Abstract
Background & Aims
Immune cells of the liver must be able to recognize and react to pathogens yet remain tolerant to food molecules and other non-pathogens. Dendritic cells (DC) are thought to contribute to hepatic tolerance. Lipids have been implicated in dysfunction of DC in cancer. Therefore, we investigated whether high lipid content in liver DC affects induction of tolerance.
Methods
Mouse and human hepatic non-parenchymal cells were isolated by mechanical and enzymatic digestion. DC were purified by fluorescence-activated cell sorting or with immunomagnetic beads. DC lipid content was assessed by flow cytometry, immune fluorescence, and electron microscopy and by measuring intracellular component lipids. DC activation was determined from surface phenotype and cytokine profile. DC function was assessed in T-cell, natural killer (NK)-cell, and NKT-cell co-culture assays, as well as in vivo.
Results
We observed 2 distinct populations of hepatic DC in mice and humans based on their lipid content and expression of markers associated with adipogensis and lipid metabolism. This lipid-based dichotomy in DC was unique to the liver and specific to DC, compared with other hepatic immune cells. However, rather than mediate tolerance, the liver DC population with high concentrations of lipid was immunogenic in multiple models—they activated T cells, NK cells, and NKT cells. Conversely, liver DC with low levels of lipid induced T-regulatory cells, anergy to cancer, and oral tolerance. The immunogenicity of lipid-rich liver DC required their secretion of tumor necrosis factor-α and was directly related to their high lipid content; blocking DC synthesis of fatty acids or inhibiting adipogenesis (by reducing endoplasmic reticular stress) reduced DC immunogenicity.
Conclusions
Human and mouse hepatic DC are comprised of distinct populations that contain different concentrations of lipid, which regulates immunogenic versus tolerogenic responses in the liver.
Keywords: immune regulation, T cells, NK cells, NKT cells
Introduction
Tolerance is the hallmark of hepatic immune function. A primary illustration of hepatic tolerance is the phenomenon of oral tolerance to ingested food1. Other entities broadly attributed to hepatic tolerance are the observations that gastrointestinal tumors most commonly metastasize to the liver and that hepatic allografts are accepted with minimal immune-suppressive therapy2, 3. However, the cellular mediators of hepatic tolerance are incompletely understood. Oral tolerance has been linked, in part, to liver sinusoidal endothelial cells. Limmer et al. demonstrated that ingested antigen leads to anergy in H-2Kb-restricted CD8+ T cells via scavenger endothelial cells4. Regulatory T cells and Kupffer cells are also important effector cells in hepatic tolerance. Breous et al. reported that IL-10 secreted by Foxp3+ Tregs renders normally immunogenic Kupffer cells unable to induce effective CTLs5. Conversely, depletion of Tregs reverses hepatic tolerance. We have recently shown that resident hepatic CD11b+Gr1+ myeloid-derived suppressor cells contribute to hepatic tolerance and allow for the deposition of hepatic metastases by creating an immune suppressive microenvironment in animals with gastrointestinal malignancies6. Recent work suggests that dendritic cells (DC) are also important mediators of hepatic tolerance7. Goubier et al. reported that liver DC contribute to tolerance by active T cell deletion8. Xia et al. showed that the unique hepatic microenvironment programs Lin−CD117+ hematopoietic progenitor differentiation into regulatory DC responsible for maintaining tolerance9. We have shown that, as a consequence of their immaturity, liver DC are poorly immunogenic when compared with DC in other compartments10.
In addition to being an important immunologic organ, the liver is a primary site of lipid storage and metabolism. Aberrations in lipid metabolism adversely affect hepatic function in diseases such as NASH. The relevance of lipids to DC function has recently been investigated. Herber et al. showed that in tumor bearing mice, as well as in cancer patients, DC accumulate lipids which endows them with tolerogenic properties, thereby providing additional avenues for cancer's evasion of immunologic attack11. In particular, DC initiation of adaptive immunity is mitigated as a direct consequence of their higher lipid content. However, the role of endogenous intracellular lipids on DC function, in the absence of cancer, is not well understood. We postulated that liver DC will be conspicuously lipid-laden, thereby accounting for their propensity to mediate tolerance. We discovered two lipid-laden hepatic DC populations that were phenotypically, molecularly, and functionally distinct. However, contrary to our expectations, our experiments show that lipid-rich hepatic DC are markedly pro-immunogenic whereas lipid-poor DC are tolerogenic suggesting a novel mechanistic role for endogenous lipids on liver DC's contribution to hepatic immunity and tolerance.
Results
Liver DC are composed of distinct populations based on lipid content
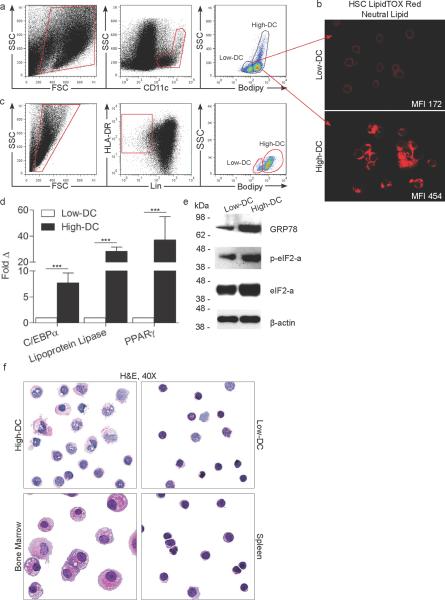
To investigate the endogenous lipid content of hepatic DC, CD11c+ hepatic non-parenchymal cells (NPC) were co-stained using BODIPY (Figure 1a) or LipidTOX Red Neutral Lipid stain (Figure 1b), each of which bind to intracellular neutral lipids12. Both assays revealed two distinct DC populations defined by high (High-DC) or low (Low-DC) lipid content (Figure 1a, b). High-DC accounted for ~75% of total murine liver DC and Low-DC for ~25%. High DC expressed increased MHC II compared with Low-DC (Figure S1a). DC were distinct from hepatic stellate cells and other leukocyte subsets (Figure S1b, c). Similar to mice, human Lin−HLA-DR+ liver DC exhibited dual populations stratified by lipid content (Figure 1c). Molecular distinction between High- and Low-DC was evident in their differential expression of genes associated with adipogenesis and lipid metabolism including Lipoprotein lipase, PPAR-γ, and C/EBPα in mice and humans (Figures 1d and S2a,b). Furthermore, consistent with their elevated lipid content13, High-DC exhibited greater endoplasmic reticulum (ER) stress as evidenced by increased expression of GRP78 (BiP), eIF2α, and peIF2α (Figure 1e).
Figure 1. Distinct liver DC populations are defined by their endogenous lipid content.
(a) Live murine liver NPC were gated and CD11c+ cells were analyzed for BODIPY staining. (b) FACS sorted CD11c+MHCII+ High-DC and Low-DC were spun onto slides and assessed by HCS LipidTOX Red neutral lipid staining. (c) Human liver NPC were co-stained using BODIPY and antibodies reactive with HLA-DR and Lineage markers. (d, e) Murine High- and Low-DC were purified by FACS and tested for expression of (d) C/EBPα, Lipoprotein lipase, and PPAR-γ by PCR (***P<0.001) and (e) for expression of GRP78 (BiP), peIF2α, eIF2α, and β-actin by Western blotting. (f) Representative purified High- and Low-DC were stained with H&E and compared with CD11c+MHCII+ spleen DC or bone marrow-derived DC.
To determine the precise component lipids in the divergent DC groups, we purified the High-DC and Low-DC populations and measured their respective phospholipid, triglyceride, and cholesterol/cholesteryl ester contents. High-DC contained elevations in phospholipids compared with Low-DC (Figures S2c). Similarly, triglycerides were approximately three-fold higher in High-DC (Figure S2d). Conversely, cholesterol and cholesteryl esters were not elevated in High-DC (Figure S2e). Further, exogenous administration of cholesterol in culture did not increase liver DC neutral lipid content (Figure S2f). Consistent with these observations, High-DC contained greater cytoplasmic area and increased vacuolization compared with Low-DC on light microscopy (Figure 1f). Transmission electron microscopy confirmed these findings and additionally revealed increased morphologic maturation and a higher density of mitochondria in High-DC (Figure S3a–m).
Liver DC lipid content is affected by the hepatic microenvironment
The fraction of High- and Low-DC populations in the liver was mutable depending on physiologic circumstance. In particular, in states associated with a lipid-rich hepatic microenvironment, the High-DC population was increased. For example, mice with NASH (Figure S4a, b) exhibited a greater fraction of High-DC (Figure S4c,d). Similarly, steatohepatitic human liver also contained an increased High-DC fraction (Figure S4e,f). Conversely, Apo-E−/− mice, which are deficient in lipid transport, had a modestly increased fraction of Low-DC (Figure S4c, d). The fraction of High-DC could also be downwardly modulated in vivo by administration of C75, an inhibitor of fatty acid synthase (Figure S4c,d).
Conditions associated with intra-hepatic inflammation were also associated with an increased fraction of High-DC. For example, inflammation from LPS administration (Figure S5a, b) as well as chronic liver fibrosis (Figure S5a,c) markedly increased the High-DC fraction. Conversely, bile duct ligation, commonly associated with immune suppression14, was associated with a decreased High-DC fraction (Figure S5a,d). Partial hepatectomy did not affect the fraction of High- and Low-DC (Figure S5a,d). Since adjusting the total liver lipid content - such as in human steatohepatitis or murine NASH - modulated the relative fractions of High- and Low-DC, we postulated that High-DC may have increased capacity for uptake from their environment, in addition to having enhanced mechanisms for adipogenesis (Figure 1d). Accordingly, High-DC exhibited modestly higher expression of lipid scavenger receptors CD204 (MSR1) and CD68 (Figure S6).
Distinct hepatic DC populations are unique
Comparison of DC with CD45−CD146+ liver sinusoidal endothelial cells as well as with other CD45+ hepatic leukocytes revealed that DC are the only resident hepatic immune competent cellular subset with distinct populations based on lipid content (Figure S7a). Total intracellular lipid was higher in DC compared with other CD45+ hepatic leukocytes but lower than in liver sinusoidal endothelial cells (Figure S7b). Similarly, analysis of DC from other compartments including spleen, lymph nodes, kidney, and epidermal Langerhans cells revealed that extra-hepatic DC do not exhibit divergent populations based on total lipid content (Figure S7c,d). Taken together, these data suggest that the dual populations of liver DC are unique within the liver and among DC sub-groups. Notably, both lipid-laden hepatic DC populations were found in similar anatomic subareas of the liver on light microscopy (Figure S7e).
Liver DC stratified by lipid content exhibit divergent immune-phenotypes
Since lipids reportedly adversely affect DC function11, we postulated distinct immune-phenotypes for liver DC subsets depending on lipid content. High-DC expressed higher levels of integrins (CD54), co-stimulatory molecules (CD40, CD80, CD86), and glycoproteins (CD1d) compared with Low-DC (Figure 2a). Both subsets had similar CD11b+ myeloid fractions but High-DC exhibited higher expression of the B220 plasmacytoid marker (Figure 2a). After 24h of in vitro culture, both DC subsets matured as expected, but High-DC matured to a greater extent (Figure S8a). Low-DC phenotype was not additionally matured after culture with exogenous triglycerides (Figure S8b). High-DC also produced markedly elevated levels of numerous pro-inflammatory cytokines and chemokines in cell culture supernatant (Figure 2b) and by intracellular cytokine analysis (Figures 2c, S8c). Conversely, Low-DC were relatively non-productive, even after 24h of in vitro culture or culture with exogenous triglycerides (Figure S8d). High-DC also produced increased inflammatory mediators compared with bulk spleen DC (Figure 2b), consistent with our previous observations10. Studies of Lin−HLA-DR+ DC isolated from the human liver confirmed that human High-DC produce elevated levels of pro-inflammatory cytokines (Figure 2d). In addition to being more activated at baseline, High-DC in both humans and mice also exhibited an exaggerated response to TLR ligation compared with Low-DC (Figure 2d,e). Consistent with this observation, High-DC exhibited augmented expression of TLR2, TLR4, TLR7, and TLR9 compared with Low-DC (Figure 2f).
Figure 2. High-DC exhibit an immunogenic phenotype.
(a) High- and Low-DC were analyzed for expression of surface markers. Median fluorescence index (MFI) for High- and Low-DC are indicated below respective histograms. (b) Liver and spleen DC production of cytokines and chemokines was measured in cell culture supernatants. (c) Liver DC populations were also assessed by intra-cellular cytokine staining for production of IL-17α and TNF-α. (d) High- and Low-DC were isolated from the human liver by FACS and cultured alone or with LPS before analysis of cytokines in cell culture supernatant. (e) Mouse liver DC production of IL-6 and TNF-α was measured in cell culture supernatant after selective TLR ligation. (f) TLR expression in High- and Low-DC was determined by flow cytometry. Experiments were repeated 3–4 times using 3 mice per group (*P<0.05; **P<0.01; ***P<0.001).
High DC are immunogenic, Low DC are tolerogenic
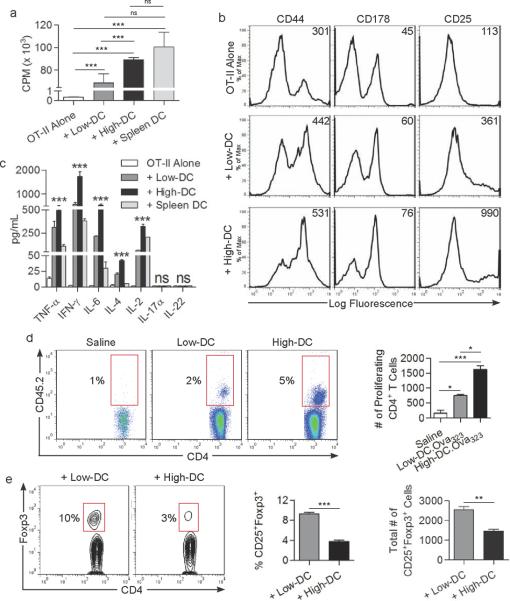
Since High-DC express elevated MHC II and co-stimulatory molecules and produce robust levels of inflammatory mediators, we suspected that, contrary to the tolerogenic properties of lipid-laden DC in cancer11, resident hepatic DC with higher endogenous lipid levels will be potently immunogenic. To test this, we assessed DC ability to induce proliferation and activation of antigen-restricted CD4+ T cells. Purified High-DC and Low-DC populations were loaded with Ova323-339 and then co-cultured with CD4+ OT-II T cells. High-DC induced more vigorous T cell proliferation than Low-DC (Figure 3a). Moreover, High-DC had far greater capacity to activate CD4+ T cell surface phenotype, including expression of CD44, CD178, and CD25 (Figure 3b) and induced elevated Th1 (IL-2, IFN-γ), Th2 (IL-4), and Th17 (IL-6, TNF-α) cytokine production (Figure 3c). However, the Th17 cytokines IL-17 and IL-22 were not detectable (Figure 3c). Notably, peptide-pulsed High-DC activated CD4+ T cells to a similar extent as bulk spleen DC (Figure 3a,c).
Figure 3. High-DC potently activate of CD4+ T cells whereas Low-DC generate Tregs.
(a) CD4+OT-II cellular proliferation, (b) surface phenotype, (c) and cytokine production was measured after co-culture with High- or Low-DC loaded with Ova323–339 peptide or culture with peptide-pulsed spleen DC. (d) CD4+OT-II T cell proliferation was also measured in vivo in the draining popliteal lymph node after footpad immunization with saline, or antigen-loaded High- or Low-DC. The fraction and total number of proliferating CD4+ T cells is shown. (e) After five days of DC co-culture with allogeneic CD4+ T cells, CD4+CD25+ cells were gated and analyzed for co-expression of Foxp3. The fraction and total number of CD25+Foxp3+ cells is shown. Experiments were performed in triplicate and repeated three times (*P<0.05; **P<0.01; ***P<0.001).
We next tested the relative capacity of the distinct hepatic DC populations to migrate to regional lymph nodes and prime CD4+ T cells in vivo. CD45.1+ mice were administered OT-II T cells i.v. followed by footpad immunization with either High- or Low-DC.Ova323-339. CD4+ T cell proliferation in the ipsilateral popliteal lymph node basin was measured at five days and found to be approximately 3-fold higher in mice that received High-DC.Ova323-339 footpad immunization compared with recipients of Low-DC.Ova323-339 immunization (Figure 3d). Non-peptide-pulsed DC controls were completely non-activating (not shown).
DC induction of Tregs is of primary importance in maintaining hepatic tolerance9, 15. To test whether liver DC's capacity to generate Tregs is affected by their endogenous lipid content, High- or Low-DC were co-cultured with allogeneic CD4+ T cells from Balb/c mice before assay for Treg generation. By day 5, approximately 10% of CD4+ T cells incubated with Low-DC expressed the CD4+CD25+Foxp3+ Treg phenotype. Conversely, High-DC prevented Treg induction (Figure 3e). Taken together, these data suggest that, with regard to their interaction with CD4+ T cells, High-DC are potently immunogenic whereas Low-DC are tolerogenic.
To explore whether the increased immunogenicity of liver DC containing high endogenous lipid levels extends to their interactions with CD8+ T cells, we loaded High- and Low-DC with Ova257-264 before either co-culture with CD8+ OT-I T cells (Figure S9a) or footpad immunization to mice that received OT-I T cells 24h earlier (Figure S9b). Peptide-pulsed High-DC induced approximately 3-fold elevated CD8+ T cell proliferation both in vitro (Figure S9a) and in the draining lymph node basin (Figure S9b) compared with Low-DC. High-DC also induced greater proliferation of allogeneic T cells in a mixed leukocyte reaction (MLR; Figure S9c). Notably, the immunogenicity of Low-DC was not enhanced after 24h in vitro culture or after culture with triglycerides (Figure S10).
High DC induce potent CTL and tumor protection, Low DC procure in vivo tolerance
To determine the relative capacity of distinct lipid-laden DC subsets to induce de novo CTL activity, naïve mice were immunized twice at weekly intervals with High- or Low-DC.Ova257-264 followed by challenge with Ova-expressing targets. We confirmed that after i.p. immunization DC reach the liver and spleen (Figure S11). Moreover, mice immunized with High-DC.Ova257-264 induced robust target lysis in both the liver and spleen (Figure 4a) and generated accelerated proliferation of Ova-restricted CD8+ effector cells in vivo (Figure 4b). The capacity of High-DC to induce CTL was similar to bulk spleen DC (Figure 4b). Conversely, animals immunized using Low-DC.Ova257-264 exhibited tolerance to Ova-expressing targets (Figure 4a,b). Analysis of cytokine production in CTL cultures similarly revealed that immunization using peptide-pulsed High-DC induced a potent cytokine response whereas Low-DC were relatively ineffectual at generating immunogenicity (Figure 4c).
Figure 4. High-DC generate potent de novo CTL, enhance pre-existing CTL, and mediate tumor protection.
(a) Lysis of Ova-expressing targets in the liver and spleen were measured in mice twice immunized with High- or Low-DC.Ova257-264. (b) Similarly, the fraction of Ova-Tetramer+ T cells among all CD8+ T cells was measured in the liver of mice immunized using peptide-pulsed High-DC, Low-DC, or bulk spleen DC. (c) Cytokines were measured in day four spleen CTL cultures that were restimulated with Ova257-264 peptide. (d) CTL lysis of Ova-expressing targets in the spleen as well as (e) IL-2 and IL-10 production in spleen CTL cultures was measured in Rag1 mice that had been reconstituted with OT-I T cells and then immunized with either High- or Low-DC.Ova257–264. Experimental results were reproduced at least three times (*P<0.05; **P<0.01; ***P<0.001). (f) Mice were challenged with EG7 after immunization with saline or High- or Low-DC or bulk spleen DC loaded with Ova257-264 peptide. Time to tumor development (P<0.01 for comparison between High- and Low-DC) and mean tumor size at 21 days are shown (mean n=6/group).
To determine whether High-DC are capable of enhancing pre-existing endogenous CTL activity and whether Low-DC suppress pre-existing CTL, we reconstituted Rag mice with OT-I T cells and subsequently used either High-DC.Ova257-264 or Low-DC.Ova257-264 for a booster immunization. Seven days after immunization, mice were tested for their ability to lyse Ova-expressing targets in vivo as above. Consistent with our previous findings, immunization using peptide-pulsed High-DC enhanced endogenous CTL lysis whereas Low-DC were anergic (Figure 4d). Non-peptide-pulsed DC had no effect (not shown). Moreover, in contradistinction to Low-DC immunization, High-DC immunization enhanced IL-2 production and suppressed IL-10 in CTL cultures (Figure 4e). Taken together, these data show that High-DC can generate potent de novo CTL and enhance endogenous CTL whereas Low-DC induce anergy in vivo.
To determine the relative capacity of liver DC subsets to protect against tumor in vivo, naive C57BL/6 mice were immunized twice with either High-DC.Ova257 or Low-DC.Ova257-264 using the same regimen as above followed by subcutaneous tumor challenge with EG7 thymoma cells, which express the chicken Ovalbumin gene. Mice immunized using Low-DC.Ova257-264 developed rapidly progressive tumors. Conversely, mice immunized using High-DC.Ova257-264 were delayed in tumor development by a median of 10 days and developed smaller lesions, similar to immunization using bulk spleen DC (Figure 4f). All mice immunized with mock-loaded DC or challenged with EL4 which does not express Ovalbumin, developed tumor at similar rates confirming the antigen-specificity of the experiment (not shown).
Low DC induce tolerance to oral antigen
Oral tolerance has been linked to liver DC8. To investigate whether Low-DC account for oral tolerance, we designed an experimental model of feeding mice Ovalbumin via oral gavage followed by transfer of liver DC subpopulations from fed mice to naïve mice. Notably, High- and Low-DC captured oral antigen at similar rates (Figure 5a); however, as predicted based on their elevated lipid content16, High-DC captured soluble antigen more efficiently than Low-DC by both generalized macropinocytosis (Ovalbumin) and via specialized mannose receptors (Mannose-Albumin) both in vitro (Figure 5b) and in vivo (Figure 5c) in mice as well as in humans (Figure S12). Moreover, in the oral tolerance experiment, Ova257-264 stimulated splenocytes harvested from mice adoptively transferred with High-DC or Low-DC derived from Ovalbumin-fed donors expressed elevated IL-2 in an antigen-restricted manner (Figure 5d), implying that liver DC can induce a modest immunogenic response to oral antigen. However, strikingly, Low-DC transfer led to splenocyte production of 1000-fold elevations of IL-10, even in the absence of fed antigen (Figure 5e), further suggesting that Low-DC are agents of tolerance in vivo.
Figure 5. Low-DC are poor at capturing antigen and mediate intra-hepatic tolerance.
(a) Hepatic DC fluorescence was measured 1h after feeding mice Ovalbumin conjugated to APC. MFIs are indicated. (b) High- and Low-DC uptake of Ovalbumin and Mannosylated-Albumin were measured in vitro at various time points and (c) in vivo at 1h after i.p. administration of the respective fluorescent antigens. (d) Restimulated spleen cultures from mice that received adoptively transferred High- or Low-DC from Ovalbumin-fed or sham-fed donors were interrogated for production of IL-2 and (e) IL-10. (f) The number of intra-hepatic Ova Tetramer+ cells was measured at 96h in mice treated with OT-I T cells followed by injection of saline, Ovalbumin, or Ovalbumin and C75. Experiments were repeated at least three times (n=3–5 mice per group; *P<0.05; **P<0.01; ***P<0.001).
Cross-presentation in-situ can be modulated by altering DC lipid content
To further test the relevance of DC lipid content to hepatic immunity, we investigated whether cross-presentation, which is DC dependant17, is affected by altering the High-DC:Low-DC ratio in situ. Mice were administered OT-I T cells and selected cohorts were additionally treated with C75 before testing for their ability to cross-present Ovalbumin in the liver as measured by assessment of the frequency of hepatic OT-I cells. Notably, increasing the fraction of Low-DC using C75 (Figure S4c,d), diminished the capacity for intra-hepatic cross-presentation nearly 10-fold (Figure 5f). These data highlight the significance of the High-DC:Low-DC ratio in maintaining the balance of liver immunity in situ.
High DC, but not Low DC, potently activate NK and iNKT cells
To investigate whether the dichotomous immune stimulatory capacity of High- and Low-DC extend to their interaction with innate immune cells we performed DC-NK co-culture experiments. High-DC induced NK cells to produce markedly high levels of IL-6 and IFN-γ (Figure 6a and S13a). Conversely, Low-DC only marginally activated NK cells above baseline. Similarly, High-DC modestly increased NK cells expression of CD69 (Figure 6b) and NKG2D (not shown) whereas Low-DC were non-activating. NK cells also gained a 3-fold enhanced capacity to lyse Yac-1 target cells after co-culture with High-DC compared Low-DC (not shown). Notably, High-DC were more effective than bulk spleen DC at activating NK cells (Figure 6a), consistent with our previous observations18.
Figure 6. High-DC activate innate immunity whereas Low DC induce anergy.
(a, b) Liver High-and Low-DC and spleen DC were co-cultured with NK cells and interrogated for production of (a) IL-6 and IFN-γ. (b) NK1.1+ cells were gated and analyzed for expression of CD69 on flow cytometry. (c, d) Liver DC-iNKT cells co-cultures were interrogated for production of (c) IL-6, IFN-γ, and TNF-α. (d) In addition, iNKT cells were gated and analyzed for expression of CD25. (e) High- and Low-DC were analyzed for expression of Notch1, Jagged1, and Delta4 by flow cytometry. (f) High- and Low-DC were also analyzed for expression of Jagged1, Delta4, and β-actin by Western blotting; (**P<0.01; ***P<0.001).
Determinants of liver DC activation of iNKT cells are incompletely understood19–20. Since High-DC express markedly elevated CD1d compared with low-DC (Figure 2a), we postulated they may have greater capacity to engage iNKT cells. Splenic iNKT cells were harvested using an α-GalCer loaded CD1d tetramer (Figure S13b) and co-cultured with α-GalCer-pulsed High- or Low-DC. High-DC induced iNKT cells to produce high levels of IL-6, IFN-γ, and TNF-α (Figure 6c). Conversely, Low-DC were unable to effectively activate iNKT cells. Similarly, High-DC increased CD25 expression on iNKT cells whereas Low-DC were non-activating (Figure 6d). Since Notch signaling governs DC-NK interaction21, we postulated that High-DC expresses elevated Notch receptors and ligands. As anticipated, High-DC expressed elevated Notch1, Jagged1, and Delta4 on flow cytometry (Figure 6e) and Western blotting (Figure 6f). High-DC derived from human liver also expressed elevated Delta4 compared with human liver Low-DC (Figure S13c).
High DC immunogenicity is TNF-α dependent and linked to adipogenesis and ER stress
TNF-α can have pleiotropic effects on liver DC function22. Since High-DC produce markedly elevated TNF-α (Figure 2b,c), we postulated that their immunogenicity may be TNF-α dependent. To test this, we repeated selected immunologic assays using a mAb directed against TNF-α and found that TNF-α blockade prevented the differential activation of antigen-restricted CD4+ OT-II T cells by peptide-pulsed High-DC (Figure 7a). Similarly, TNF-α blockade negated the elevated NK cell activation induced by High-DC (Figure 7b,c).
Figure 7. High-DC immunogenicity is abrogated by blocking TNF-α or lessening ER stress.
(a) CD4+OT-II T cells were cultured alone or co-cultured with High- or Low-DC.Ova323-339 in the presence or absence of an mAb against TNF-α. IFN-γ was measured in cell culture supernatant. (b, c) Similarly, DC-NK co-cultures were performed in the presence of TNF-α blockade before measuring (b) IFN-γ and (c) IL-6. (d) Peptide-pulsed High-DC, alone or with chaperone, were used to stimulate OT-II T cells. TNF-α was measured in cell culture supernatant. Experiments were repeated 2–3 times (*P<0.05; **P<0.01; ***P<0.001).
We next investigated whether the inherent capacity of High-DC for immunogenicity may be contingent on their ability to synthesize fatty acids. To test this, we co-cultured DC with TOFA, an inhibitor of Acetyl-CoA carboxylase, before measuring DC cytokine production or performing in vitro CD4+ T cell and NK cell activation assays. As expected, TOFA decreased DC fatty acid synthesis (Figure S14a) as well as cellular lipid content on electron microscopy (Figure S14b). TOFA did not induce apoptosis or necrosis of liver DC (Figure S14c). Moreover, blockade of fatty acid synthesis mitigated High-DC production of TNF-α and IL-6 (Figure S14d) and prevented their modestly elevated expression of TLRs (Figure S14e). Furthermore, TOFA reduced High-DC ability to induce immunogenic responses in antigen-restricted CD4+ T cells (Figure S14f,g) and NK cells (Figure S14h, i).
High-DC have elevated ER stress (Figure 1e) which has been linked to enhanced antigen presentation in APC23. Since the chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response and decreasing ER stress24, we postulated this may mitigate the increased immunogenicity of High-DC. Accordingly, we found that the pre-incubation with 4-phenylbutyrate reduced T cell activation by High DC (Figure 7d). T cell stimulation by Low-DC was not affected (not shown). Taken together, these data show that blocking fatty acid synthesis or inhibiting adipogenesis by lowering ER stress, decreases the immunogenic capacity of High-DC suggesting that lipids are causal to liver DC induction of immunogenicity or tolerance.
Antigenic challenge results in High-DC apoptosis
We and other groups have shown that, as a whole, liver DC are tolerogenic and serve as a foundation for hepatic tolerance9–10, 25. A central question arising from the current data is, if High-DC are potently immunogenic and make up roughly 75% of liver DC, why are liver DC tolerogenic? It is likely that High-DC cannot overcome the strong tolerogenic influences of Low-DC on immune effector cells (Figure 5e). However, we also noted that, whereas High-DC predominate in the steady-state, upon antigenic challenge in vivo, there is an effective diminution of DC lipid content (Figure S15a). To determine whether DC readily convert from the higher lipid-laden fraction to the lower fraction, we cultured High- and Low-DC in vitro for 18h and reassessed their lipid content. Notably, more than 80% of Low-DC remained “Low” after culture (Figure S15b). However, approximately 40% of High-DC converted to “Low” (Figure S15b). Taken together, these data suggest that High-DC decrease their lipid content in vivo upon antigenic stimulation and during in vitro culture. As High-DC exhibit elevated ER stress which can induce apoptosis 26, we postulated that High-DC may also have a greater propensity towards apoptosis. We found that the fraction of apoptotic High-DC was higher than that of Low-DC both immediately upon isolation (Figure S15c) and after 18h of in vitro culture (Figure S15d). Further, the ratio of apoptotic High-DC:Low-DC further increased upon in vivo antigenic challenge (Figure S15e). These data suggest that antigen administration results in downward modulation of the DC lipid content via direct conversion to Low-DC and elevation of High-DC apoptosis, which may act to maintain the balance of hepatic tolerance.
Discussion
We demonstrate that DC are the only hepatic leukocyte group with distinct populations based on lipid content. Furthermore, mouse and human liver DC are unique when compared with DC in other compartments in exhibiting high- and low-lipid populations. The mechanism for the dichotomous lipid levels among hepatic DC is uncertain; however, the elevated expression of scavenger receptors on High-DC as well as upward skewing of the High-DC fraction in mouse models of NASH and in human steatohepatitis suggest that High-DC have an increased capacity to incorporate lipid from their microenvironment. Nevertheless, the findings that in vivo inhibition of fatty acid synthase lowers the fraction of High-DC, as well as the observation that inhibition of Acetyl-CoA carboxylase modulates High-DC function, suggest that intracellular fatty acid synthesis within hepatic DC may also determine their fractionation. This is supported by our finding that High-DC exhibit higher expression of genes associated with adipogenesis and lipid metabolism including Lipoprotein lipase, PPAR-γ, and C/EBPα.
This work uncovers the critical finding that endogenous lipid content determines liver DC propensity for immunogenicity or tolerance. The hallmark of hepatic immunology is antigenic tolerance and this has, in part, been attributed to liver DC7. However, our investigations show that whereas Low-DC induce innate immune anergy and adaptive T cell tolerance, High-DC are potently immunogenic. As evidence for the existence of an immunogenic liver DC population associated with elevated lipid content, High-DC exhibit an activated surface phenotype, produce markedly elevated levels of immune-modulatory cytokines and chemokines compared with Low-DC in humans and mice, express elevated levels of Toll-like receptors and differentially produce inflammatory mediators in response to TLR ligation. High-DC also induce robust allogeneic T cell proliferation, elevated antigen-restricted CD4+ and CD8+ T cell proliferation and activation in vitro and in vivo, potent CTL responses in a variety of contexts, enhanced capacity for cross-presentation in situ, as well as tumor protection in adoptive transfer immunization models. Conversely, Low-DC induce the generation of Tregs and stimulate T cells to produce robust levels of IL-10 after in vivo adoptive transfer. The dichotomous functional capacity of High- and Low-DC extends to their interaction with innate immunity as NK cells and iNKT cells are differentially activated by High-DC whereas Low-DC are entirely ineffectual at engaging innate effector cells. Interestingly, we found that High-DC express elevated IL-17α which is predominately thought to be produced by Th17-deviated T cells. However, this was only detectable by intra-cellular cytokine staining and not secreted at detectible levels (Figure 2b, c). Therefore, its physiologic significance requires further study.
We and others have shown that after hepatic injury and during periods of intense intrahepatic inflammation, liver APC acquire enhanced immunogenic and pro-inflammatory function22, 27. For example, in hepatic fibrosis we previously found that DC increase their expression of immunogenic cytokines and gain enhanced capacity for induction of innate and adaptive immunity25. Our current investigations suggest that in liver fibrosis as well as after injection of pro-inflammatory agents such as LPS, the High-DC:Low-DC ratio is upwardly modulated. Changes in the relative fraction of lipid-laden DC may plausibly contribute to the augmented hepatic DC immune function in the context of hepatic inflammation and chronic liver disease. In support of this notion, we show that modulating the fraction of High-DC in situ has impressive implications on cross-presentation in the murine liver. Moreover, these data suggest that manipulation of the High-DC:Low-DC ratio may be an attractive strategy to explore in experimental therapeutics in various contexts including inflammatory and autoimmune liver diseases and in transplantation. However, these experimental approaches may be hindered by the differential rates of apoptosis and plasticity of DC subgroups we observed. It is also interesting that in vitro modulation of Low-DC lipid content using triglycerides did not enhance their immunogenicity. However, the physiologic relevance of exogenous modification of liver DC in culture is uncertain.
The capacity of High-DC to produce TNF-α is primary for their enhanced immunestimulatory capacity. Moreover, we show that high lipid content is not only associated with increased DC immunogenicity but determines it. For example, the inherent ability of High-DC to synthesize fatty acids is necessary for their elevated immunogenicity as evidenced by the finding that blockade of Acetyl-CoA carboxylase, the rate limiting step in fatty acid synthesis, diminishes the augmented capacity of High-DC to express elevated TLRs, produce cytokines, and engage both innate and adaptive immunity. Further, chemical chaperones which decrease ER stress by reducing adipogenesis similarly diminish High-DC capacity to activate T cells. Elevated ER stress has previously been linked to higher immunogenicity in macrophages23. In addition, the relationship between DC lipid content and immunogenicity may also imply an association between increased energy stores and higher immune function. This has been previously described on a whole-organism level as Drazen et al. delineated an association between exogenous administration of leptin, which results in decreased fat mass, and decreased humoral immune function28.
Our results contrast significantly with an important recent report which found that elevated DC lipid content, in the context of cancer, is associated with poorly immunogenic DC. Herber et al. found that DC cultured in tumor conditioned media as well as DC harvested from tumor bearing mice or cancer patients exhibited high lipid content, primarily elevated triglycerides, and had reduced capacity to process antigens and stimulate CD4+ and CD8+ T cells11. The confluence of the current report with that of Herber et al. suggests that there is a dichotomy between the effects of endogenous intracellular lipids in hepatic DC, which enhance immunogenicity, from the context of cancer, where exogenous “toxic” lipid accumulation reduces DC capacity to mount an anti-tumor T cell response11. It is conceivable that within the context of cancer, a host of additional well-characterized and undetermined factors may suppress DC immunogenicity29. It is also clear that DC lipid content cannot be regarded universally as pro-immunogenic. As evidence of this concept, we show that DC in diverse compartments have considerable variations in their total lipid levels. For example, liver DC have higher overall lipid content than spleen DC (Fig. S2d). Nevertheless, numerous previous reports have found that, in both humans and rodents, bulk liver DC are poorly immunogenic in comparison to spleen DC10, 30–31. Hence, whereas endogenous lipid content in hepatic DC subsets overtly defines their immunogenic potential - and have important experimental and potential therapeutic implications as delineated above - lipid content cannot be considered universally beneficial or toxic to DC ability to engage immunity across clinical or experimental contexts. However, differentiating these determinants, outside the liver requires further experimentation.
Methods
Animal Models
Six week-old C57BL/6, OT-I, and OT-II mice were purchased from Taconic (Germantown, New York). CD45.1, Apo-E−/−, and Rag1 mice were purchased from Jackson (Bar Harbor, Maine). NASH was induced with a methionine-choline deficient diet (Dyets, Bethlehem, Pennsylvania) for 6 weeks. Liver fibrosis was induced using i.p. thioacetamide (250 mg/kg; Sigma-Aldrich, St. Louis, Missouri) for 12 weeks. For partial hepatectomy experiments, mice underwent left lobectomy or sham laparotomy. Bile duct ligation was accomplished using 3mm surgical micro-clips and livers examined at 24h. In selected experiments, mice were treated with C75 (10 mg/kg; Sigma-Aldrich). Animal procedures were approved by the NYU IACUC.
Human Cellular Isolation
Liver was collected from patients undergoing hepatic resection. Patients with viral hepatitis were excluded. For patients with metastatic liver deposits, tissues harvested were ≥ 5 cm away from the nearest implant. To isolate hepatic non-parenvhymal cells (NPC), liver tissue was flushed with HBSS containing 1% Type IV Collagenase and DNase, mechanically minced, and then incubated at 37 °C for 30 min. The cell pellet was reconstituted, and the hepatocytes were removed via low speed spin centrifugation (30g). Mononuclear cells from the remaining NPC were isolated by Ficoll-Paque density centrifugation. Studies using human tissue were approved by the NYU IRB.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by a Liver Scholar Award from the American Liver Foundation (GM), a Society of University Surgeons Junior Faculty Grant (GM), and NIH Awards DK085278 (GM), CA155649 (GM) and CA108573 (ABF).
Abbreviations
- (DC)
Dendritic cells
- (MFI)
Median fluorescence index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions: Junaid Ibrahim1,2, Andrew H. Nguyen1,2,3, Adeel Rehman1, Atsuo Ochi1, Ashim Malhotra1, Justin R. Henning1,4,, Christopher S. Graffeo1,2,3, Aaron Mitchell1, Andrea S. Bedrosian1, Constantinos P. Zambirinis1,2, Rocky Barilla1,4, Mohsin Jamal1, Sana Badar1, Radghavendra Rao1, Devrim Acehan1, Alan B. Frey4,5, George Miller2,3,4,5
1acquisition of data, 2drafting of manuscript, 3interpretation of data, 4critical revision, 5study design
Reference List
- 1.Tsuji NM, et al. Oral tolerance: intestinal homeostasis and antigen-specific regulatory T cells. Trends Immunol. 2008;29:532–40. doi: 10.1016/j.it.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Zavadsky KE, et al. Liver metastases from colorectal carcinoma: incidence, resectability, and survival results. Am Surg. 1994;60:929–33. [PubMed] [Google Scholar]
- 3.Solari MG, et al. Human dendritic cells and transplant outcome. Transplantation. 2008;85:1513–22. doi: 10.1097/TP.0b013e318173a768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limmer A, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–81. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 5.Breous E, et al. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–21. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly MK, et al. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. Journal of leukocyte biology. 2010;87:713–25. doi: 10.1189/jlb.0909607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson AW, et al. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 8.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia S, et al. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112:3175–85. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillarisetty VG, et al. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 11.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai J, et al. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 14.Katz SC, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187:1150–6. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeki C, et al. Accumulation of functional regulatory T cells in actively inflamed liver in mouse dendritic cell-based autoimmune hepatic inflammation. Clin Immunol. 2010;135:156–66. doi: 10.1016/j.clim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Khandelwal, et al. Distinct MHC class II molecules are associated on the dendritic cell surface in cholesterol-dependent membrane microdomains. J Biol Chem. 2010;285:35303–10. doi: 10.1074/jbc.M110.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plitas G, et al. Dendritic cells are required for effective cross-presentation in the murine liver. Hepatology. 2008;47:1343–51. doi: 10.1002/hep.22167. [DOI] [PubMed] [Google Scholar]
- 18.Miller G, et al. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 2002;62:5260–6. [PubMed] [Google Scholar]
- 19.Joyee AG, et al. Invariant NKT cells preferentially modulate the function of CD8 alpha+ dendritic cell subset in inducing type 1 immunity against infection. J Immunol. 2010;184:2095–106. doi: 10.4049/jimmunol.0901348. [DOI] [PubMed] [Google Scholar]
- 20.Diana J, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–99. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Kijima M, et al. Dendritic cell-mediated NK cell activation is controlled by Jagged2-Notch interaction. Proc Natl Acad Sci U S A. 2008;105:7010–5. doi: 10.1073/pnas.0709919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly MK, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–25. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vabulas RM, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–53. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 24.Basseri S, et al. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. Journal of lipid research. 2009;50:2486–501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson AW, et al. Tolerogenic dendritic cell-regulatory T-cell interaction and the promotion of transplant tolerance. Transplantation. 2009;87:S86–90. doi: 10.1097/TP.0b013e3181a2dcec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devries-Seimon T, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bataller R, et al. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drazen DL, et al. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–75. doi: 10.1210/endo.142.7.8271. [DOI] [PubMed] [Google Scholar]
- 29.Pinzon-Charry A, et al. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunology and cell biology. 2005;83:451–61. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 30.Bamboat ZM, et al. Human liver dendritic cells promote T cell hyporesponsiveness. Journal of immunology. 2009;182:1901–11. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connell PJ, et al. Type-1 polarized nature of mouse liver CD8alpha− and CD8alpha+ dendritic cells: tissue-dependent differences offset CD8alpha-related dendritic cell heterogeneity. European journal of immunology. 2003;33:2007–13. doi: 10.1002/eji.200323379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.