Abstract
5′-Ester derivatives of the potent adenosine agonists N6-[4-[[[[4-[[[(2-acetylaminoethyl)amino] carbonyl] methyl] anilino] carbonyl] methyl] phenyl] adenosine (N-AcADAC; 1) and N6-cyclopentyladenosine (CPA; 2) were prepared as prodrugs. Both alkyl esters or carbonates (designed to enter the brain by virtue of increased lipophilicity) and 1,4-dihydro-1-methyl-3- [(pyridinylcarbonyl)oxy] esters designed to concentrate in the brain by virtue of a redox delivery system were synthesized. In the 5′-blocked form, the adenosine agonists displayed highly diminished affinity for rat brain A1-adenosine receptors in binding assays. The dihydropyridine prodrug 29 was active in an assay of locomotor depression in mice, in which adenosine agonists are highly depressant. The behavior depression was not reversible by peripheral administration of a non-central nervous system active adenosine antagonist. In an assay of the peripheral action of adenosine (i.e., the inhibition of lipolysis in rats), the parent compounds were highly potent and the dihydropyridine prodrug was much less potent.
Adenosine acts as a neuromodulator in the central nervous system (CNS)1 and as a regulator of the activity of other organs, for example the heart2 and kidneys.3 The role of adenosine is generally as a protective depressant of the activity of the organ in response to excessive oxygen demand and/or diminished oxygen supply.4 Centrally-acting adenosine agonists have sedative,1 antipsychotic,5 anticonvulsant,6 and cerebroprotective properties.7 For example, the adenosine agonist N6-cyclohexyladenosine has been reported to protect gerbils from damage to the hippocampus and to increase survival following ischemic injury.7,8 Thus, there is much interest in the therapeutic potential of adenosine agonists in the CNS.
Although central effects1,7,9 of potent adenosine agonists are highly pronounced at very low doses, there is an apparently contradictory observation that the common N6-substituted adenosine agonists are poorly absorbed into the brain, if at all.10 We reasoned that delivery of the adenosine agonist to the CNS through a prodrug scheme might allow brain-selective agents to be designed. The ubiquity of adenosine receptors makes a delivery scheme highly attractive for this class of drugs. Earlier attempts to use receptor-subtype-selective adenosine agonists in a therapeutic capacity were impeded by side effects at other organ sites.11 We examined the feasibility of the prodrug approach for adenosine agonists with known prodrug delivery schemes, including the dihydropyridine redox system developed and applied to many different classes of drugs by Bodor and colleagues.12
Results
Chemistry
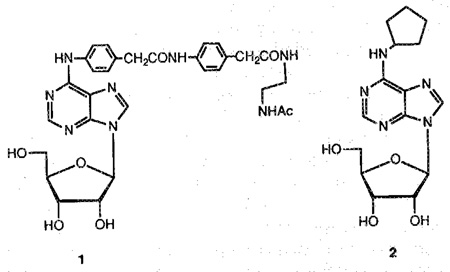
The synthesis of esters or carbonates of an alcohol is a general approach for developing prodrugs. This derivatization can increase stability13 and/or lipophilicity relative to the parent compound, thus improving the diffusion through plasma membrane or lipid barriers such as the blood-brain barrier. 5′-Esters of the adenosine agonist N6-(2,2-diphenylethyl)adenosine (CI-936), which has antipsychotic-like activity in rats,14 have been suggested as prodrugs with CNS activity. Therefore the synthesis of 5′-esters and 5′-carbonates of the potent adenosine agonists N6- [4- [[[[4- [[[(2-acetylaminoethyl)-amino]carbonyl]-methyl]anilino] carbonyl] -methyl] -phenyl]-adenosine15 (N-AcADAC; 1) and N6-cyclopentyladenosine (CPA; 2; see structures) may provide an approach for developing prodrugs of various A1-selective agonists.
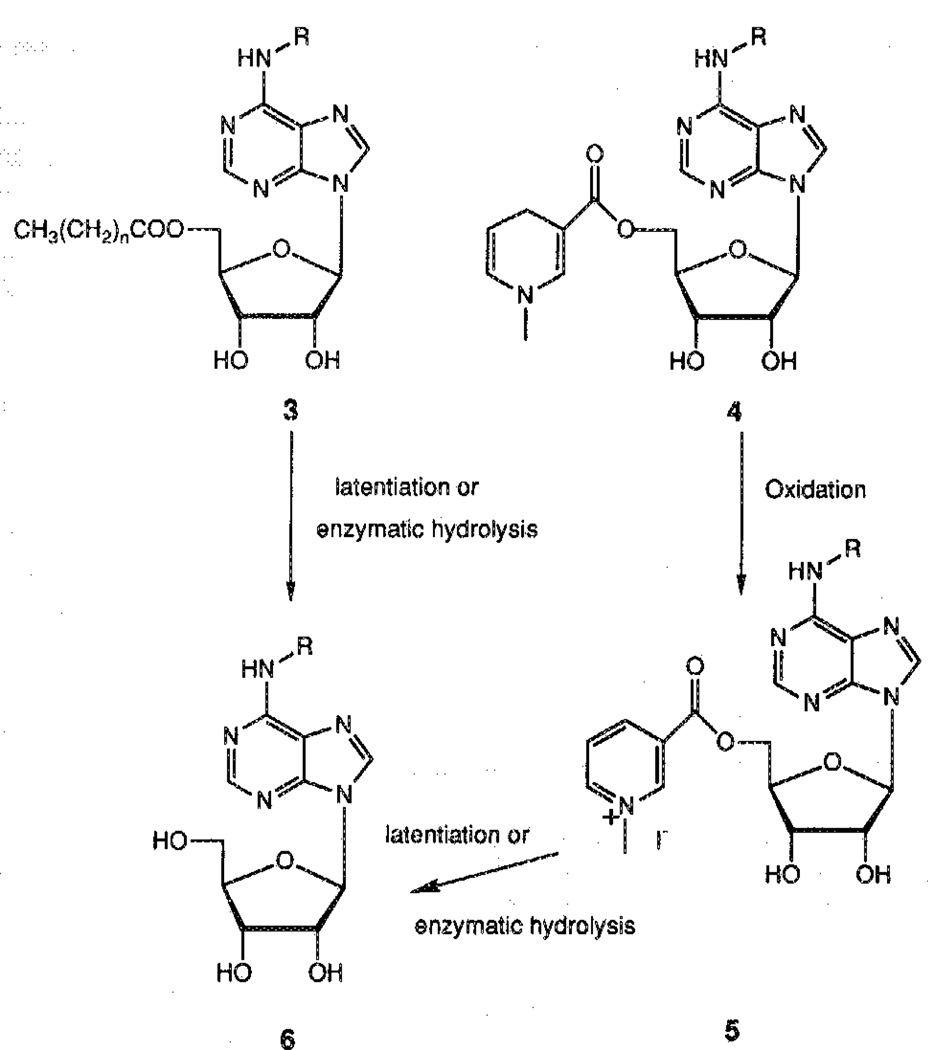
Both esters and carbonates, designed to enter the brain by virtue of increased lipophilicity, and l,4-dihydro-l-methyl-3-[pyridhiylcarbonyl)-oxy]esters, designed to concentrate in the brain by virtue of a redox delivery system,12 were synthesized. The intended mechanisms of activation of these prodrugs are shown in Scheme 1. The ester function may be hydrolyzed chemically (latentiation) or by an enzymatic mechanism (i.e., esterases). In the case of 5′-esters of alkyl carboxylic acids, we first attempted direct acylation of the free nucleoside 1 at the 5′-position by adding one equivalent of the appropriate acyl chloride or anhydride to a solution of nucleoside in a 1:1 mixture of pyridine and N,N-dimethylformamide (DMF). These conditions have been previously described for the selective 5′-(O-acylation) of purine nucleosides.16 Unfortunately, in both cases we obtained a mixture of uncharacterized products of poly(O-acylation), even at low temperature, and only a small amount of the desired product.
Scheme 1.
R = alkyl or aryl.
An alternative for the synthesis of 5′-O-acyl nucleosides was the sequence of (a) protection of both 2′- and 3′-hydroxyls, (b) acylation, and (c) deprotection of the 2′,3′-diol system. At first, the acetonide was chosen as the protecting group and introduced by reacting N-AcADAC 1 with 2,2-dimethoxypropane in the presence of camphorsulphonic acid. The resulting 2′,3′-acetonide 7 then reacted with acetyl chloride in the presence of 4-dimethylaminopyridine (DMAP) to give the corresponding 5′-acetyl derivative 8 in 42% overall yield.
Hydrolysis of the isopropylidene group required acidic conditions such as 1N HCl or Dowex 50H+, which also surprisingly caused a great deal of depurination. This sensitivity toward acidic medium may be explained by the influence of the N6 substituent on the overall basicity of the purine ring and hence the stability of the glycosidic bond.13 No deprotection was observed in refluxing water for 2h. No mildly acidic conditions for the deprotection that did not also cause depurination were found.
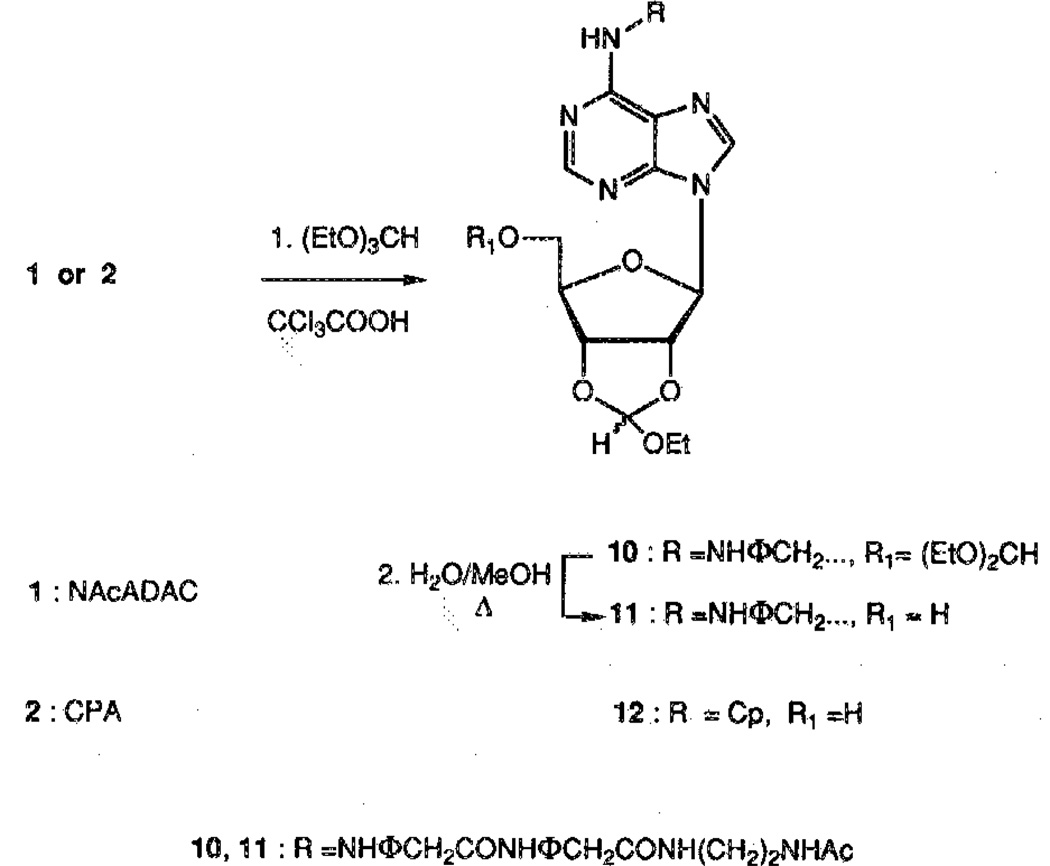
In an attempt to overcome this difficulty, a more labile group was introduced for the protection of the 2′,3′-diol system. Thus, the 2′,3′-ethoxymethynyl group was selected17 and was introduced by reaction of 1 with triethylorthoformate, which was used as a cosolvent because of the low solubility of 1 in dioxane. However, these conditions favored the formation of the undesired product 10 (Scheme 2). This problem was circumvented by refluxing the crude mixture in H2O/MeOH to selectively deprotect the 5′-OH of 10, giving 11 in 45% overall yield. In the case of CPA (2), the 5′-blocked by-product was converted to the desired compound during the purification on silica gel, giving the protected derivative 12 in one step with an 86% yield (Scheme 2).
Scheme 2.
Compounds 11 and 12 were subsequently treated with the appropriate anhydride in presence of a catalytic amount of DMAP in DMF to give esters 13–16 in high yield (Scheme 3). These conditions have been shown to be more efficient than the system of acyl chloride/DMF/pyridine previously used.16 The final deprotection to give 19–22 was carried out under mild conditions [5% trifluoroacetic acid (TFA) in chloroform/methanol]. The final product generally precipitated from this solvent mixture in yields of 85–94% (Scheme 3).
Scheme 3.
The carbonate derivatives were obtained in two steps starting from 12. Reaction with 1,1′-carbonyldiimidazole gave the activated esters that reacted in situ with the appropriate alcohol followed by treatment with 5% TFA to give 25–26 (Scheme 4).
Scheme 4.
We have employed the diol protection scheme involving the acid labile 2′,3′-ethoxymethynyl group to obtain the nicotinyl derivatives 17 and 18. This follows a general route previously applied to derivatize the 5′-hydroxyl group of nucleosides, as in the synthesis of the 3′-azidothymidine (AZT) derivative.18 Nicotinic anhydride, prepared from nicotinic acid and phosgene,19 reacted with 11 or 12 to give the corresponding esters 17 or 18, respectively (Scheme 3). The nicotinyl ester was then quaternized using methyliodide in acetone under pressure. The protecting group was removed with 5% TFA before the reduction step because of the instability of the final derivatives 29 and 30 in acidic medium (Scheme 5) to provide the trigonellate esters 27 and 28, respectively, in roughly 70% yield.
Scheme 5.
We attempted the synthesis of the pyridinium derivatives 27 and 28 as well by the direct esterification of 1 or 2 with trigonelline (1-methylpyridinium-3-carboxylate) with a carbodiimide with 1-hydroxybenzotriazole included as a catalyst. The reaction failed, probably because the positive charge on the nitrogen of the trigonelline diminished its reactivity in coupling.
Attempts to reduce the pyridinium salt to the 1,4-dihydropyridine in basic medium according to a known procedure12,18 were unsuccessful. The pyridinium derivatives 27 and 28 were unstable in basic medium, as is usually required to stabilize the dihydropyridine formed in the reduction step, and decomposed to form the starting materials 1 and 2. We found that by carrying out this reaction in a pH 7 buffered solution, both the pyridinium salt and the dihydropyridine formed were stable (Scheme 5). In the case of the N-AcADAC derivative, the desired product 29 precipitated from the reaction mixture. In the case of the CPA derivative 30, a biphasic system was used, and the ethyl acetate layer containing the product was regularly withdrawn during the course of the reaction.
Competitive Binding Assays
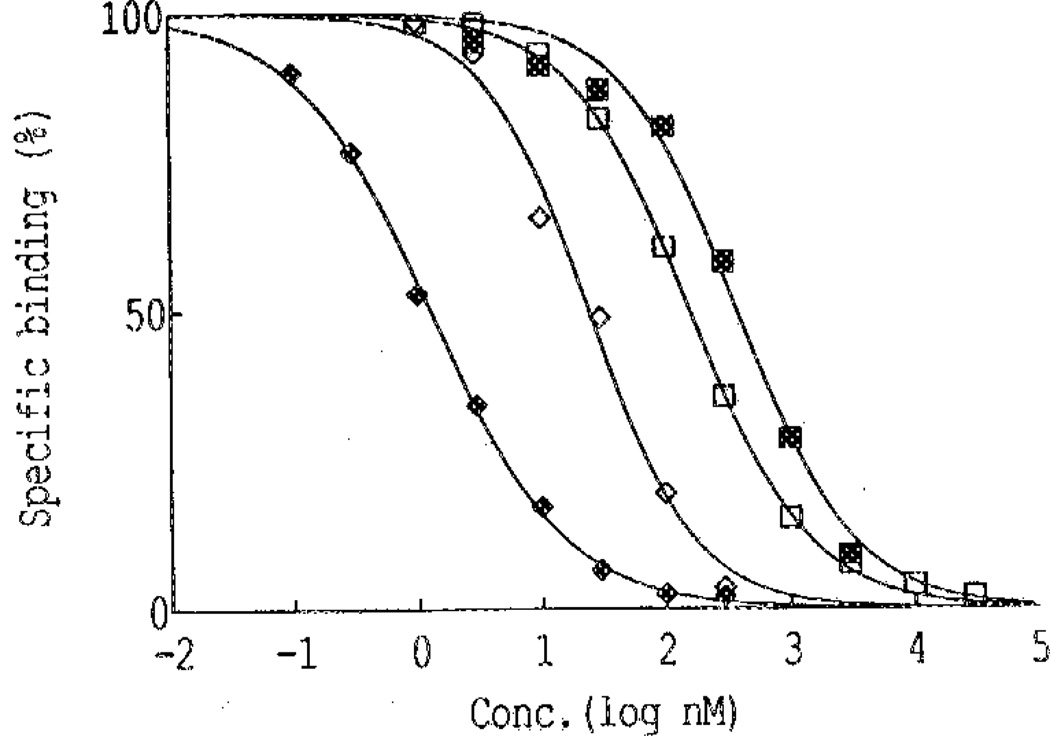
The analogues were tested in a radioligand binding assay for affinity at A1-adenosine receptors in rat cerebral cortical membranes. Blocking either the 2′,3′-diol function or the 5′-hydroxyl function greatly diminished the receptor affinity. In the displacement of [3H]-N6-phenylisopropyladenoeine ([3H]PIA), an A1-selective agonist, N-AcADAC (1) displayed a Ki (inhibition constant) value of 9.3 ± 1.7 nM (Table 1). The intermediates 11 and 13, both containing the ethoxymethyne protecting group at the 2′,3′-position, resulted in incomplete displacement curves, and 50% inhibitory concentration (IC50) values were not obtained in the concentration range studied. The corresponding 5′-O-acyl blocked derivatives of N-AcADAC, 19–21, and the 5′-O-carbonates of CPA, 25–26, displaced the radioligand with a characteristic curve with an apparent Ki value ranging from 57 to 140 nM (figure 1).
Table 1.
Effects of Derivatization of N-AcADAC and CPA on the Affinity at A1-Adenosine Receptors
| Compound |
A1-Receptora |
|
|---|---|---|
| Number | Name | Ki, nM |
| 1 | N-AcADAC | 9.3 ± 1.7 |
| 11 | 2′,3′-EM-N-AcADACb | > 1000 |
| 13 | 5′-Acetyl-2′,3′-EM-N-AcADACb | > 1000 |
| 19 | 5′-Acetyl-N-AcADAC | 140 ± 6.0 |
| 20 | 5′-Valeroyl-N-AcADAC | 57.6 ± 67 |
| 21 | 5′-Stearoyl-N-AcADAC | 125 ± 4.5 |
| 27 | 5′-Pyridinium ester of N-AcADAC | 44.8 ± 6.0 |
| 29 | 5′-DHP ester of N-AcADAC | 140 ± 6.8 |
| 31 | ADAC | 0.85 ± 035c |
| 2 | CPA | 0.59 ± 0.02d |
| 22 | 5′-Acety1-CPA | 0.43 ± 0.19 |
| 25 | 5′-Butyloxycarbonyl-CPA | 74.7 ± 5.8 |
| 26 | 5′-Benzyloxycarbonyl-CPA | 113 ± 10 |
IC50 values for A1 receptors were obtained from competition for binding of 1 nM [3H] R(−)PIA to cerebral cortical membranes; Ki = IC50/1 + Concentration [3H] R(−)PIA/Kd for R(−) PIA (the Kd value is equal to ≈ 1 nM). Values are means ± SEM for two of three experiments, each of which included duplicate or triplicate determinations for each point of the curve.
EM = ethoxymethynyl.
From ref 15.
From ref 20.
Figure 1.
Displacement of specific [3H]R-PIA binding to A1 receptors in rat cerebral cortical membranes by 22 (♦), 1 (◊), 25 (□), and 21 (■). The data are expressed as a percentage of total specific binding. Incubation was carried out for 60 min at pH 7.4 and 37 °C with a 1 nM concentration of radioligand. Data for 19 and 20, and 26 are very similar to those for 21 and 25, respectively.
Compound 22 had an apparent affinity in the nanomolar range. The stability of two prodrugs 22 and 19 in binding assay conditions was investigated by HPLC. For compound 22 after 1.5 h, ~60% had been converted to CPA (2) when the ratio [prodrug]/[tissue] was 45 times higher than in the competitive binding assay (limit of the HPLC detection). Total hydrolysis to CPA (2) may therefore be expected during the binding assay and may be the reason for the potent displacement of radioligand (Figure 1). In the case of 19, 35% hydrolysis occurred for a [prodrug]/[tissue] ratio 10 times higher than that in the competitive binding assay. Therefore, we can assume that the potency of the 5′-acetyl prodrugs is lower than indicated by the Ki values measured.
Locomotor Studies
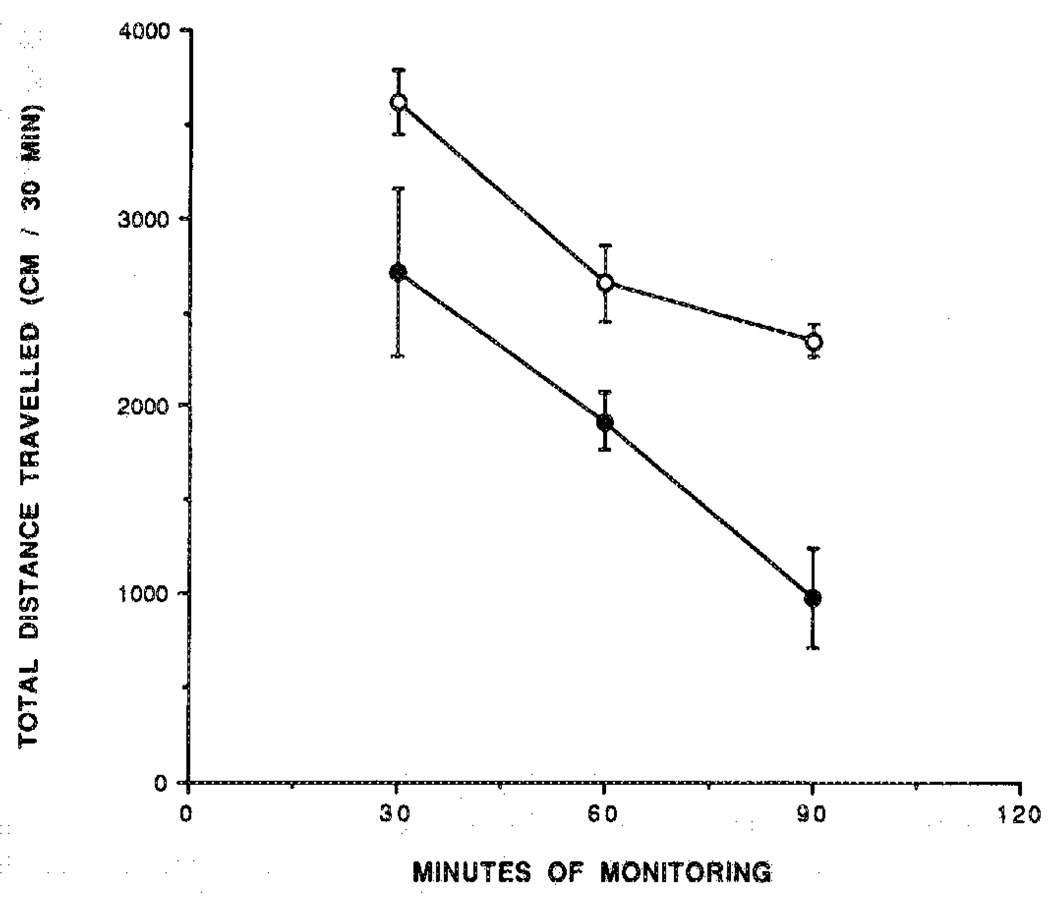
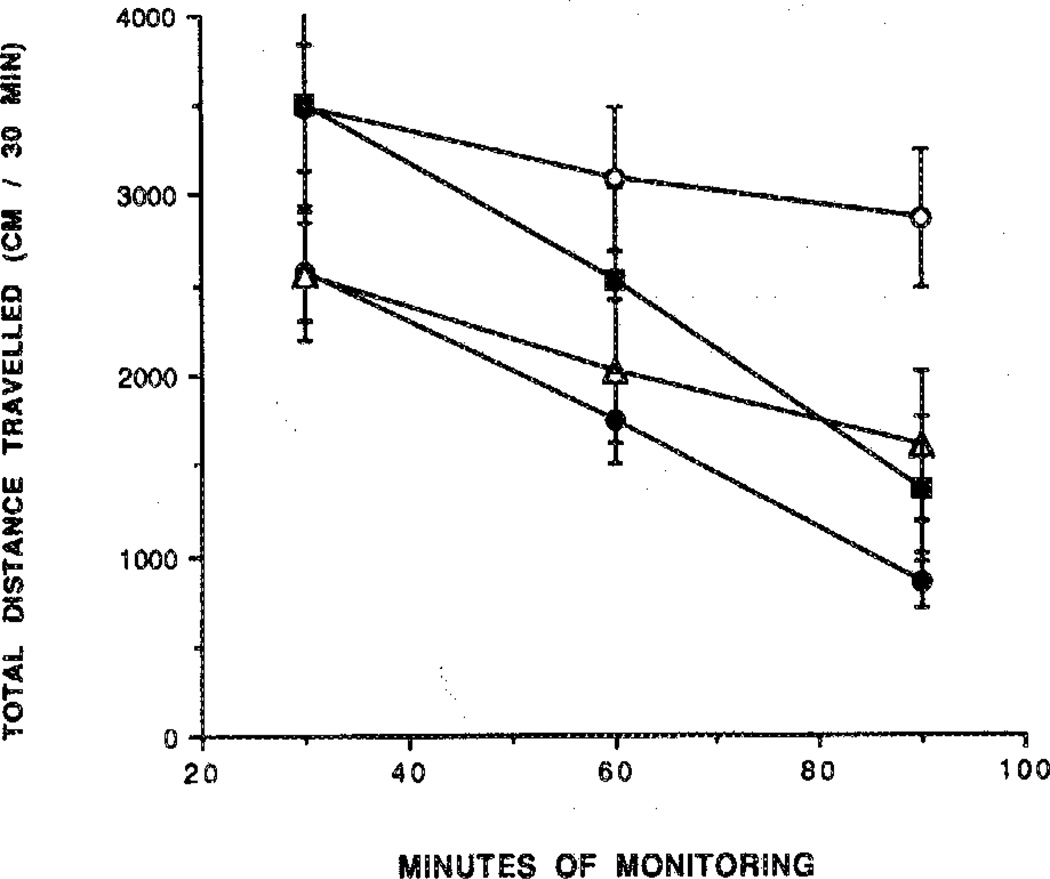
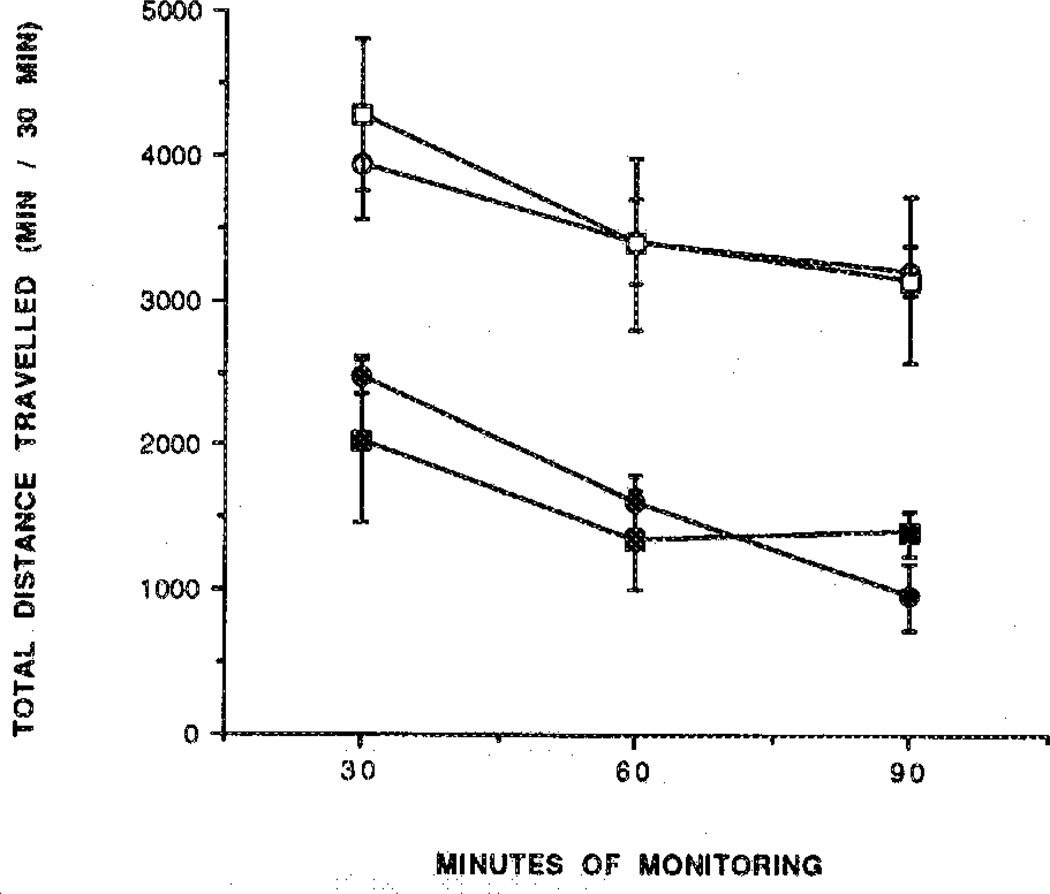
Adenosine agonists cause intense behavioral effects at low doses (1–100 µg/kg body weight in mice).1,9 Several derivatives were examined in a behavioral assay,9 with a computerized animal activity monitor cage. The locomotor activity of mice, control and drug treated, was followed over a 90-min time course, with measurements made in 10-min intervals (Figures 2–6). The dihydropyridine 29 caused locomotor depression in mice (Figure 3), and the time course of the effect was similar to previously examined adenosine agonists.20 The locomotor depression elicited by 29 was partly reversed by a centrally acting antagonist (8-cyclopentyl-1,3-dipropylxantbine; CPX) at a dose of 1 mg/kg during the first 60-min period (Figure 3). Interpretation of these results is complicated by the surprising depressant effect of CPX. This locomotor depression was not antagonized by the peripherally selective adenosine antagonists 8-(p-sulfophenyl)-theophylline (8-PST)2 at a dose of 5 mg/kg (Figure 4) and 1,3-dipropyl-8-(p-(Carboxyethynyl)phenyl)xanthine (BW1433) at a dose of 4 mg/kg (data not shown). The pyridinium derivative 27 did not produce significant depression during a 2.5-h period (Figure 5). The acetyl ester prodrug 19 did not have a depressant effect (Figure 5), whereas 5′-O-acetyl-CPA, 22 (Figure 6), elicited locomotor depression during the first 50 min of the monitoring period.
Figure 2.
Locomotor activity in control mice (○) and in mice injected ip with the adenosine agonist N-AcADAC (1; 440 nmol/kg; ●) during 90 min. Activity counts for total distance traveled (mean ± SEM) are shown in relation to time elapsed since the beginning of monitoring in a Digiscan activity monitor that was initiated 10 min after ip injection (n = 6–11).
Figure 6.
Locomotor activity over 2 h for control mice (○) and for mice injected ip with the prodrug 22 (●; 305 nmol/kg). Activity counts for total distance traveled (mean ± SEM) are shown in relation to time elapsed sine the beginning of monitoring in a Digiscan activity monitor that was initiated 10 min after ip injection (n = 6–10).
Figure 3.
The effects of the A1-selective adenosine antagonist CPX on locomotor depression induced by 29. Mice ware injected ip with the prodrug 29 (365 nmol/kg) in the absence (●) or presence (■) of CPX (1 mg/kg). The locomotor activity in control mice in the absence (○) or presence (Δ) of CPX (1 mg/kg) is represented by the open symbols. The antagonist or vehicle was injected 10 min before the agonist. Activity counts for total distance traveled (mean ± SEM) are shown in relation to time elapsed since the beginning of monitoring in a Digiscan activity monitor that was initiated 10 min after ip injection (n = 5–8). The level of significance was p < 0.005.
Figure 4.
Non-reversal by the peripherally selective adenosine antagonist 8-PST of the locomotor depression by 29. Mice were injected ip with the prodrug 29 (365 nmol/kg) in the absence (●) or presence (■) of 8-PST (5 mg/kg). The locomotor activity in control mice in the absence (○) or presence (□) of 8-PST (5 mg/kg) is represented by the open symbols. The antagonist or vehicle was injected 10 min before the agonist. Activity counts for total distance traveled (mean ± SEM) are shown in relation to time elapsed since the beginning of monitoring in a Digiscan activity monitor that was initiated 10 min after ip injection (n = 5–8). The level of significance was p < 0.005.
Figure 5.
Locomotor activity over 2.5 h in control mice (○) and for mice injected ip with the 5′-O-acetyl prodrug 19 (●; 410 nmol/kg) or pyridinium compound 27 (□; 310 nmol/kg). Activity counts for total distance traveled (mean ± SEM) are shown in relation to time elapsed since the beginning of monitoring in a Digiscan activity monitor that initiated 10 min after ip injection (n = 5–10).
Serum Glycerol Studies
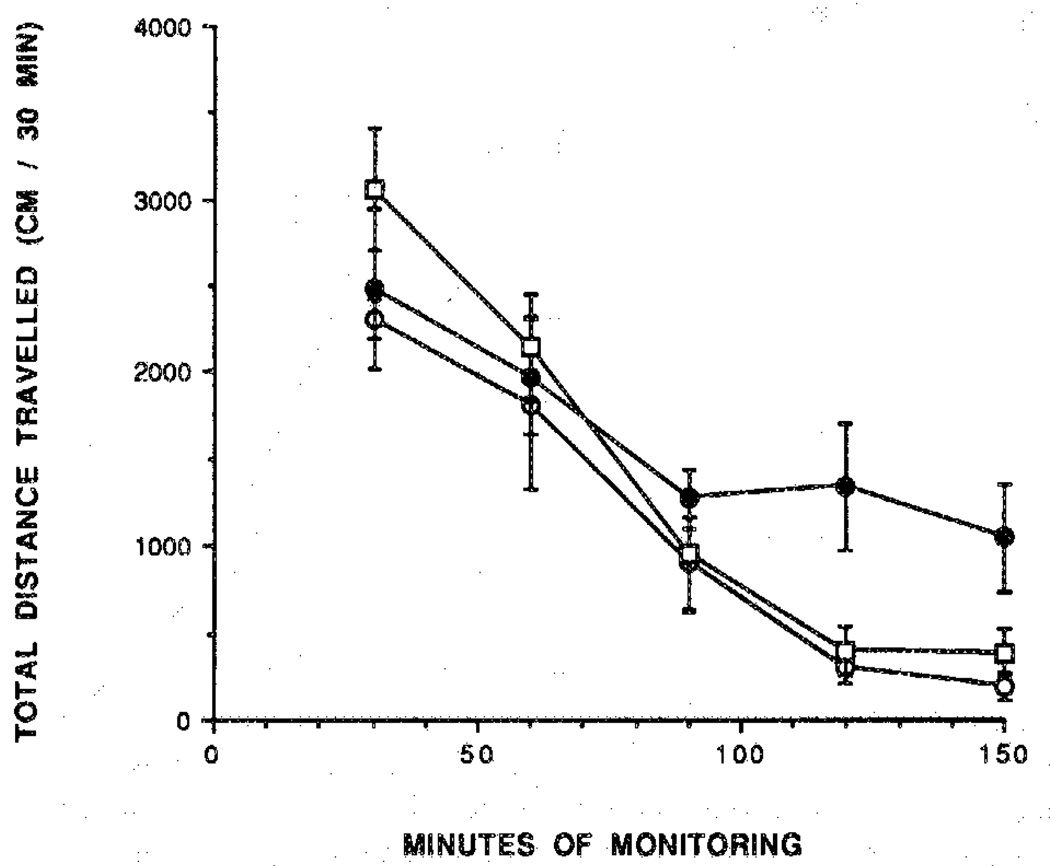
Activation of A1 receptors in adipocytes is known to inhibit lipolysis.22 Two potent adenosine agonists (1 and 2) were examined as inhibitors of lipolysis in vivo in rats at 25–45 mmol/kg and were found to lower serum glycerol levels. The maximum effect was seen at 30–60 min post intraperitoneal (ip) injection (Figure 7). After 60 min, the effects diminished slowly. Three prodrugs (19,22, and 29) were tested in this assay at approximately a 10-fold dose relative to the parent drug. Compounds 19 and 22 resulted in a rapid decrease of the serum glycerol level. This effect persisted over the 90-min period (Figure 8). The dihydropyridine derivative 29 (Figure 9) had a slower onset of action and was much less potent than the corresponding 19 at a comparable dose. The antilipolytic effect elicited by adenosines agonists such as 19 and 22 was partly or fully antagonized by the peripherally selective adenosine antagonist BW143323 (Figure 8).
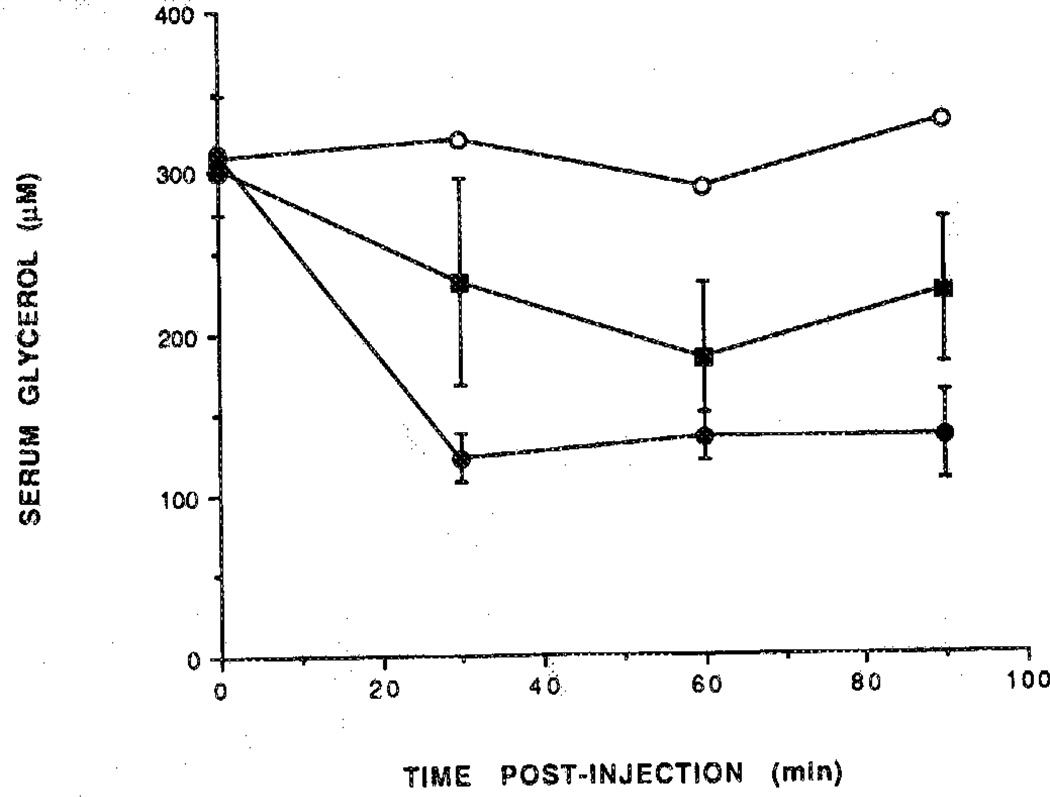
Figure 7.
Time course of glycerol level. Rats were injected with (■) N-AcADAC (1; 48 nmol/kg) or (●) CPA (2; 27 nmol/kg) and compared with control (○). Blood samples were taken from the tail vein at regular intervals over a 90-min period and assayed for glycerol (n = 3).
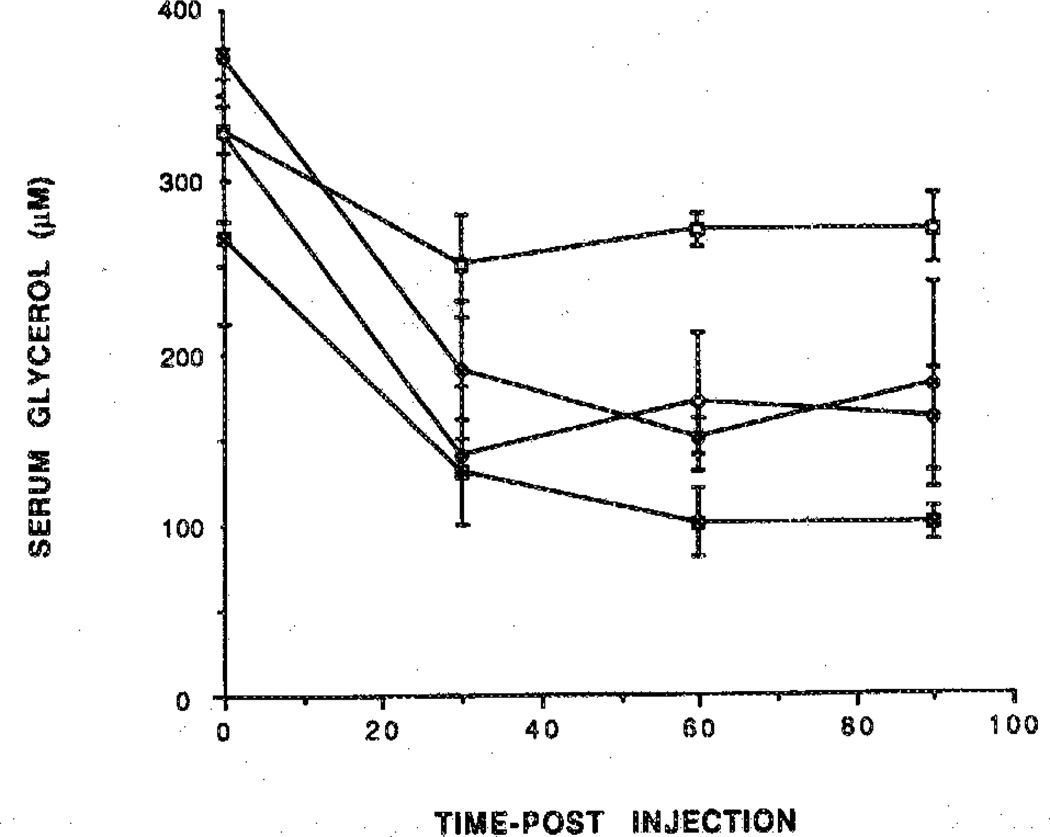
Figure 8.
Reversal by the peripherally selective adenosine antagonist BW 1433 of inhibition of lipolysis elicited by prodrugs of adenosine agonists. Each agonist was injected immediately after the antagonist. The activity reflects the serum glycerol concentration over a 90-min period after the injection. Rats were treated with the prodrug 19 (300 nmol/kg) in the absence (○) or presence of BW 1433 (●; 4 mg/kg) or with the prodrug 22 (320 nmol/kg) in the absence (□) or presence (■) of BW 1433 (4 mg/kg). Blood samples were taken from the tell vein at regular intervals and assayed for glycerol (n= 3).
Figure 9.
Time course for glycerol levels in rats injected with the prodrug 29 at three doses [270 nmol/kg (○), 540 nmol/kg (●), or 810 nmol/kg (■)] compared with control (□). Blood samples were taken from the tail vein at regular intervals over a 90-min period and assayed for glycerol (n=3).
Discussion
Two approaches to enhancing brain delivery of adenosine agonists have been explored: (1) increasing lipophilicity through formation of an ester or a carbonate at the 5′-hydroxyl position,14 designed to be cleaved by nontissue specific mechanisms, and (2) applying a dihydropyridine chemical delivery system developed by Bodor and co-workers.12 The latter approach has been demonstrated to be a successful CNS prodrug scheme for a variety of drug classes.17 Two prototypical adenosine agonists were subjected to this derivatization: (1) CPA, known to be highly A1-selective,24 and (2) N-AcADAC, a moderately A1 selective functionalized congener derivative.25
Adenosine agonists, when administered peripherally, cause locomotor depression through a centrally mediated mechanism. Evidence for central mediation of the depressant effect is that low agonist doses (i.c.v.) are effective and that peripherally selective antagonists, such as p-sulfophenylxanthine derivatives and BW1433, are inactive in reversing the CNS-mediated effects of adenosine agonists in previous studies.26 The nonselective xanthines caffeine and theophylluse and the A1-selective antagonist, CPX, which freely cross the blood brain barrier,21 reverse adenosine–agonist-induced behavioral depression.9,26
The locomotor depressant effect elicited by the dihydropyridine prodrug 29 demonstrates that a sufficient among of an active drug is generated to produce an effect comparable to 1. A biotransformation is most likely occurring because 29 is much less active on its own. The lack of reversal by 8-PST and the partial reversal by the antagonist CPX of locomotor depression induced by 29 suggest that this effect occurs mainly via central adenosine receptors. The absence of locomotor depression observed in the case of the quaternary derivative 27 can be attributed to the inability of this ionic derivative to cross the blood–brain barrier.
Furthermore the lack of locomotor effects elicited by 27 shows that cleavage of this pyridinium 5′-ester-linked prodrug moiety does not occur readily in the circulatory system. In the CPA series, the prodrug 22 elicited rapid locomotor depression that is consistent with the rapid cleavage of the prodrug moiety (acetyl ester), as observed under conditions of the binding assay.
The effect of adenosine agonists to diminish serum glycerol levels in vivo is predominantly peripheral.26 The relative activity of 1 and 2 in inhibiting lipolysis is consistent with the binding data for these potent agonists. The cleavage of the prodrug moiety of 19 in the periphery to generate the parent compound and/or the intrinsic activity of the prodrug can account for the inhibition of lipolysis measured. Because 19 is not centrally active, we can rule out the possibility of rapid cleavage of the prodrug moiety in the circulatory system and we may have measured here a peripheral action elicited by the prodrug itself. Compound 22, on the other hand, is probably rapidly cleaved to give 2, as expected on the basis of its instability in the binding assay, resulting in activity with rapid onset both in CNS and in the periphery. The lower potency of 29 in the peripheral activity (lipolysis) may result from elimination of the adenosine derivative from the general circulation after oxidation of the carrier moiety to form the ionic pyridinium salt 27.
In summary, we have synthesized simple 5′-ester derivatives of potent A1-adenosine agonists and dihydropyridinium esters intended to be activated through oxidation and cleavage according to the redox scheme of Bodor and co-workers.12 The simple esters, especially derivatives of CPA, tended to be cleaved nonselectively and did not accumulate in the brain as a result of enhanced lipophilicity. In general, 5′-ester derivatives of N-AcADAC were more stable both in vitro and in vivo than ester derivatives of CPA. In contrast to the simple esters, the dihydropyridine ester of N-AcADAC displayed considerable CNS depressant activity typical of potent adenosine agonists. The periperal effects of this derivative were present but less pronounced; thus, the dihydropyridine derivative of N-AcADAC was not totally selective for the CNS. These data indicate that the dihydropyridine chemical delivery system reduces peripheral action of an A1-agonist without reducing its central-mediated action.
Experimental Section
Chemistry
New compounds were characterized (and resonances assigned) by 300 MHz 1H NMR spectroscopy with a Varian XL-300 FT-NMR spectrometer. Unless noted, chemical shifts are expressed as ppm downfield from tetramethylsilane. Synthetic intermediates were characterized by chemical ionization mass spectrometry (CIMS, NH3) with a Finnigan 1015 mass spectrometer modified with EXTREL electronics or on a Finnigan 4500 MS. The 252Cf-plasma-desorption mass spectra were obtained as previously described.27 Accurate mass [with fast atom bombardment (FAB)-MS] was measured on a JEOL SX102 high-resolution mass spectrometer. HPLC analyses were run on a HP 1090 with diode array detector. Melting points were determined on a Thomas-Hoover melting point apparatus (uncorrected). The C, H, and N analysis was carried out by Atlantic Microlabs (± 0.4% acceptable). Dragendorff’s spray reagent was from Sigma (St. Louis, MO).
N6-[4-[[[4-[[[N-Acetyl(2-aminoethyl)amino]carbonyl]methyl]anilino]-carbonyl]-methyl]phenyl]-adenosine (1) was prepared by the route previously described,15 starting from 6-chloropurine riboside in a 15% overall yield. An improvement was made in the preparation of the methyl ester intermediate by recrystallizing from a mixture of methanol: chloroform (1:1). CPA (2) was synthesized as described previously.28
N6-[4-[[[4-[[[N-Acetyl(2-aminoethyl)amino]carbonyl]-methyl]anilino]carbonyl]methyl]phenyl]-2′,3′-O-ethoxy-methylene adenosine (11)
A suspension of N-AcADAC (1; 300 mg, 0.48 mmol) in dioxane (15 mL) was sonicated for 10 min. After adding trichloroacetic acid (160 mg, 0.96 mmol), triethylorthoformate in excess, and 4 Å molecular sieves, the temperature of the suspension was raised to 110 °C over a 5-min period and kept at this temperature for 10 min more until the solution became clear. After cooling, the solution was poured into 120 mL of ether. The precipitate 10 (characterized by 1H NMR) and MS (252Cf-plasma-desorption; m/z 799 MNa) was collected and immediately dissolved in 10 mL of water:methanol (1:1) and heated at 80 °C for 10 min. After concentration, the crude product was adsorbed onto silica gel and purified by flash chromatography on silica gel, with 200 mL of methanol:chloroform (1:9) as an eluent. Evaporation of the solvent gave 160 mg (47%) of 11; 1H NMR δ (DMSO-d6): 1.1 & 1.2 (t, 3H, CH3 endo/exo, J = 7 Hz), 1.78 (s, 3H, acetamide), 3.06 (bs, 4H, CH2CH2), 3.28 (s, 2H, CH2CO), 3.58 (s, 2H, CH2CO), 3.6–3.7 (m, 4H, CH2O & 5′-H), 4.2 & 4.3 (m, 1H, 4′-H), 4.95 & 5.08 (dd, 1H, 3′-H endo/exo, J3′,4′ = 3.5 Hz, J3′,2′ = 7.3 Hz), 5.12 (t, 1H, OH), 5.42 (dd, 1H, 2′-H, J1′,2′ = 3.1 Hz), 6.15 & 6.22 (s, 1H, O2CH endo/exo), 6.20 & 6.32 (d, 1H, 1′-H), 7.15, 7.28 & 7.50 (d. 6H, arom), 7.82 (m, 3H, arom & NH), 8.0 (bs, NH), 8.30,8.39,8.50 & 8.56 (s, 2H, 2-H & 8-H), 9.90 & 10.10 (s, 2H, NH); MS (252Cf-plasma-desorption): m/z 697.3 MNa.
Anal.—Calcd (C33H38N8O3·1.33 H2O) H, N.
N6-Cyclopontyl-2′,3′-O-ethoxymethylene-adenosine (12)
CPA (2; 500 mg, 1.5 mmol) and trichloroacetic acid (500 mg, 3 mmol) were dissolved in dioxane (25 mL), and the mixture was stirred at room temperature over molecular sieves. Triethylorthoformate (2 mL, 12 mmol) was added, and the solution was heated at 60 °C for 1 h under a nitrogen atmosphere. Sodium bicarbonate (1 g, 12 mmol) was added at 0 °C and, after 1 h of vigorous stirring, the mixture was filtered and the solvent evaporated. The residue was purified by flash column chromatography on silica gel with chloroform/methanol (2%) as an eluent. Evaporation of the solvent from the pure fraction afforded 480 mg of 12 in nearly quantitative yield; mp, 61–64 °C; 1H NMR δ (DMSO-d6): 1.1 & 1.2 (t, 3H, CH3 endo/exo, J = 7 Hz), 1.6, 1.7 & 1.95 (m, 8H, Cp), 3.5–3.7 (m, 4H, CH2O endo/exo & 5′-H), 4.2 & 4.3 (m, 1H, 4′-H), 4.5 (m, 1H, CHN), 4.95 & 5.05 (dd, 1H, 3′-H endo/exo, J3′,4′ = 3.5 Hz, J3,′,2′ = 7.1 Hz), 5.40 & 5.46 (dd, 1H, 2′-H endo/exo, J2′,1′ = 3.1 Hz), 6.17 & 6.21 (s, 1H, O2CH endo/exo), 6.28 (d, 1H, l′-H), 7.78 & 8.2 (bs, 1H endo/exo, NH), 8.32 & 8.4 (s & d, 2H, 2-H & 8-H); MS (CI-NH3): 392 M + 1, 204 Base + 2; high-resolution MS [FAB/3-nitrobenzyl alcohol (NOBA) matrix]: m/e 392.1945, M + 1,392.1934 calcd for C18H26N5O5.
Anal.—C18H25N5O5·0.5CHCl3) C, H.
N6-[4-[[[4-[[[N-Acetyl(2-aminoethyl)amino]carbonyl]-methyl]anilino]carbonyl]methyl]phenyl]-5′-O-(3-pyridinylcarbonyl)-2′,3′-O-ethoxy-methylene-adenosiae (17)
To a solution of 11 (265 mg, 0.31 mmol) and DMAP (10 mg, 0.06 mmol) in DMF (12 mL) was added nicotimc anhydride (105 mg, 0.36 mmol), prepared by treatment of nicotinic acid with phosgene.19 The reaction mixture was stirred at room temperature overnight and the solution was poured into 150 mL of ether; the white solid obtained was collected, washed with ether, and dried under reduced pressure (270 mg, 88%) mp, 152–153 °C; 1H NMR δ (DMSO-d6): 1.12 & 1.22 (t, 3H, CH3 endo/exo, J = 7 Hz), 1.75 (s, 3H, acetamide), 2.95 (bs, 4H, CH2CH2), 3.05 (s, 2H, CH2CO), 3.58 (s, 2H, CH2CO), 3.4–3.6 (q, 2H, CH2O), 4.40–4.60 (m, 2H, 5′-H), 4.68 (m, 1H, 4′-H), 5.24 (dd, 1H, 3′-H endo/exo, J3′,4′ = 3.5 Hz, J3′,2′ = 7.1 Hz), 5.65 (m, 1H, 2′-H endo/exo), 6.21 & 6.28 (s, 1H, O2GH endo/exo), 6.36 (d, 1H, l′-H, J2′,1′ = 2.7 Hz), 7.15, 7.26 & 7.50 (d, 6H, arom), 7.55 (m, 1H, 5″-H), 7.85 (m, 3H, arom & NH), 8.0 (bs, NH), 8.22 (d, 1H, 4″-H), 8.44 & 8.50 (s, 2H, 2-H & 8-H), 8.80 (d, 1H, 8.32, 6″ -H), 9.15 & 9.40 (bs, lH, 2″-H), 9.90 & 10.10 (s, 2H, NH); MS (252Cf-plasma-desorption): m/z 802.4 MNa.
Anal.—Calcd (C39H41N9O9·1 H2O) H, N.
N6-Cyclopentyl-5′-O-(3-pyridinylcarbonyl)-2′;3′-O-ethoxymethylene-adenoaine (18)
The procedure described for 17 was followed with 440 mg of 12 (1.27 mmol), 350 mg of nicotinic anhydride (1.52 mmol), and DMAP (31 mg, 0.25 mmol) to yield 18 (329 mg, 59%) as a colorless oil. An analytical sample was obtained after passage through a short column of silica gel (eluent: 2% methanol in chloroform).
General Procedure for 5′-Alkylesters of N6-[4-[[[4-[[[N-Acetyl-(2-aminoethyl)amino]carbonyl]methyl]anilino]carbonyl]methyl]-phenyl]-adenosine (19–21)
Compound 11 (47 mg, 0.07 mmol) was suspended in 2 mL of DMF and treated with a solution of stearic anhydride (42 mg, 0.08 mmol) in 2 mL of methylene dichloride and DMAP (8 mg, 0.07 mmol). The mixture was warmed to 40 °C until the solution became clear and then stirred for 2 h at 40 °C. The product was isolated upon addition of 15 mL of phosphate buffer (pH 7). The resulting precipitate was filtered, washed with ether, and dried. The crude yield of 15 was 30 mg (46%). This material (30 mg, 0.025 mmol) was dissolved in 4 mL of a mixture of chloroform and methanol (1:1). TFA (200 mL, 5%) was added at 0 °C, and the mixture was allowed to reach room temperature overnight. The precipitation of a white solid was completed by adding 1 mL of water. The precipitate 21 (28 mg, 100%) was collected, triturated with ethyl acetate, and dried under reduced pressure overnight; mp, 227–229 °C; 1H NMR δ (DMSO-d6); 0.8 (m, 3H, CH3Stearyl), 1.20 (m, 28H, CH2Stearyl), 1.45 (m, 2H, CH2-Stearyl), 1.75 (s, 3H, acetyl), 2.30 (t, 2H, CH2Stearyl), 3.08 (bs, 4H, CH2CH2), 3.30 (s, 2H, CH2CO), 3.60 (s, 2H, CH2CO), 4.08 (m, 1H, 4′-H), 4.20 (dd, 1H, 5′-H, J5′,4′ = 6.1 Hz, Jgem = 11 Hz), 4.30 (m, 2H, 3′-H & 5′-H), 4.70 (m, 1H, 2′-H), 5.38 (d, 1H, OH), 5.60 (d, 1H, J1′,2′ = 4.5 Hz), 5.98 (d, 1H, 1′-H), 7.15, 7.28 & 7.50 (d, 6H, arom), 7.85 (d, 2H, arom), 8.0 (bs, 1H, NH), 8.38 & 8.48 (s, 2H, 2-H & 8-H), 9.90 & 10.10 (s, 2H, NH); MS (FAB.Pos.): m/z 885 MH.
Anal.—Calcd (C48H68N8O8·0.5 CHCl3) C, H, N.
N6-Cyelopeatyl-5′-O-acyl-adeaosine (22)
To a solution of 12 (80 mg, 0.20 mmol) and DMAP (5 mg, 0.01 mmol) in DMF (3 mL) was added acetic anhydride (25 mL, 0.21 mmol), and the reaction mixture was stirred at room temperature overnight. Water was added and the solution was extracted with ether (2 × 10 mL). The combined ether layers were washed with water (3 × 5 mL). The organic phase was dried over Na2-SO4 and filtered. The solvent was evaporated to yield, after drying under reduced pressure overnight, 16 (80 mg, 90%) as a white solid. Compound 16 (600 mg, 1.59 mmol) was dissolved in 12 mL of chloroform, TFA (500 mL, 4%) was added at 0 °C, and the mixture was stirred at 8 °C for 2 days. The solvent was coevaporated with ethanol, and the residue obtained was purified by flash chromatography with ethyl acetate followed by ethyl acetate:methanol (9:1). After concentration of the desired fraction and trituration of the oil with hexane, 310 mg of the product 22 (56%) were isolated; mp, 114–116 °C; 1H NMR δ (DMSO-d6): 1.5 to 1.7 (m, 8H, Cp), 2.0 (s, 3H, Acetyl), 4.10 (m, 1H, 4′-H), 4.20 (dd, 1H, 5′-H, J5′,4′ = 6.0 Hz, Jgem = 11 Hz), 4.25 (t, 1H, 3′-H, J3′,4′ = 4.9 Hz, J3′,2′ = 4.9 Hz), 4.30 (dd, 1H, 5′-H, J5′,4′ = 3.5 Hz), 4.65 (t, 1H, 2′-H, J2′,1′ = 4.9 Hz), 5.94 (d, 1H,-1′-H), 8.27 & 8.39 (s, 2H, 2-H & 8-H); MS (CI-NH3): 378 M + 1, 204 Base + 2.
Anal.—Calcd (C17H23N5O5) C, H, N.
General Procedure for 5′-Carbonates of N6-Cyclopentyladenosine (25–26)
Compound 12 (0.25 mmol) was treated with 1.1 equivalent of 1,1′-carbonyldiimidazole in DMF for 1 h at room temperature. After addition of an excess of the appropriate alcohol, the reaction mixture was refluxed overnight. Water was added, and the solution was extracted with ether. The combined ether layers were washed with water, dried over Na2SO4, and concentrated. The residue was purified by flash column chromatography on silica gel with hexane: ethyl acetate (1:2) as an eluent. Evaporation of the solvent from the pure fraction afforded 23 and 24 in moderate and good yields, respectively. Subsequent deprotection using the same conditions as described for 22 gave 25 (mp, 65 °C) and 26 (mp, 95 °C) in almost quantitative yields; 25: 1H NMR δ (DMSO-d6): 0.85 (t, 3H, CH3), 1.35 (2H, CH2), 1.55 (m, 6H, CH2 & Cp), 1.7 & 1.95 (m, 4H, Cp), 4.10 (m, 3H, 4′-H & CH2O), 4.25 (m, 2H, 5′-H & 3′-H), 4.40 (dd, 1H, 5′-H, J5′,4′ = 3 Hz, Jgem = 11 Hz), 4.80 (t, 1H, 2′-H, J2′,1′ = J2′,3′ = 5.6 Hz), 5.92 (d, 1H, 1′-H), 8.20 & 8.30 (s, 2H, 2-H & 8-H); MS (CI-NH3): 435 M + 1, 204 Base + 2.
Anal.—Calcd for (C20H29N5O6·0.75 TFA) C, H, N.
26: 1H NMR d (DMSO-d6): 1.5–1.7 (m, 8H, Cp), 4.10 (m, 1H, 4′-H), 4.22 (t, 1H, 3′-H, J3′,4′ = J3′,2′ = 4.5 Hz), 4.32 (dd, 1H, 5′-H, J5′,4′ = 6.4 Hz, Jgem = 12 Hz), 4.42 (dd, 1H, 5′-H, J5′,4′ = 4.0 Hz), 4.80 (t, 1H, 2′-H), 5.12 (s, 2H, CH2O), 5.92 (d, 1H, 1′-H), 7.30 (s, 5H, arom), 8.20 & 8.30 (s, 2H, 2-H & 8-H).
Anal.—Calcd (C23H27N5O6·0.75 TFA) C, H, N.
N6-[4-[[[4-[[[N-Acetyl(2-aminoethyl)amino]carbonyl]-methyl]anilino]carbonyl]methyl]phenyl]-5′-O-(1-methyl-3-pyridiniumcarbonyl)adenosine (27)
Compound 17 (250 mg, 0.34 mmol) was suspended in acetone (13 mL) and treated with methyl iodide (430 mL, 6.8 mmol). The system was closed, and the reaction mixture was heated to 50 °C for 7 h. As the reaction mixture cooled, the product separated as a yellow precipitate, which give a positive test with Dragendorffs spray reagent. The precipitate was removed by filtration and dried under reduced pressure (220 mg, 74%). This solid (210 mg, 0.32 mmol) was then dissolved in 20 mL of a mixture of chloroform, methanol, and water (1.5:1.5:0.5, v/v). TFA (1.5 mL) was added at 0 °C, and the mixture was allowed to reach room temperature overnight. The solution was concentrated to dryness, and the residue coevaporated three times with 15 mL of ethanol without heating. The oil obtained was then triturated several times with ether to obtain a hygroscopic yellow solid, which was dried overnight under reduced pressure to give in nearly quantitative yield (225 mg) of 27; 1H NMR δ (DMSO-d6): 1.75 (s, 3H, acetamide), 2.95 (bs. 4H, CH2CH2), 3.08 (s, 2H, CH2CO), 3,60 (s, 2H, CH2CO), 4.29 (m, 1H, 4′-H), 4.39 (s, 3H, NMe), 4.5 (t. 1H, 3′-H, J3′,4′ = J3′,2′ = 5.0 Hz), 4.63 (dd, 1H, 5′-H, J5′,4′ = 6.5 Hz, Jgem = 10.4 Hz), 4.78 (dd, 1H, 5′-H, J5′,4′ = 2.5 Hz), 4.82 (t, 1H, 2′-H, J1′,2′ = 4.8 Hz), 6.0 (s, 1H, 1′-H), 7.15,7.25 & 7.50 (d, 6H, arom), 7.85 (m, 3H, arom & NH), 8.0 (bs, NH), 8.23 (dd, 1H, 5″ -H), 8.30 & 8.52 (s, 2H, 2-H & 8-H), 8.92 (d, 1H, 4″-H, J4″,5″ = 8.4 Hz), 9.18 (d, 1H, 6″-H. J6″,5″ = 6.3 Hz), 9.49 (s, 1H, 2″-H), 9.90 & 10.10 (s, 2H, NH); MS (252Cf-plasma-desorption): m/z 738.8 M; high-resolution MS (FAB/NOBA matrix): m/e 738.2996, M, 738.3000 calcd for C37H40N9O8I.
Anal.—Calcd (C37H40N9O8I·H2O) C, H.
N6-Cyclopentyl-5′-O-(1-methyl-3-pyridiniumcarbonyl)adenosine (28)
A solution of 18 (150 mg, 0.3 mmol) in 8 mL of acetone was treated with methyl iodide (375 mL, 6 mmol) in a closed system at 50–55 °C for 4.5 h. The solvent was removed, and the residue was treated with ether, forming a slurry to provide 170 mg (88 %) of a yellow solid. The product give a positive test with Dragendorff’s spray reagent.
The procedure described for 27 was then followed. The oil obtained was triturated with ether giving in nearly quantitative yield 28 as a yellow hygroscopic solid; 1H NMR δ (DMSO-d6): 1.6–1.8 & 2.0 (m, 3H, Cp), 4.3 (m, 2H, 3′-H & 4″-H). 4.5 (s, 3H, NMe), 4.75 (m, 3H, H-5′ & H-2′), 6.05 (bs, 1H, 1′-H). 8.22 (dd, 1H, 5″-H), 8.30 & 8.50 (s, 2H, H-2 & H-8), 8.87 (m, 1H, 4″-H. J4″,5″ = 9 Hz). 9.20 (d. 1H. 6″-H, J6″,5″ = 6 Hz), 9.48 (s, 1H, 2″-H); MS (FAB/NOBA matrix): 455 M, 318 (M -Trigonelline), 204 Base + 2.
Anal.—Calcd (C22H27N6O5I·1.25 TFA) C, N.
N6-[4-[[[4-[[[N-Acetyl(2-aminoethyl)amino]carbonyl]-methyl]anilino]carbonyl]methyl]phenyl]-5′-O-[(1,4-dihydro-1-methyl-3-pyridinyl)carbonyl]-adenosine (29)
Compound 27 (60 mg, 0.069 mmol) was dissolved in 10 mL of phosphate buffer [pH 7.1, (I) = 0.4], which had been previously deaerated with a stream of N2 for 1 h. Then, Na2S2O4 (32 mg, 0.18 mmol) was added at 0 °C over a period of 40 min while keeping N2 bubbling into the solution. The yellow precipitate formed was isolated by centrifugation followed by suction filtration, washed with cold water, and dried under reduced pressure; 27 mg (55% yield) of the product 29 were obtained as a yellow powder; UV λmax (MeOH): 297.9 nm ε = 15,300): 1H NMR δ (DMSO-d6): 1.78 (s, 3H, acetamide), 2.50 (bs, 5H. CH3N & H-4″), 2.95 (bs, 4H. CH2CH2), 3.08 (s, 2H, CH2CO), 3.60 (s, 2H. CH2CO), 4.11 (m. 1H, 4′-H), 4.18 (dd, 1H, 5′-H, J5′,4′ = 5.4, Jgem, = 10.8 Hz), 4,29 (t, 1H. 3′-H, J3′,4′ = J3′,2′ = 4.5 Hz), 4.35 (dd, 1H, 5′-H, J5′,4′ = 3.6 Hz), 4.70 (m, 2H. 2′-H & 5″-H), 5.83 (d, 1H, 6″-H, J6″,5″ = 8 Hz), 5.97 (s, 1H, 1′-H, J1′,2′ = 4.6 Hz), 7.0 (s, 1H, 2″-H), 7.16, 7.28 & 7.50 (d, 6H, arom), 7.85 (m, 3H, arom & NH), 8.0 (bs, NH), 8.38 & 8.48 (s, 2H, 2-H & 8-H), 9.90 & 10.10 (s, 2H, NH); MS (Cf): m/z 762 MNa, 738 MH-H2, 138 C7H8NO2 (trigoelline); high-resolution MS (FAB/NOBA matrix): m/e found 738.3002 MH-H2, 738.3000 calcd for (C37H40N9O8).
N6-Cyclopentyl-5′-O-[(1,4-dihydro-1-methyl-3-pyridinyl)carbonyl]-adenosine (30)
To 100 mg of 28 (0.17 mmol) in 10 mL of deaerated phosphate buffer (pH 7.1, I = 0.4) and 10 mL of deaerated ethyl acetate were added portionwise at 0 °C over a period of 40 min Na2S2O4 (100 mg, 0.57 mmol). The mixture was kept under nitrogen bubbling throughout the reaction. The ethyl acetate layer was removed and replaced with fresh ethyl acetate before addition of the next portion of dithionite, washed with water, and dried (Na2SO4). The combined organic extracts were concentrated and passed through a column (10 mm) of silica gel with a mixture of chloroform:methanol 9:0.5 as an eluent. The eluate was evaporated to dryness to give 29 mg of 30 as a yellow oil (37%); UV λmax (CH2Cl2): 273.8 nm (ε = 6474); 1H NMR δ (DMSO-d6): 1-6–1.8 & 2.0 (m, 8H. Cp), 2.94 is, 3H. CH3N). 3.18 (d. 2H, H-4″), 4.11 (m, 1H, 4′-H). 4.18 (dd, 1H, 5′-H. J5′,4′ = 5.2 Hz, Jgem = 13 Hz), 4.29 (m. 1H, 3′-H), 4.34 (dd, 1H, 5′-H, J5′,4′ = 3.9 Hz), 4.65 (m, 2H, 2′-H), 4.72 (d, 1H, 5″-H, J5″,4″ = 3 Hz, J5″,6″ = 8 Hz), 5.84 (d, 1H, 6″-H), 5.97 (s, 1H, 1′-H, J1″,2″ = 4.5 Hz). 7.1 (s, 1H, 2″-H), 7.7 (d. 1H, NH), 8.28 & 8.34 (s, 2H, H-2 & H-8); MS (FAB) NH3: m/z 455 MH-H2, 318 MH-C7H9NO2, 204 BH2, 138 C7H8NO2; high-resolution MS (FAB/NOBA matrix): found 455.2053 MH-H2, 455.2043 calcd for (C22H37N6O5).
Receptor Binding
Rat cerebral cortical membranes were prepared15 and treated with adenosine deaminase (0.5 U/mL) for 20 min at 37 °C prior to radioligand binding studies or incorporation studies. Inhibition of binding of 1 nM [3H]-N6-phenylisopropyladenosine (Dupont New England Nuclear, Boston, MA) to A1-adenosine receptors in rat cerebral cortex membranes was measured.21 Membranes (150 µg) were incubated for 90 rain at 37 °C in a total volume of 2 mL, containing 50 µL of radioligand of the indicated concentration and 50 µL of the competing ligand (dissolved as a stock solution in DMSO). Samples of the prodrugs were dissolved freshly from solid and stored at −80 °C. The DMSO solutions were diluted to a concentration of <0.1 mM prior to adding to aqueous medium. Bound and free radioligand was separated by addition of 4 mL of a solution containing 50 mM Tris hydrochloride at pH 7.4 and 5 °C followed by vacuum filtration on glass filters with additional washes totaling 12 mL of buffer. Nonspecific binding was determined with 10 µM 2-chloroadenosine. At least seven different concentrations, spanning four orders of magnitude, adjusted appropriately for the IC50 of each compound, were used. The IC50 values, computer-generated with a nonlinear regression formula of the Graph-PAD program, were converted to apparent Ki value with the Cheng-Prusoff equation, assuming a Kd (dissociation constant) value for [3H] PIA of 1.0 nM.
In Vitro Stability Determination in Brain Homogenate and Phosphate Buffer
Prodrugs 19 or 22 (as a 1- or 5-mg/mL standard in CH3CN) were added to give an initial concentration of either 10 or 50 µg/mL (detection limit for the HPLC system), respectively, in 0.5 mL of phosphate buffer (pH 7.4) and 0.5 mL of rat brain homogenate maintained at 37 °C on a shaking water bath. One-hundred-microliter aliquots were withdrawn at time zero and 0.5, 1, and 1.5 h after adding the prodrugs. The aliquots were filtered and injected onto the HPLC system for analysis of the prodrug and the parent drug moieties.
Chromatographic separations were achieved on an Adsorbosphere Nucleotide-Nucleoside column (7 µm 4.6 × 10 mm). The mobile phase consisted of a 5–22% gradient of CH3CN in 0.1 N triethylammonium acetate (TEA acetate) at pH 7.0 (flow rate, 1.0 mL/min).
Locomotor Studies
Individual adult male mice of the NIH (Swiss) strain between 5 and 6 weeks of age and weighting at least 25 g were studied in a Digiscan activity monitor (Omnitech Electronics Inc., Columbus, OH) with computerized data analysis (ILAM software). Animals were placed in the cage immediately after injection and monitored for nine intervals of 10 min, each animal was initiated after 10 min. Total distance traveled, which indicates the distance in centimeters traveled by the animal, was used as a measure of locomotor activity in an open field assay as previously described.9 Adenosine analogues were dissolved in a 20:80 (v/v) mixture of Alkamuls EL-620 (Rhôone-Poulenc, Cranbury, NJ) and phosphate buffered saline and injected ip in a volume of 5 mL/kg body weight. Control values for vehicle-injected animals were determined for each experiment. Statistical analysis was performed with the t test.31 The results are reported as means ± SEM for each point.
Lipid Metabolic Studies
The following protocol was used for determination of adenosine agonist effects on in vivo lipolysis. Sprague Dawley rats (200–250 g) were fasted overnight. A blood sample was taken from the tail vein 10 s before the subcutaneous injection of a drug in vehicle (DMS). Additional blood samples were taken from the tail vein at 30, 60, and 90 min post-injection and were kept on ice and centrifuged within 0.5 h. A perchloric acid extract (3 %) of plasma was neutralized with KOH and 3-[N-morpholino]propanesulfonic acid and then assayed for glycerol as previously described.30
Acknowledgements
This work was presented in part at the American Chemical Society, 202nd National Meeting, August 26–30, 1991, New York, NY; Abstract MEDI 115, M.M. is grateful for financial support from Cortex Pharmaceuticals, Inc.
References and Notes
- 1.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Proc. Natl. Acad. Sci., U.S.A. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belardinelli L, Linden J, Berne RM. Prog. Cardiovasc. Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- 3.Spielman WS, Arend LJ. Hypertension. 1991;17:117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Bruns RF. Nucleosides Nucleotides. 1991;10:931–943. [Google Scholar]
- 5.Bridges AJ, Moos WH, Szotek DL, Trivedi BK, Bristol JA, Heffner TG, Bruns RF, Downs DA. J. Med. Chem. 1987;30:1709–1711. doi: 10.1021/jm00393a003. [DOI] [PubMed] [Google Scholar]
- 6.Dragunow M, Goddard GV, Laverty R. Epilepsia. 1985;26:480–487. doi: 10.1111/j.1528-1157.1985.tb05684.x. [DOI] [PubMed] [Google Scholar]
- 7.von Lubitz DKJE, Marangos PJ. J. Mol. Neurosci. 1990;2:53–59. doi: 10.1007/BF02896926. [DOI] [PubMed] [Google Scholar]
- 8.Phillis JW, Walter GA, Simpson RE. J. Neurochem. 1991;56:644–650. doi: 10.1111/j.1471-4159.1991.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 9.Nikodijević O, Sarges R, Daly JW, Jacobson KA. J. Pharmacol. Exp. Ther. 1991;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- 10.Brodie MS, Lee K, Fredholm BB, Stahle L, Dunwiddie TV. Brain Res. 1987;415:323–330. doi: 10.1016/0006-8993(87)90214-9. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA, Trivedi BK, Churchill PC, Williams M. Biochem. Pharmacol. 1991;41:1399–1410. doi: 10.1016/0006-2952(91)90555-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodor N, Farag HH. J. Med. Chem. 1983;26:313–318. doi: 10.1021/jm00357a002. [DOI] [PubMed] [Google Scholar]
- 13.Remaud G, Zhou X-X, Chattopadhyaya J, Oivanen M, Lönnberg H. Tetrahedron. 1987;34:4453–4461. [PubMed] [Google Scholar]
- 14.Hamilton HW, Hawkins LD, Patt WC, Johnson SA, Trivedi BK, Heffner T, Wiley J, Bruns RF. American Chemical Society National Meeting. Los Angeles, CA: 1988. Sept. Medi 80. [Google Scholar]
- 15.Jacobson KA, Kirk KL, Padgett WL, Daly JW. J. Med. Chem. 1985;28:1341–1345. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DC, Haskell TH, Putt SR. J. Med. Chem. 1978;21:1218–1221. doi: 10.1021/jm00210a009. [DOI] [PubMed] [Google Scholar]
- 17.Holy A. Coll. Cze. Chem. Commun. 1967;32:3064. [Google Scholar]
- 18.Torrence PF, Kinjo J, Lejiak K, Balzarini J, DeClercq E. FEBS Lett. 1988;234:135–140. doi: 10.1016/0014-5793(88)81319-x. [DOI] [PubMed] [Google Scholar]
- 19.Rinderknecht H, Gutenstein M. In: Organic Synthesis. Baumgarten HE, editor. Vol. V. New York: Wiley; 1973. pp. 822–824. Collect. [Google Scholar]
- 20.Jacobson KA. Comprehensive Medicinal Chemistry. Vol. 3. London: Pergamon; 1990. pp. 601–642. [Google Scholar]
- 21.Baumgold J, Nikodijevic O, Jacobson KA. Biochem. Pharmacol. 1992;43:889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vannucci SJ, Klim CM, Martin LF, LaNoue KF. Am. J. Physiol. 1989;257:E871. doi: 10.1152/ajpendo.1989.257.6.E871. [DOI] [PubMed] [Google Scholar]
- 23.Linden J. The FASEB Journal. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- 24.Bruns RF, Lu GH, Pugsley TA. Mol. Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- 25.Daly JW, Jacobson KA. In: Adenosine Receptors in the Nervous System. Ribeiro JA, editor. London: Taylor & Francis; 1989. pp. 41–52. [Google Scholar]
- 26.Jacobson KA, Nikodijevic O, Ji X-D, Berkich DA, Eveleth D, Dean RL, Hiramatsu KI, Kassell NF, van Galen PJM, Lee KS, Bartus R, Daly JW, LaNoue KF, Maillard M. J. Med. Chem. 1992;35:4143–4149. doi: 10.1021/jm00100a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannell LK, Sokoloski EA, Fales HM, Tate RL. Anal. Chem. 1985;57:1060–1067. doi: 10.1021/ac00283a022. [DOI] [PubMed] [Google Scholar]
- 28.Kusachi S, Thompson RD, Bugni WJ, Nobuyuki Y, Olsson RA. J. Med. Chem. 1985;28:1636–1643. doi: 10.1021/jm00149a016. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YC, Prusoff WH. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 30.Eggstein M, Kuhlman E. In: Methods of Enzymatic Analysis. Bermeyer HU, editor. Verlag Chemic: Weinheim; 1974. pp. 1825–1831. [Google Scholar]
- 31.Finney DJ. Probit Analysis. 3rd. Cambridge, England: Cambridge University; 1971. [Google Scholar]