Abstract
Objective
To estimate the effects of prostaglandin E1 (PGE1) and E2 (PGE2) on myometrial contractility and structure in vitro.
Study design
Myometrial strips from 18 women were incubated with PGE1 (10−5 mol/l), PGE2 (10−5 mol/l) or solvent (CTR) for up to 360 min in organ chambers for isometric tension recording. The area under the contraction curve (AUC), total collagen content and the percentage of the area covered by connective tissue were calculated at various time periods.
Results
PGE1 significantly increased in vitro myometrial contractility up to 90 min when compared to PGE2 and CTR (p < 0.01), and up to 180 minutes as compared to PGE2 (p < 0.05). After 360 min, CTR and PGE1 samples had lower total collagen content and area covered by connective tissue than PGE2 (p < 0.01).
Conclusion
The effects of prostaglandins on the uterus cannot be solely explained by contractility. Treatment with PGE1 significantly increased myometrial contractions, decreased both total collagen content and the area covered by connective tissue. Such findings may explain the higher rates of vaginal delivery, tachysystole and uterine rupture associated with PGE1 use.
Keywords: prostaglandin E1, prostaglandin E2, collagen, uterine contractility, organ chamber
INTRODUCTION
Induction of labor is an increasingly common practice in the United States, and accounts for at least 20% of all births. Providers generally resort to induction of labor when the risks to the mother and/or the fetus with pregnancy continuation outweigh the risks involved with the intervention (1). While oxytocin is an effective drug in patients with favorable Bishop scores, other pharmacological or mechanical agents are frequently utilized in the event of an unripe cervix (2). Local administration of prostaglandin E1 (misoprotol, PGE1) or E2 (dinoprostone, PGE2) has been used for decades to achieve cervical ripening and labor induction (3, 4). A recent meta-analysis showed that women receiving PGE1 are more likely to deliver vaginally, and require less oxytocin for labor augmentation when compared to those receiving PGE2 (5, 6). Although some of the complications associated with the use of prostaglandins (PGs) are reversible if rapidly addressed (tachysystole), their use for labor induction in women with prior cesarean delivery (CD) has been associated with uterine rupture (7 – 10). Such rare but catastrophic complication has been observed more frequently with the administration of misoprostol, which is also responsible for increased frequency of uterine tachysystole and meconium stained amniotic fluid (5, 6). The American Congress of Obstetricians and Gynecologists encourages trial of labor in women with prior cesarean section, and while it does not preclude induction of labor for those with an unfavorable cervix, ACOG does not recommend the use of misoprostol because of the increased risk of uterine rupture (9). Cervical ripening induced by PGE1 and PGE2 is associated with an increase in inflammatory mediators in the cervix, and remodeling of the cervical extracellular matrix through a decrease in collagen cross links and cervical glycosaminoglycans (11 – 13). However, the effects of PGs on the human myometrium are not as clearly defined. In vitro, PGE2 does not affect contractility of uterine tissue collected from term pregnancies; and both contractile and relaxant responses have been reported in myometrial samples from non-pregnant women (14, 15). Studies investigating the effects of misoprostol on myometrial contractility, as well as the effects of PGs on myometrial structure, particularly in women with prior CD, are lacking. Finally, the clinical recommendations regarding the frequency of PG application, as well as the waiting period prior to starting oxytocin, are not based on experimental evidence.
Therefore, we sought in this study to estimate the effects of PGE1 and PGE2 on myometrial contractile function and structure over a clinically-relevant time period using tissues collected from term pregnancies undergoing scheduled repeat CD.
MATERIALS AND METHODS
The study was approved by the institutional review board of the University of Texas Medical Branch. Patients undergoing scheduled repeat cesarean section at term gestation (38–41 weeks) between July 1st and December 31st 2010 were approached for consent. Exclusion criteria were presence of more than 3 contractions per hour, rupture of membranes, placenta previa, and uterine conditions/abnormalities affecting contractility (such as leyomiomas).
In consenting women, 2×2×4 cm biopsies were taken from the upper edge of the transverse incision in the lower uterine segment, placed in Hanks’ balanced salt solution (HBSS; Gibco BRL Products, Rockville, MD, USA), and transported to the lab. The biopsies were then prepared into9×2×2 mm strips suspended in organ chambers for isometric tension recording.
Tissue organ chamber
Myometrial strips were mounted vertically in 10 mL organchambers containing Krebs–Henseleit solution and prepared for isometric tension recording using stainless steel hooks and surgical silk sutures. One end of the strip was attached to a fixed support at the bottom of the chamber, while the other end was connected to an isometric force transducer. The temperature in the organ bath was maintained at 37°Cand the solution was continuously bubbled with 5% CO2 in air(pH 7.4). Strips were equilibrated at the passive tension of 1g in Krebs’ solution. Isometric tension was measured with Harvard isometric force transducers (Harvard Apparatus, South Natik, MA, USA) connected to a computer. The data were acquired and analyzed using Windaq data acquisition system (Dataq Instruments, Inc., Akron, OH, USA). The specimens were equilibrated for 1–2 hours until uniform, regular and rhythmic contractions had developed for at least 40 min (contraction stabilization). The bath solution was changed every 30 min to maintain myometrial viability before incubation with PGs. Tissues with poor spontaneous contractile activity were discarded with the assumption that they represented scar tissue or that the tissue was not viable at the time of the experiment. The remaining tissue samples were incubated with PGE1 at 10−5 mol/l, PGE2 at 10−5 mol/l or distilled water as control (CTR). The PG concentrations used in this study were based on our preliminary dose response studies testing myometrial strips with cumulative concentrations (10 −10M to 10 −5M) of PGE1 and PGE2 (16).
The area under the curve (AUC) defining myometrial contractions (integral activity) was used as a measure of uterine contractility. Baseline activity was calculated as the integral activity over the 40 min following stabilization of myometrial contractions, before the addition of any drug; uterine contractility was then measured in time CTR and in samples exposed to PG after completion of baseline activity. Tissue responses to PGE1 and PGE2 over time were calculated cumulatively as the integral activity over 30, 90, 180 and 360 minutes after PG addition to the tissue baths. To correct for time-induced decay in contractility, the same study intervals were used in the CTR samples. The contractile responses at 30, 90, 180, and 360 min were expressed as percentages of the baseline activity, dividing the integral activity calculated in these time periods by the baseline contractility; time-response curves were then generated.
Some of the tissue samples were removed from the organ chambers after 30 and 360 min, and stored at −80°C for biochemical assessment of total collagen, or fixed in formalin for histological examination.
Total collagen content (TCC) by hydroxyproline measurement
Collagen contains approximately 14% hydroxyproline (17), thus the amount of total collagen in tissue samples can be inferred determining the concentration of this aminoacid. Hydroxyproline was measured in tissue hydrolysates by a modification of the method developed by Woessner (18).
Myometrial strips were placed in an oven at 80°C to dry overnight; their weight was then calculated prior to being hydrolyzed in 6N HCL for 3 hours at 130°C. Aliquots of L-hydroxyproline (0.5–5 μg/mL) were prepared in water from a stock solution. One hundred μL of tissue hydrolysate in 6 N HCL was neutralized with 3 N NaOH. The samples and standards were oxidized for 20 minutes at room temperature by the addition of 50 μL chloramine T reagent (1.41 g chloramine T in 20 mL water, 50 mL citrate-acetate buffer and 30 mL 2-methoxyethanol). The reaction was stopped by the addition of 50 μL 3.15 M perchloric acid. Color development was achieved over a 20-minute incubation at 65°C with 100 μL fresh Ehrlich’s reagent. One hundred μL of reacted sample or standard was added in duplicate to a 96-well microtiter plate, and the absorbance was read at 557 nm using the SpectraMax 190 (Molecular Devices, Downingtown, PA, USA). Hydroxyproline induces a change in the color of the Ehrlich’s reagent from bright yellow to magenta. All measurements were done in a single assay to eliminate interassay variability. The intra-assay variability was 2%. Collagen concentrations were expressed in milligram per tissue gram dry weight.
Histology
Myometrial strips were fixed in 10% buffered formalin (Fisher Chemical, Fairlawn, NJ) for 24 hours, and then changed to 70% ethanol and embedded in paraffin. Paraffin sections were cut to a thickness of 5 μm and stained with Masson Trichrome. All slides were examined by two reviewers that were masked to the identity of the specimens. The amount of connective tissue was estimated using image analysis: Aperio Scan Scope (Aperio, Vista, CA, USA) was used for slide scanning, Image Pro Plus 6.0. (Media Cybernetics Inc, Bethesda, MD, USA) for area measurements. Briefly, whole-slide scanning was used to generate a digital slide image, and the area covered by connective tissue was measured as percent of the total tissue section area. Slides from normal controls were used for system calibration after staining with Masson trichrome. Background removal was used to select only the area of the slide that was covered with tissue. To determine the area of the section actually occupied by connective tissue (stained in blue), lower and upper thresholds were set on the blue tone curve. The tone and intensity thresholds were verified on control specimens, before examining the study samples. After measurements were completed, the identity of the specimens was unmasked and data were compared.
Drugs and Solutions
Dinoprostone (Sigma, St Louis, MO, USA) was dissolved and diluted in distilled water, while misoprostol (Sigma, St Louis, MO, USA) was dissolved in ethanol and then diluted in Krebs’ solution. Stock solutions were aliquoted, kept at −20°C, and sonicated for 5 min to aid solubilization immediately prior to use. Citrate-acetate buffer was prepared by adding 50 g citric acid, 12 mL glacial acetic acid, 34 g sodium hydroxide, and 120 g sodium acetate trihydrate to 1 L of water with the pH adjusted to 6.0. The Ehrlich’s reagent was prepared by suspending 20 g of 4-dimethylaminobenzaldehyde in 100 mL 2-methoxyethanol.
Data analysis
As this was an exploratory study, no formal power calculation was performed. We assessed normality of the data using the Shapiro – Wilk test. Because of the sample size limitation and failed normality test, we used one-way and/or two-way Kruskal-Wallis analysis of variance on ranks followed by post hoc tests to compare the different groups. Data were expressed as median and interquartile range [IQR]. Two-sided p value < 0.05 was considered statistically significant.
RESULTS
Uterine biopsies from 18 patients were included in the analysis. The demographic characteristics of the study participants are summarized in table 1. Twenty six strips were discarded due to poor contractile activity or inadequate tension recording (7 of which before the 30 min time point). At the 30 min mark, 25 strips were removed for total collagen quantification, and 24 for histology. Isometric tension was recorded after completion of baseline activity for 30, 90, 180 and 360 min in 151, 84, 79, and 76 myometrial strips, respectively. No significant differences in the number of tissue samples collected from each patient was observed among the study groups (Table 2).
Table 1.
Characteristics of study participants
| Age (years) | 32 ± 3.8 |
| Gravidity | 3.5 [2–5] |
| Parity | 1 [1–2] |
| Number of prior CD | 1 [1–1] |
| Gestational age (weeks) | 38.8 ± 0.7 |
| Race/Ethnicity | |
| Caucasian | 3 (16.7%) |
| African American | 1 (5.5%) |
| Hispanic | 14 (77.8%) |
CD = cesarean delivery; data are reported as mean +/− SD, median [IQR], or n (%)
Table 2.
Number of myometrial samples collected from each patient and used to assess cumulative uterine contractility, total collagen content and the percentage of the area covered by connective tissue at different time intervals
| Time (min) | CTR | PGE1 | PGE2 | P* | |
|---|---|---|---|---|---|
| Uterine contractility | |||||
| 30 | 3 [2–3] | 3 [2.5–4] | 3.5 [2–4] | 0.1 | |
| 90 | 2 [1–2] | 2 [2–2] | 2 [1–2] | 0.1 | |
| 180 | 2 [1–2] | 2 [2–2] | 2 [1–2] | 0.1 | |
| 360 | 2 [1–2] | 2 [2–2] | 2 [1–2] | 0.1 | |
| TCC | |||||
| 30 | 1 [1-1] | 1 [1-1] | 1 [1-1] | 1 | |
| 360 | 1 [1-1] | 1 [1-1] | 1 [1-1] | 1 | |
| CT% | |||||
| 30 | 1 [1-1] | 1 [1-1] | 1 [1-1] | 1 | |
| 360 | 1 [1-1] | 1 [1-1] | 1 [1-1] | 1 |
TCC: total collagen content; CT%: percentage of the area covered by connective tissue.
Kruskal-Wallis One Way Analysis of Variance on Ranks. Data are reported as median [IQR].
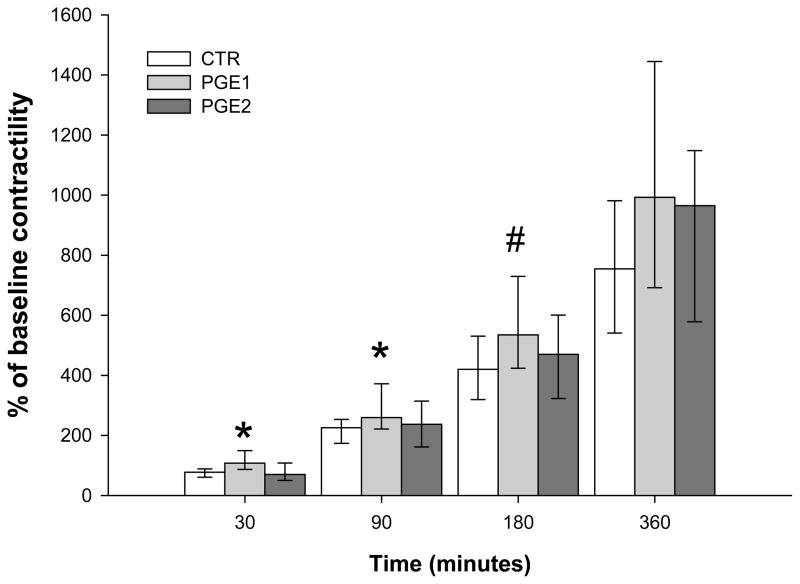
When cumulative uterine contractility was studied by measuring the AUC from the time PG was added to the tissue baths (0 min), misoprostol induced significantly higher contractility as compared to dinoprostone and CTR in the first 30 (p < 0.01) and 90 (p < 0.01) minutes. When evaluating the first 180 minutes, myometrial contractility in samples exposed to PGE1 remained higher than CTR but not PGE2 (p = 0.02). No significant differences were detected between the study groups when the entire 360 minutes period was evaluated (Figure 1).
Figure 1. Cumulative uterine contractility at 30, 90, 180, 360 minutes from baseline.
Kruskal-Wallis One Way Analysis of Variance on Ranks followed by Dunn’s post hoc test * p< 0.01; PGE1 vs PGE2 & CTR; # p<0.05; PGE1 vs CTR. Data are reported as median [IQR].
Contractility was then analyzed by successive time periods (baseline, 0–30 min, 30–90 min, 90–180 min, and 180–360 min). There were no statistically significant differences in baseline contractile activity between the 3 study groups (Table 3). Incubation with PGE1 significantly increased myometrial contractility in the 0 to 30 min period when compared with PGE2 and time CTR (p < 0.01). In the 30 to 90 min period, uterine activity in the PGE1 group remained higher than in the PGE2 and CTR groups (p = 0.04). No differences were detected between the groups in the 90 to 180 min and 180 to 360 min periods (Table 3).
Table 3.
Baseline myometrial contractility and cumulative uterine contractility during different time intervals.
| Time (minutes) | CTR | PGE1 | PGE2 | P* |
|---|---|---|---|---|
| Area under the curve (g) | ||||
| Baseline (− 40 to 0) | 546.1 [461.4–848.3] | 566.1 [396.4–752.2] | 618.1 [482.7–942.9] | 0.2 |
| % of baseline activity | ||||
| 0 to 30 (n = 151) | 77.5 [60.6–88.5] | 107.7 [86.7–149.4] | 70.2 [49.9–108] | < 0.01 |
| 30 to 90 (n = 84) | 153 [131.1–211.7] | 276.5 [217.3–456.4] | 175.5 [103.8–210.8] | 0.04 |
| 90 to 180 (n = 79) | 222.5 [187–382.1] | 372.1 [300–501.1] | 232.5 [150–295.6] | 0.06 |
| 180 to 360 (n = 76) | 372.2 [250–702.7] | 514.4 [358.1–612.7] | 433.8 [242.7–567.7] | 0.3 |
Time 0 represents the time at which PGs were added.
Kruskal-Wallis One Way Analysis of Variance on Ranks followed by Dunn’s post hoc test.0 to 30 min:PGE1 vs PGE2 p < 0.01, PGE1 vs CTR p < 0.01, 30 to 90 min: PGE1 vs CTR p < 0.05 Data are reported as median [IQR].
Total collagen was quantified by hydroxyproline assay in myometrial strips collected from 12 patients at 30 (CTR n = 9; PGE1 n = 8; PGE2 n = 8) or 360 (CTR n = 13; PGE1 n = 14, PGE2 n = 14) min after incubation. CTR and PGE1 samples had lower TCC at 360 min compared with 30 min (CTR: 66 [34.5–71.1] vs 139.8 [ 138.2–152.7] mcg/g dry tissue, p = 0.02; and PGE1: 51.8 [21.6–68.5] mcg/g dry tissue, p = 0.04). In the myometrial strips collected at 360 min, TCC in CTR and PGE1 samples were significantly lower when compared with PGE2 (66 [34.5–71.1] vs. 51.8 [21.6–68.5] vs. 104.2 [78.5–176.4] mcg/g dry tissue, p < 0.01; Figure 2)
Figure 2. Total collagen concentration (mcg/g dry tissue) in samples removed at 30 or 360 minutes.
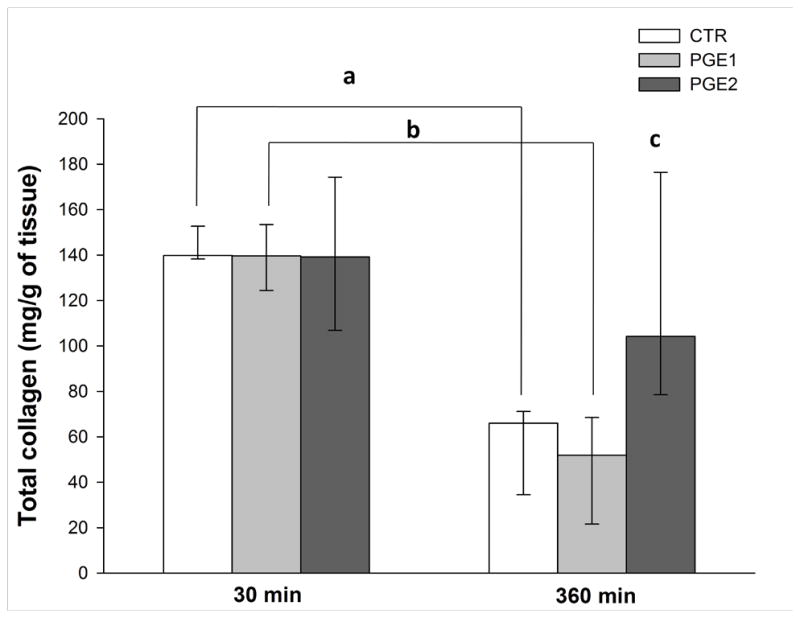
Two Way Analysis of Variance on Ranks followed by Holm-Sidak post hoc test. 30 min vs 360 min p < 0.01; a: p = 0.002; b: p = 0.004; c: PGE2 vs PGE1 and CTR at 360 min p < 0.01. Data are reported as median [IQR].
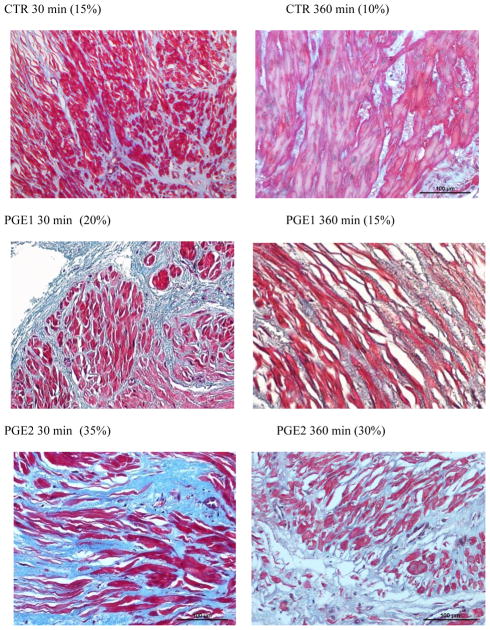
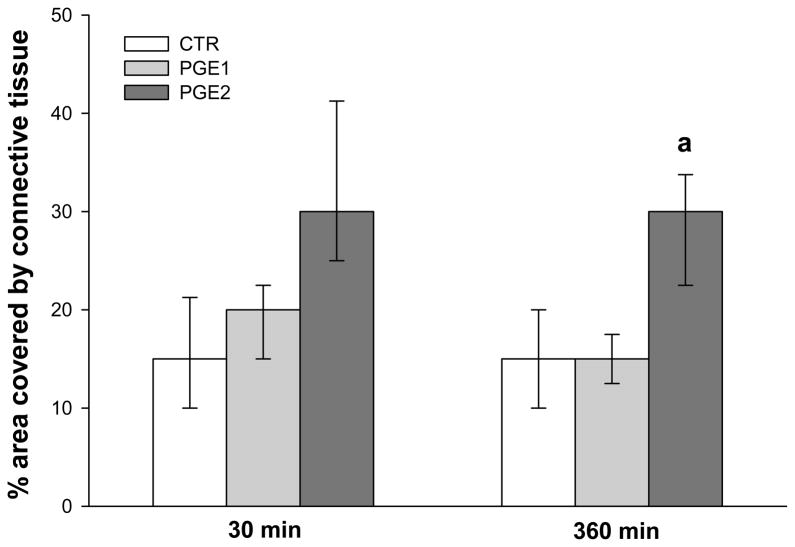
The percentage of area covered by connective tissue was calculated in myometrial strips from 9 patients at 30 (CTR n = 9; PGE1 n = 8; PGE2 n = 7) and 360 (CTR n = 9; PGE1 n = 8; PGE2 n = 7) min. CT% was significantly lower in CTR and in PGE1 samples when compared to PGE2 at 360 min (15 [10–20]% vs 15 [12.5–17.5]% vs 30 [22.5–33.7]%, p < 0.01; Figures 3, 4). There were no differences in CT% among the various samples at 30 min as opposed to 360 min.
Figure 3. Representative histology from myometrial samples treated with PGE1 and PGE2, as well as CTR collected at 30 and 360 min and stained with Masson Trichrome.
After generating a digital image, the area covered by connective tissue (stained in blue) was measured as percent of the total section area; muscle fibers were stained red. Original magnification: 20X
Figure 4. Percentage area covered by connective tissue in samples removed at 30 or 360 minutes.
Two Way Analysis of Variance on Ranks followed by Holm-Sidak post hoc test. a: PGE2 vs PGE1 and CTR at 360 min p < 0.01. Data are reported as median [IQR].
CONCLUSION
Using uterine biopsies from women at term undergoing scheduled repeat CD, we found that PGE1 significantly increased in vitro contractility over the first 30 and the first 90 min when compared with PGE2 and CTR, and up to 180 minutes when compared with CTR. The total collagen concentration and the CT% were lower in CTR and samples treated with PGE1 as compared to PGE2. Prostaglandin E1 and CTR samples also had lower TCC at 360 compared with 30 min.
Clinical studies have demonstrated that when compared to placebo, both misoprostol and dinoprostone increase vaginal delivery rates and improve cervical Bishop scores (3, 4), and when compared to oxytocin infusion they also improve the chance of vaginal delivery within 24 hours in the event of an unripe cervix (19). The ability of dinoprostone and misoprostol to induce labor cannot be explained solely by their effects on myometrial contractility, as we have demonstrated that PGE1 but not PGE2 promotes uterine contractions in vitro. Similarly, the clinical use of PGs is not consistent with their in vitro effects on myometrial contractility as the in vitro response to misoprostol lasted up to 180 min in our study, while clinically it is recommended that the frequency of administration should not exceed 3–6 hour intervals (2). The discrepancy between in vitro data and clinical findings suggest that PGs mediate induction of labor through an elaborate process that involves various targets, and necessitates a complex interplay between the uterine cervix and the myometrium. As different PG receptors may lead to myometrial contraction or relaxation, different uterine responses to PGE1 and PGE2 could depend on distinct receptor expression or activity. Instead, animal models have shown that PG synthesis is the main factor regulating uterine contractility as contractile receptors did not increase, nor changed in sensitivity to PGs at the onset of labor (20). Further studies are needed to determine whether the increased uterine contractility is an indirect response to cervical ripening rather than a direct effect of PG administration, and whether increased uterine contractility can affect cervical structure and function. Our results may account for the higher incidence of uterine tachysystole (5, 6) and success of labor induction (5) observed with misoprostol, as PGE1 was associated with increased in vitro uterine contractility.
Rupture of the pregnant uterus is a catastrophic event, affecting between 0.7 and 1.9% of women with previous CD attempting a trial of labor (2). The healing process of the human myometrium after CD is still subject to controversy and intense speculation. Beginning with Kroenig’s description of the lower uterine segment incision (21), physicians have continued to debate the process of scar formation in the uterine wall. Most authors believe that the repair of the muscular tissue occurs by scar formation and that the healing process of the human myometrium is subject to large variance, depending not only on the number of prior CDs, but also on the specific genetic makeup of each individual (22). Buhimschi et al. compared the lower uterine segment (LUS) myometrial structure of laboring women undergoing primary low-transverse CD (LTCD) for labor dystocia, nonlaboring women undergoing primary elective LTCD and subjects undergoing elective repeat LTCD at term. The myometrium from scarred LUS had a higher collagen content compared with unscarred myometrium from laboring women, but not unscarred myometrium from nonlaboring women (23). Our findings additionally suggest that in women with previous CD, myometrial contractions are associated with a decrease in total myometrial collagen and possibly connective tissue content, and that incubation with misoprostol accentuates such effect, while exposure to dinoprostone does not. The more pronounced contractile response and decrease in collagen content observed with misoprostol may explain the higher incidence of uterine rupture observed with its use in women with previous CD, who usually experience uterine rupture at the site of their old scar when treated with PGs for cervical ripening as compared to other agents (24). Similarly, the milder effects of dinoprostone on collagen content seem to suggest that it may represent a safer choice for labor induction in the setting of a previous CD. Further studies are necessary to determine if the decrease in collagen and connective tissue associated with uterine contractions is a pure mechanical phenomenon or if it depends on the activation of enzymes responsible for the extracellular matrix breakdown such as matrix metallopeptidase 2 and 9 (MMP 2 and 9).
The strengths of our study include selection of a homogeneous study population that consisted of non laboring women with term pregnancies undergoing scheduled repeat CD, and our analysis of myometrial contractility for longer time periods, comparable to the intervals of PG administration used in clinical practice. Our study also had some weaknesses in that we analyzed the effects of PGE1 and PGE2 on myometrial contractility and structure only, whereas the physiology of labor induction indicates that cervical ripening and uterine contractions often occur concurrently after PG administration. Data on total collagen content and the percentage of area covered by connective tissue were not available prior to isometric tension recording, immediately after collection. We also analyzed total collagen concentrations and therefore cannot comment on changes in the different types of collagen or the cross links between collagen fibers.
In summary, our study contributes to explain some of the advantages and risks associated with various PGs used for labor induction. When evaluating the role of PGs in cervical ripening and labor induction, their effects on the myometrium should not be ignored. Further studies are needed to evaluate the interaction between the uterine cervix and myometrium during labor induction and the mechanisms through which myometrial contractions and PG exposure affect collagen concentration. A better understanding of the mechanisms of action of PGs would allow clinicians to perform safer and more effective inductions of labor.
Acknowledgments
Sources of Financial support: Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, TX
Footnotes
Disclosure: None of the authors have a conflict of interest
References
- 1.Martin JA, Hamilton BE, Sutton PD. Births: final data for 2006: National vital statistics reports. 7. Vol. 57. National Center for Health Statistics; Hyattsville, MD: 2009. [Google Scholar]
- 2.American College of Obstetricians and Gynecologists, Induction of labor. ACOG practice bulletin no.107. Obstet Gynecol. 2009;114:386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 3.Kelly AJ, Malik S, Smith L, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2009;7(4):CD003101. doi: 10.1002/14651858.CD003101.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010;10:CD000941. doi: 10.1002/14651858.CD000941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin SC, Sanchez-Ramos L, Adair CD. Labor induction with intravaginal misoprostol compared with the dinoprostone vaginal insert: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202:624. e1–9. doi: 10.1016/j.ajog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Vaknin Z, Kurzweil Y, Sherman D. Foley catheter balloon vs locally applied prostaglandins for cervical ripening and labor induction: a systematic review and meta analysis. Am J Obstet Gynecol. 2010;203:418–29. doi: 10.1016/j.ajog.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Akhan SE, Iyibozkurt AC, Turfanda A. Unscarred uterine rupture after induction of labor with misoprostol: a case report. Clin Exp Obstet Gynecol. 2001;2:118–20. [PubMed] [Google Scholar]
- 8.Khabbaz AY, Usta IM, El-Hajj MI, Abu-Musa A, Seoud M, Nassar AH. Rupture of an unscarred uterus with misoprostol induction: case report and review of the literature. J Matern Fetal Med. 2001;10:141–5. doi: 10.1080/714904310. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists ACOG Practice bulletin no. 115. Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 Pt 1):450–63. doi: 10.1097/AOG.0b013e3181eeb251. [DOI] [PubMed] [Google Scholar]
- 10.Al-Zirqi I, Stray-Pedersen B, Forsén L, Vangen S. Uterine rupture after previous caesarean section. BJOG. 2010;117:809–20. doi: 10.1111/j.1471-0528.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 11.Stjernholm YM, Sahlin L, Eriksson HA, Byström BE, Stenlund PM, Ekman GE. Cervical ripening after treatment with prostaglandin E2 or antiprogestin (RU486). Possible mechanisms in relation to gonadal steroids. Eur J Obstet Gynecol Reprod Biol. 1999;84:83–8. doi: 10.1016/s0301-2115(98)00329-7. [DOI] [PubMed] [Google Scholar]
- 12.Ji H, Dailey TL, Long V, Chien EK. Prostaglandin E2-regulated cervical ripening: analysis of proteoglycan expression in the rat cervix. Am J Obstet Gynecol. 2008;198:536. e1–7. doi: 10.1016/j.ajog.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Sahlin L, Stjernholm-Vladic Y, Roos N, Masironi B, Ekman-Ordeberg G. Impaired leukocyte influx in cervix of postterm women not responding to prostaglandin priming. Reprod Biol Endocrinol. 2008;2:6–36. doi: 10.1186/1477-7827-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon-Wright AP, Elder MG. Effect of prostaglandin E2 and its metabolites on lower segment myometrium in vitro. Eur J Obstet Gynecol Reprod Biol. 1980;10:297–302. doi: 10.1016/0028-2243(80)90076-3. [DOI] [PubMed] [Google Scholar]
- 15.Popat A, Crankshaw DJ. Variable responses to prostaglandin E(2) in human non-pregnant myometrium. Eur J Pharmacol. 2001;23:145–52. doi: 10.1016/s0014-2999(01)00852-4. [DOI] [PubMed] [Google Scholar]
- 16.Chiossi G, Costantine MM, Bytautiene E, Betancourt A, Hankins GDV, Saade GR, Longo M. Misoprostol potentiates spontaneous uterine contractility in vitro. Reproductive Sciences. 2011;18:129a. [Google Scholar]
- 17.Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 18.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 19.Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;4:CD003246. doi: 10.1002/14651858.CD003246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palliser HK, Hirst JJ, Rice GE, Dellion NL, Escalona RM, Parkington HC, Young IR. Prostaglandin E and F receptor expression and myometrial sensitivity at labor onset in the sheep. Biol Reprod. 2005;72(4):937–43. doi: 10.1095/biolreprod.104.035311. [DOI] [PubMed] [Google Scholar]
- 21.Kronig B. Transperitonealer, cervikaler kaiserschnitt. In: Doderlein A, Kronig B, editors. Operative gynakologie. 3. Leipzig: Georg Thieme; 1912. pp. 870–886. [Google Scholar]
- 22.Buhimschi CS, Zhao G, Sora N, Madri JA, Buhimschi IA. Myometrial wound healing post-Cesarean delivery in the MRL/MpJ mouse model of uterine scarring. Am J Pathol. 2010;177:197–207. doi: 10.2353/ajpath.2010.091209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhimschi CS, Buhimschi IA, Yu C, Wang H, Sharer DJ, Diamond MP, Petkova AP, Garfield RE, Saade GR, Weiner CP. The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment. Am J Obstet Gynecol. 2006;194:873–83. doi: 10.1016/j.ajog.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Buhimschi CS, Buhimschi IA, Patel S, Malinow AM, Weiner CP. Rupture of the uterine scar during term labour: contractility or biochemistry? BJOG. 2005;112:38–42. doi: 10.1111/j.1471-0528.2004.00300.x. [DOI] [PubMed] [Google Scholar]