayden M.R. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge K.E., Tian H., Graf G.A., Yu L., Grishin N.V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 6.Lambert J.E., Parks E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta. 2012;1821:721–726. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J., Lee B., Buhman K.K., Cheng J.X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J. Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez C., Lindholm M., Krogh M., Lucas S., Larsson S., Osmark P., Berger K., Boren J., Fielding B., Frayn K., Holm C. Disturbed cholesterol homeostasis in hormonesensitive lipase-null mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E820–E831. doi: 10.1152/ajpendo.90206.2008. [DOI] [PubMed] [Google Scholar]
- 9.Sekiya M., Osuga J., Yahagi N., Okazaki H., Tamura Y., Igarashi M., Takase S., Harada K., Okazaki S., Iizuka Y., Ohashi K., Yagyu H., Okazaki M., Gotoda T., Nagai R., Kadowaki T., Shimano H., Yamada N., Ishibashi S. Hormone-sensitive lipase is involved in hepatic cholesteryl ester hydrolysis. J. Lipid Res. 2008;49:1829–1838. doi: 10.1194/jlr.M800198-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer F.B. Adrenal cholesterol utilization. Mol. Cell. Endocrinol. 2007;265–266:42–45. doi: 10.1016/j.mce.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Grober J., Lucas S., Sorhede-Winzell M., Zaghini I., Mairal A., Contreras J.A., Besnard P., Holm C., Langin D. Hormone-sensitive lipase is a cholesterol esterase of the intestinal mucosa. J. Biol. Chem. 2003;278:6510–6515. doi: 10.1074/jbc.M208513200. [DOI] [PubMed] [Google Scholar]
- 12.Buchebner M., Pfeifer T., Rathke N., Chandak P.G., Lass A., Schreiber R., Kratzer A., Zimmermann R., Sattler W., Koefeler H., Frohlich E., Kostner G.M., Birner-Gruenberger R., Chiang K.P., Haemmerle G., Zechner R., Levak-Frank S., Cravatt B., Kratky D. Cholesteryl ester hydrolase activity is abolished in HSL −/− macrophages but unchanged in macrophages lacking KIAA1363. J. Lipid Res. 2011;51:2896–2908. doi: 10.1194/jlr.M004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lass A., Zimmermann R., Oberer M., Zechner R. Lipolysis — a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2010;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haemmerle G., Zimmermann R., Strauss J.G., Kratky D., Riederer M., Knipping G., Zechner R. Hormone-sensitive lipase deficiency in mice changes the plasma lipid profile by affecting the tissue-specific expression pattern of lipoprotein lipase in adipose tissue and muscle. J. Biol. Chem. 2002;277:12946–12952. doi: 10.1074/jbc.M108640200. [DOI] [PubMed] [Google Scholar]
- 15.Harada K., Shen W.J., Patel S., Natu V., Wang J., Osuga J., Ishibashi S., Kraemer F.B. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1182–E1195. doi: 10.1152/ajpendo.00259.2003. [DOI] [PubMed] [Google Scholar]
- 16.el Marjou F., Janssen K.P., Chang B.H., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 17.Chandak P.G., Obrowsky S., Radovic B., Doddapattar P., Aflaki E., Kratzer A., Doshi L.S., Povoden S., Ahammer H., Hoefler G., Levak-Frank S., Kratky D. Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim. Biophys. Acta. 2011;1811:1011–1020. doi: 10.1016/j.bbalip.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patankar J.V., Chandak P.G., Obrowsky S., Pfeifer T., Diwoky C., Uellen A., Sattler W., Stollberger R., Hoefler G., Heinemann A., Battle M., Duncan S., Kratky D., Levak-Frank S. Loss of intestinal GATA4 prevents diet-induced obesity and promotes insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2011;300:E478–E488. doi: 10.1152/ajpendo.00457.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandak P.G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., Tabas I., Levak-Frank S., Kratky D. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 2011;285:20192–20201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis H.R., Jr., Zhu L.J., Hoos L.M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S.P., Lam M.H., Lund E.G., Detmers P.A., Graziano M.P., Altmann S.W. Niemann–Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann R., Lass A., Haemmerle G., Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim. Biophys. Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E.F., Klingenspor M., Hoefler G., Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 24.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K., Schreiber R., Eichmann T., Kolb D., Kotzbeck P., Schweiger M., Kumari M., Eder S., Schoiswohl G., Wongsiriroj N., Pollak N.M., Radner F.P., Preiss-Landl K., Kolbe T., Rulicke T., Pieske B., Trauner M., Lass A., Zimmermann R., Hoefler G., Cinti S., Kershaw E.E., Schrauwen P., Madeo F., Mayer B., Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoiswohl G., Schweiger M., Schreiber R., Gorkiewicz G., Preiss-Landl K., Taschler U., Zierler K.A., Radner F.P., Eichmann T.O., Kienesberger P.C., Eder S., Lass A., Haemmerle G., Alsted T.J., Kiens B., Hoefler G., Zechner R., Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 2009;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J.W., Wang S.P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K.G., Yang G., Mitchell G.A. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 28.Mahan J.T., Heda G.D., Rao R.H., Mansbach C.M., II The intestine expresses pancreatic triacylglycerol lipase: regulation by dietary lipid. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1187–G1196. doi: 10.1152/ajpgi.2001.280.6.G1187. [DOI] [PubMed] [Google Scholar]
- 29.Du H., Heur M., Duanmu M., Grabowski G.A., Hui D.Y., Witte D.P., Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J. Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 30.Sieber M.H., Thummel C.S. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson S., Wierup N., Sundler F., Eliasson L., Holm C. Lack of cholesterol mobilization in islets of hormone-sensitive lipase deficient mice impairs insulin secretion. Biochem. Biophys. Res. Commun. 2008;376:558–562. doi: 10.1016/j.bbrc.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 32.Kraemer F.B., Fong L., Patel S., Natu V., Komaromy M.C. Overexpression of hormone-sensitive lipase in Chinese hamster ovary cells leads to abnormalities in cholesterol homeostasis. J. Lipid Res. 1997;38:1553–1561. [PubMed] [Google Scholar]
- 33.Nguyen L.B., Shefer S., Salen G., Tint G.S., Ruiz F., Bullock J. Mechanisms for cholesterol homeostasis in rat jejunal mucosa: effects of cholesterol, sitosterol, and lovastatin. J. Lipid Res. 2001;42:195–200. [PubMed] [Google Scholar]
- 34.Turley S.D., Valasek M.A., Repa J.J., Dietschy J.M. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1012–G1022. doi: 10.1152/ajpgi.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Repa J.J., Buhman K.K., Farese R.V., Jr., Dietschy J.M., Turley S.D. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- 36.Lutton C., Champarnaud G. Cholesterol synthesis and high density lipoprotein uptake are regulated independently in rat small intestinal epithelium. Gut. 1994;35:343–346. doi: 10.1136/gut.35.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.