Abstract
Steroid avoidance is safe and effective in children receiving kidney transplants in terms of graft function and survival, but the effects on allograft histology are unknown. In this multicenter trial, 130 pediatric renal transplant recipients were randomized to steroid-free (SF; n=60) or steroid-based (SB; n=70) immunosuppression, and underwent renal allograft biopsies at time of graft dysfunction and per protocol at implantation and 6, 12 and 24 months after transplantation. Clinical follow-up was 3 years post-transplant. Subclinical acute rejection was present in 10.6% SF vs. 11.3% SB biopsies at 6 months (p=0.91), 0% SF vs. 4.3% SB biopsies at 1 year (p=0.21) and 0% vs. 4.8% at 2 years (p=0.20). Clinical acute rejection was present in 13.3% SF and 11.4% SB patients by 1 year (p=0.74) and in 16.7% SF and 17.1% SB patients by 3 years (p=0.94) after transplantation. The cumulative incidence of antibody-mediated rejection was 6.7% in SF and 2.9% in SB by 3 years after transplantation (P=0.30). There was a significant increase in chronic histological damage over time (p<0.001), without difference between SF and SB patients. Smaller recipient size and higher donor age were the main risk factors for chronic histological injury in post transplant biopsies.
Keywords: histology, chronic allograft nephropathy (CAN), steroid free immunosuppression, pediatric transplantation, kidney allograft
INTRODUCTION
Kidney transplantation is the first-choice therapy for end-stage renal disease in children (1). Interstitial fibrosis and tubular atrophy (IFTA) remain a principal cause of progressive chronic histological damage and graft loss after 5 years post transplantation (2-4). Both immune and non-immune phenomena instigate this multifactorial process (5). The evolution of chronic renal allograft histology can therefore be regarded as a valuable surrogate marker for long-term graft outcome (6).
Chronic steroid usage is a mainstay of current immunosuppressive regimens for kidney transplantation, but is associated with important side effects, including diabetes mellitus, hyperlipidemia, hypertension, osteoporosis, cataract and disfigurement. This is especially important in pediatric and adolescent renal allograft recipients, who suffer from steroid-associated growth impairment (7;8). Immunosuppression protocols that completely avoid the use of steroids have been adopted in several studies in adult renal allograft recipients. These protocols appeared to be safe in terms of short-term renal allograft function and survival, with variable results for acute rejection incidence. The appearance of acute rejection is dependent on the length of induction therapy (9-11). In pediatric kidney transplantation, steroid-free immunosuppression was shown efficacious and safe in a single-center non-randomized trial (12;13).
A recent 3-year prospective, randomized, multicenter trial showed that complete steroid avoidance with prolonged IL-2 receptor blockade was safe and effective in children receiving kidney transplants in terms of graft function and survival (14). Another study showed that early steroid withdrawal (by day 4 after transplantation) in pediatric kidney transplantation was associated with favorable linear growth without increased risk for acute T-cell mediated rejection, at least in the first 6 months after transplantation (15).
There is conflicting data whether steroid avoidance influences the evolution of chronic histological transplant injury (16-18). In the current multicenter, prospective, randomized trial in pediatric renal allograft recipients, mentioned above, the performance of serial protocol biopsies allowed for the serial evaluation of histology and for assessment of the clinical and subclinical determinants of the 2-year evolution of acute and chronic histological damage between a steroid-free (SF) and a steroid-based (SB) immunosuppressive protocol. The clinical safety and efficacy end points of this study are reported separately (Sarwal et al, submitted).
METHODS
Study design and patients
We carried out a randomized, prospective, open-label, multicenter study in pediatric and adolescent renal-transplant recipients (14). Written informed consent was obtained for all patients and the study was approved by the institutional review boards of the collaborating centers. Patients between the ages of 1 and 21 years who received a primary, single-organ renal transplant from either a living donor or a deceased donor were eligible. Patients who were previously treated with steroids within 6 months of the proposed transplant, or had a peak PRA (panel reactive antibodies) > 20%, were excluded from this study. Patients who were to receive kidneys from HLA-identical donors, from non-heart beating deceased donors, from donors > 55 years of age or with prolonged cold ischemia time (>20 hours for simple cold storage; >30 hours for machine perfusion), were also excluded.
Patients were randomly assigned to one of two treatment groups by a web-based data entry system maintained by the clinical coordinating center: a steroid-free arm (SF) and a steroid-based arm (SB). For both the SF arm and the SB arm, oral tacrolimus was administered pre-operatively to recipients >5 years of age at a starting dose of 0.1 mg/kg/dose BID for living donor recipients and 0.1 mg/kg/dose QD for deceased donor recipients. Recipients <5 years of age received tacrolimus at 0.15 mg/kg/dose BID for living donor recipients and 0.15 mg/kg/dose QD for deceased donor recipients. Postoperatively, the oral tacrolimus dose was 0.07 mg/kg/dose BID adjusted subsequently to achieve target levels of 12-14 ng/ml from day 0-7, 10-12 ng/ml from week 2-8, 7-10 ng/ml from week 9-12 and 5-7 ng/ml after 12 weeks. Evidence of tacrolimus toxicity on any protocol biopsy resulted in a further lowering of the tacrolimus target level to 4-6 ng/ml before the first year and 3-5 ng/ml after the first year post-transplantation. Intravenous MMF was dosed at 1200 mg/m2/day in 2 divided doses pre-operatively and for the first 48 hours post-operatively. Oral MMF was dosed at 600-900 mg/m2/day in 2 divided doses, the dose ranging because of tolerability and side effects of MMF. This regimen was used in both the SF and the SB arm. Extended daclizumab (Zenapax®, Hoffman-La Roche) dosing was the investigational product for evaluation (BB-IND-10127 held by MS from 1999-2004, and NIAID from 2004-current). The dosing for daclizumab for the SF arm was 2 mg/kg pre-transplant followed by 1 mg/kg at weeks 2, 4, 6, 8, 11 and months 4, 5, and 6, in concordance with previous single-center experience (12;13). For the SB arm, daclizumab was given at a dose of 1mg/kg peri-operatively and then at week 2, 4, 6 and 8. In the SB arm, MMF and tacrolimus were dosed in a manner similar to the SF protocol. In the SB arm, prednisone 10 mg/kg was given peri-operatively followed by 2 mg/kg/day in subjects weighing <40 kg and 1.5 mg/kg/day in subjects weighing >40 kg. The prednisone dosing was tapered as follows: by the end of weeks 1, 2, 4, 6, 12, and 16, dosages were 0.5, 0.4, 0.3, 0.2, 0.15, and 0.1 mg/kg/day respectively. The prednisone dose of 0.1 mg/kg was achieved by no later than 6 months post transplant.
Concomitant medications included intravenous gancyclovir or oral valgancyclovir for anti-viral prophylaxis for minimum the first 100 days post-transplantation and trime-thoprim/sulfamethoxazole (Septra®) for pneumocystis prophylaxis for a minimum first 6 months post-transplantation. Further details are provided in the report on the safety and efficacy of this study (14).
Histological evaluation
“Protocol” kidney biopsies were performed at the time of transplantation (prior to reperfusion) and at 6, 12 and 24 months after transplantation. In addition, “indication” biopsies were performed at times of renal allograft dysfunction (i.e. increase in serum creatinine of >10% from baseline on 2 consecutive readings). Hematoxylin eosin and periodic acid-Schiff (PAS) stained sections obtained from formalin fixed, paraffin embedded tissue were sent to the central pathologist (N.K) at Stanford University, who remained blinded for any clinical information or timing of the biopsies. Immunohistochemical staining was performed on all biopsies with antiserum to C4d (dilution 1:20, catalog #04-B1-RC4D, Biomedica Gruppe, Austria), CD20 (dilution 1:1000, Catalog # M07SS, DAKO, Carpenteria, CA) and SV40 (for BK polyoma virus, dilution 1:100, Catalog # DPO2, Calbiochem, San Diego, CA).
The revised Banff criteria (Banff ‘07) (19) were used to semi-quantitatively score the severity of histological lesions (interstitial inflammation, tubulitis, intimal arteritis, glomerulitis, tubular atrophy [TA] and interstitial fibrosis [IF] (IF/TA grade), vascular intimal thickening, arteriolar hyalinosis, increase in mesangial matrix, transplant glomerulopathy), and to establish the diagnosis of acute T-cell mediated rejection, borderline changes and/or of antibody-mediated changes. Acute antibody-mediated rejection was diagnosed if C4d positivity in peritubular capillaries was combined with either glomerulitis or peritubular capillaritis, or both. Chronic antibody-mediated rejection was diagnosed when glomerular double contours were observed together with C4d positivity in peritubular capillaries. The number of globally sclerosed glomeruli was counted and classified into 4 groups (0% = 0; <25% = 1; 26–50% = 2; >50% = 3). In addition, the presence or absence of isometric tubular vacuolization, tubular microcalcifications and of ischemic glomerular changes (shrinkage of the glomerular capillary tuft, wrinkling and thickening of the capillary walls and thickening of Bowman’s capsule or pericapsular fibrosis) was scored separately. A chronic damage score was calculated according to the previously described addition of interstitial fibrosis, tubular atrophy, vascular intimal thickening and glomerulosclerosis; score range 0-12 (20). For each specimen, the single high-power field with the highest CD20+ cell count was identified, and cell counts of more than 275 and less than 100 were chosen arbitrarily as definitions of CD20+ and CD20i status, so that the high threshold was more than 2.5 times the low threshold, as was described previously (21). In addition, the Chronic Allograft Damage Index (CADI) score was calculated in all biopsies (score range 0–18) (22), as well as a recently proposed chronic calcineurin inhibitor nephrotoxicity (cCNIT) score (score range 0–15) (4).
All patients with clinical episodes (associated with graft dysfunction) of Banff grade ≥ I acute cellular rejections were treated with pulsed doses of methylprednisolone. Graft dysfunction was defined as an increase in serum creatinine of >10% from baseline on 2 consecutive readings. Subclinical acute cellular rejection (in protocol biopsies, at time of stable graft function) was treated with intensification of baseline immunosuppression without additional pulse therapy. After treatment of acute rejection, patients remained in the SF group without maintenance steroid therapy, unless two biopsy proven acute cellular rejections occurred within 3 months, in which case subject switched to SB arm. Patients in the SF study group who switched to steroids were treated with “reduced follow-up” (no later protocol biopsies were performed in these patients), and therefore, these patients were no longer included in the evaluation of chronic lesions progression. No treatment adjustments were advocated for the appearance or progression of chronic histological lesions as the precise etiology of these lesions is not clear.
Statistical analysis
All randomized patients were evaluated on an intent-to-treat basis. The association between time post transplantation and the prevalence of different grades of histological lesions was analyzed with the Cochran–Mantel–Haenszel statistic. To model the risk factors (recipient and donor age and gender, recipient size, donor source, graft quality at implantation, cold ischemia time, treatment group and acute rejection) for the different histological patterns by time after transplantation, and to control for repeated samples from the same patient, the GENMOD procedure with a generalized-estimating-equations (GEE) approach was used, with an independent correlation structure of the GEE model and a repeated statement to take into account the repeated measures and to allow entrance of patients with missing data in the analysis; final models were constructed after backward elimination. IF/TA grade, cCNIT score, CADI score and glomerulosclerosis were analyzed as ordinal variables, vascular intimal thickening, arteriolar hyalinosis, ischemic glomeruli and tubular microcalcifications were dichotomized for analysis. For purposes of analysis, donors were divided into 3 groups according to donor age: <25 years, 25-35 years and >35 years. For variance analysis of continuous variables in different groups, nonparametric Wilcoxon-Mann-Whitney U non-parametric one-way ANOVA and parametric one-way ANOVA were used, as appropriate. Dichotomous variables were compared using the chi-square test. Survival distributions were compared using the log-rank test and plotted using the Kaplan-Meier method. For chronic histology lesions, patients were censored at the time of their last biopsy. Correlations between ordinal variables were assessed by Spearman correlation analysis. Data are expressed as mean and standard deviation (SD), odds ratios from the GEE analysis are presented with a 95% confidence interval. The odds ratios presented in the multivariate models are adjusted for the other independent variables included in these final models. In a power analysis based on a previous probability of IF/TA presence of 75% at 2 years after transplantation (4), 32 patients were needed in each group to detect a difference in IF/TA probability at 2 years of 40%, with alpha=0.05 and a power of 70%. All P values were two-sided, and those less than 0.05 were considered to indicate statistical significance. Data analysis was performed using SAS software (SAS version 9.1; SAS institute, Cary, NC).
RESULTS
Patients
From March 2004 to July 2006, a total of 130 patients from 12 U.S. sites underwent randomization, 70 patients to the SB arm and 60 patients to the SF arm (Figure 1). More SB patients were enrolled in two centers, resulting in unequal final numbers in the 2 arms. Both groups were well matched with respect to demographic characteristics, without significant inter-group differences (Table 1). Due to the preferential allocation of younger deceased donor kidneys to pediatric recipients, there was a significant correlation between donor age and donor source (living versus deceased donor): living donors were significantly older than deceased donors (35.8 ± 9.0 vs. 23.1 ± 7.0 years; P<0.001). Five patients from the SF study group (8.3%) switched to steroids. They remained in the study but were treated with “reduced follow-up” (no later protocol biopsies were performed in these patients).
Figure 1. Study design and numbers of biopsies included.
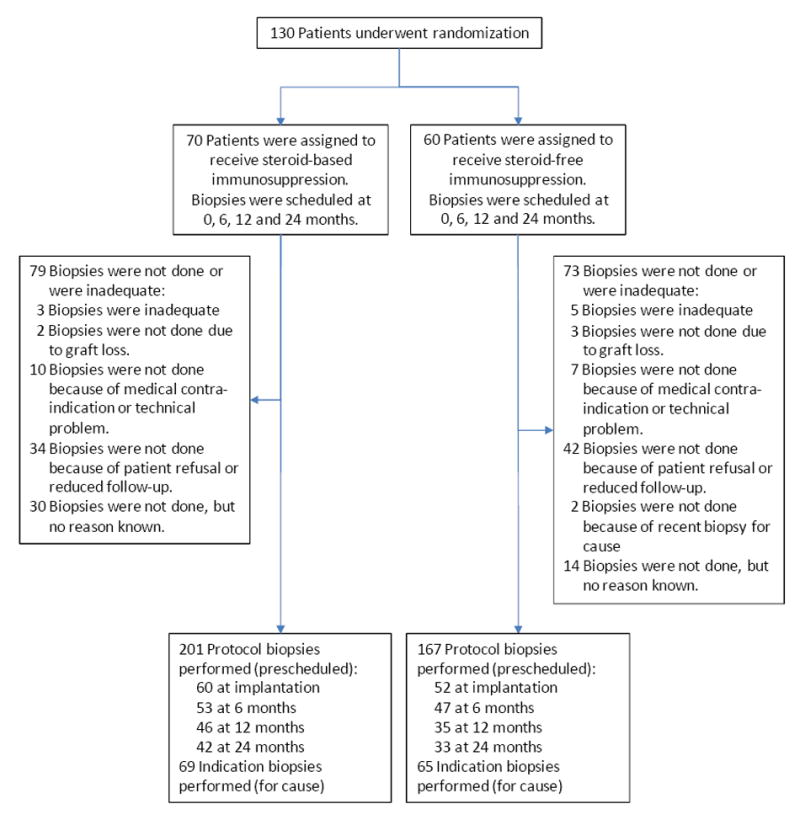
All patients who underwent randomization and transplantation were included in the intent-to-treat analysis.
TABLE 1. Baseline Patient Characteristics.
| Steroid-free treatment group | Steroid-based treatment group | P value | |
|---|---|---|---|
| Number of patients | 60 | 70 | |
| Recipient age | 11.8 ± 5.42 | 11.9 ± 6.08 | 0.93 |
| Infant: <yrs old (%) | 18.3% | 21.4% | 0.66 |
| 5-12 years old (%) | 30% | 22.9% | 0.36 |
| >12 years old (%) | 51.7% | 55.7% | 0.64 |
| Recipient weight (kg) | 40.2 ± 18.9 | 40.7 ± 22.9 | 0.86 |
| Recipient height (cm) | 138 ± 26.5 | 136 ± 33.3 | 0.77 |
| Recipient size (BSA in m2) | 1.21 ± 0.41 | 1.22 ± 0.49 | 1.00 |
| Recipient race | 0.56 | ||
| White | 33/60 (55%) | 35/70 (50%) | |
| African American | 15/60 (25%) | 19/70 (27%) | |
| Asian | 5/60 (8%) | 3/70 (4%) | |
| Other | 7/60 (12%) | 13/70 (19%) | |
| Recipient gender (female) | 20/60 (33%) | 28/70 (40%) | 0.42 |
| Cause of ESRD (1,2,3,4,5,6,7,8)* | 8%,3%,18%,2%,12%,15%,3%,38% | 10%,1%,16%,11%,11%,9%,9%,33% | 0.33 |
| Donor age | 29.1 ± 10.1 | 27.4 ± 10.0 | 0.34 |
| Donor gender (female) | 28/60 (47%) | 28/70 (40%) | 0.44 |
| Living donor | 25/60 (42%) | 27/70 (39%) | 0.59 |
| Cold ischemia time (mins) | 705 ± 479 | 716 ± 417 | 0.90 |
| Total number of biopsies | 3.8 ± 2.1 | 3.9 ± 1.8 | 0.84 |
| Number of indication biopsies | 1.0 ± 1.4 | 1.1 ± 1.2 | 0.86 |
| Number of protocol biopsies | 2.8 ± 1.2 | 2.8 ± 1.3 | 0.91 |
| Biopsy at implantation | 52/60 (87%) | 60/70 (86%) | >0.99 |
| IFTA grade 1 | 1/52 (1.9%) | 1/60 (1.7%) | >0.99 |
| Arteriolar hyalinosis | 2/52 (3.9%) | 2/60 (3.3%) | >0.99 |
| Glomerulosclerosis (%) | 1.5 ± 3.3 | 1.7 ± 3.5 | 0.71 |
| Vascular intimal thickening | 1/52 (1.9%) | 3/60 (5.0%) | 0.62 |
| Tubular microcalcifications | 1/52 (1.9%) | 1/60 (1.7%) | >0.99 |
1=glomerulonephritis; 2=polycystic kidney disease; 3=dysplasia; 4=reflux nephropathy; 5=obstructive uropathy; 6=FSGS; 7=congenital nephrotic syndrome; 8=others
Histological evolution
After exclusion of inadequate biopsies (N=7), 502 biopsies were available, 368 protocol biopsies (112 at implantation, 100 at 6 months, 81 at 12 and 75 at 24 months after transplantation) and 134 indication biopsies (obtained at time of graft dysfunction, median202 days after transplantation). Patients who did not have biopsies at the protocol biopsy time points did not have more biopsies for cause during follow-up, neither did they experience more or less episodes of acute T-cell mediated rejection or borderline changes (Supplemental Table 1).
At implantation, 98% of biopsies had IFTA grade 0. The calculated chronic histological damage score is shown in Table 1 and Supplemental Table 2, and demonstrates the excellent quality of the kidneys at time of transplantation.
Biopsy-proven acute clinical T-cell mediated rejection (diagnosed ina biopsy for cause) occurred in 13.3% SF patients vs. 11.4% SB patients in the first year after transplantation (P=0.74), and in 16.7% SF patients vs. 17.1% SB patients by 3 years after transplantation (P=0.94) (Table 2 and Figure 2A). Of all indication biopsies performed, 28/134 (20.9%) showed acute T-cell mediated rejection, and 27/134 (20.1%) showed borderline changes. This percentage did not differ between study arms. C4d stains were available for 116/134 indication biopsies and for 86/100 biopsies at 6 months, 75/81 at 12 months and 72/75 at 24 months. In 8/116 (6.9%) of indication biopsies, antibody-mediated rejection was diagnosed. Six indication biopsies showing antibody-mediated rejection had concomitant acute T-cell mediated rejection, two other biopsies from a single patient showed concomitant borderline changes. Acute and chronic antibody-mediated changes were observed together in 3/8 biopsies with antibody-mediated rejection. Two patients, both in the SF group, had repeated antibody-mediated rejection. No statistically significant differences were observed between the SF and SB patients. It should however be mentioned that at 3 years after transplantation, the cumulative incidence of acute antibody-mediated rejection in indication biopsies was numerically higher in the SF group (6.7%) compared to the SB group (1.4%), without reaching statistical significance (Table 2).
TABLE 2. Acute clinical and subclinical rejection prevalence according to treatment arm.
This table illustrates how many patients had at least one biopsy with borderline changes, acute T-cell mediated rejection, acute antibody-mediated rejection and chronic antibody-mediated rejection. P-values were obtained using the Chi Square test. The cumulative incidence is reporting on the number of patients who had at least one biopsy with rejection within the given time frame. Patients with more than one biopsy showing rejection were counted once.
| Steroid-free treatment group | Steroid-based treatment group | P value* | |
|---|---|---|---|
| Number of patients | 60 | 70 | |
| Indication biopsies: associated with graft dysfunction | |||
| Cumulative incidence of acute T-cell mediated rejection | |||
| At 1 year | 8/60 (13.3%) | 8/70 (11.4%) | 0.74 |
| At 3 years | 10/60 (16.7%) | 12/70 (17.1%) | 0.94 |
| Cumulative incidence of borderline changes in patients without T-cell mediated rejection | |||
| At 1 year | 4/60 (6.67%) | 5/70 (7.14%) | 0.92 |
| At 3 years | 6/60 (10.0%) | 8/70 (11.4%) | 0.79 |
| Cumulative incidence of acute T-cell mediated rejection or borderline changes | |||
| At 1 year | 12/60 (20.0%) | 13/70 (18.6%) | 0.84 |
| At 3 years | 16/60 (26.7%) | 20/70 (28.6%) | 0.81 |
| Cumulative incidence of acute antibody-mediated rejection | |||
| At 1 year | 3/60 (5.00%) | 1/70 (1.43%) | 0.24 |
| At 3 years | 4/60 (6.67%) | 1/70 (1.43%) | 0.12 |
| Cumulative incidence of chronic antibody-mediated rejection | |||
| At 1 year | 0/60 (0.0%) | 1/70 (1.43%) | 0.35 |
| At 3 years | 1/60 (1.67%) | 1/70 (1.43%) | 0.91 |
| Protocol biopsies: with stable graft function | |||
| Prevalence of subclinical acute T-cell mediated rejection | |||
| At 6 months | 5/47 (10.6%) | 6/53 (11.3%) | 0.91 |
| At 12 months | 0/35 (0.0%) | 2/46 (4.3%) | 0.21 |
| At 24 months | 0/33 (0.0%) | 2/42 (4.8%) | 0.20 |
| Prevalence of subclinical borderline changes | |||
| At 6 months | 4/47 (8.5%) | 5/53 (9.4%) | 0.87 |
| At 12 months | 6/35 (17.1%) | 2/46 (4.3%) | 0.06 |
| At 24 months | 4/33 (12.1%) | 1/42 (2.4%) | 0.09 |
| Prevalence of acute antibody-mediated rejection | |||
| At 6 months | 0/41 (0.0%) | 1/45 (1.89%) | 0.34 |
| At 12 months | 0/33 (0.0%) | 0/42 (0.0%) | NA |
| At 24 months | 0/33 (0.0%) | 0/39 (0.0%) | NA |
| Prevalence of chronic antibody-mediated rejection | |||
| At 6 months | 0/41 (0.0%) | 1/45 (1.89%) | 0.34 |
| At 12 months | 0/33 (0.0%) | 0/42 (0.0%) | NA |
| At 24 months | 0/33 (0.0%) | 0/39 (0.0%) | NA |
| All biopsies (indication and protocol biopsies together) | |||
| Cumulative incidence of acute T-cell mediated rejection | |||
| At 1 year | 12/60 (20.0%) | 15/70 (21.4%) | 0.84 |
| At 3 years | 14/60 (23.3%) | 20/70 (28.6%) | 0.50 |
| Cumulative incidence of borderline changes in patients without acute T-cell mediated rejection | |||
| At 1 year | 9/60 (15.0%) | 8/70 (11.4%) | 0.55 |
| At 3 years | 11/60 (18.3%) | 9/70 (12.9%) | 0.39 |
| Cumulative incidence of acute T-cell mediated rejection or borderline changes | |||
| At 1 year | 21/60 (35.0%) | 23/70 (32.9%) | 0.80 |
| At 3 years | 25/60 (41.7%) | 29/70 (41.4%) | 0.98 |
| Cumulative incidence of acute antibody-mediated rejection | |||
| At 1 year | 3/60 (5.00%) | 2/70 (2.86%) | 0.53 |
| At 3 years | 4/60 (6.67%) | 2/70 (2.86%) | 0.30 |
| Cumulative incidence of chronic antibody-mediated rejection | |||
| At 1 year | 0/60 (0.0%) | 2/70 (2.86%) | 0.19 |
| At 3 years | 1/60 (1.67%) | 2/70 (2.86%) | 0.65 |
| Cumulative incidence of acute or chronic antibody-mediated rejection | |||
| At 1 year | 3/60 (5.0%) | 2/70 (2.86%) | 0.53 |
| At 3 years | 4/60 (6.67%) | 2/70 (2.86%) | 0.30 |
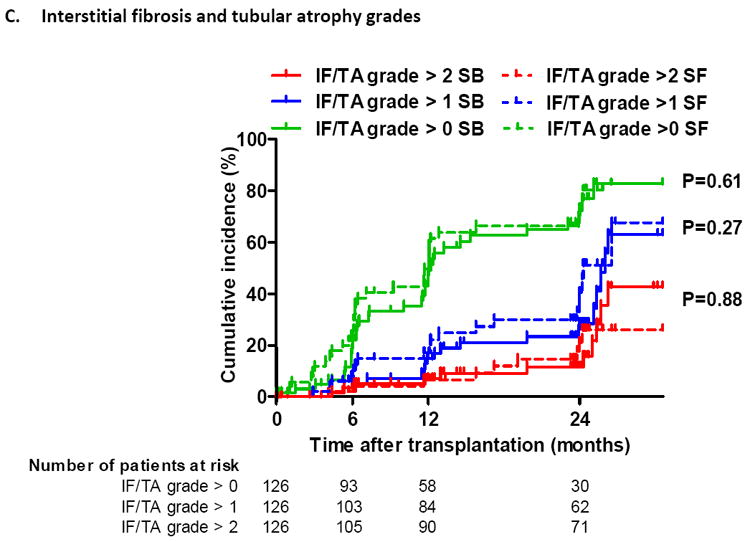
Figure 2. Cumulative incidence of acute and chronic histological lesions by time after transplantation.
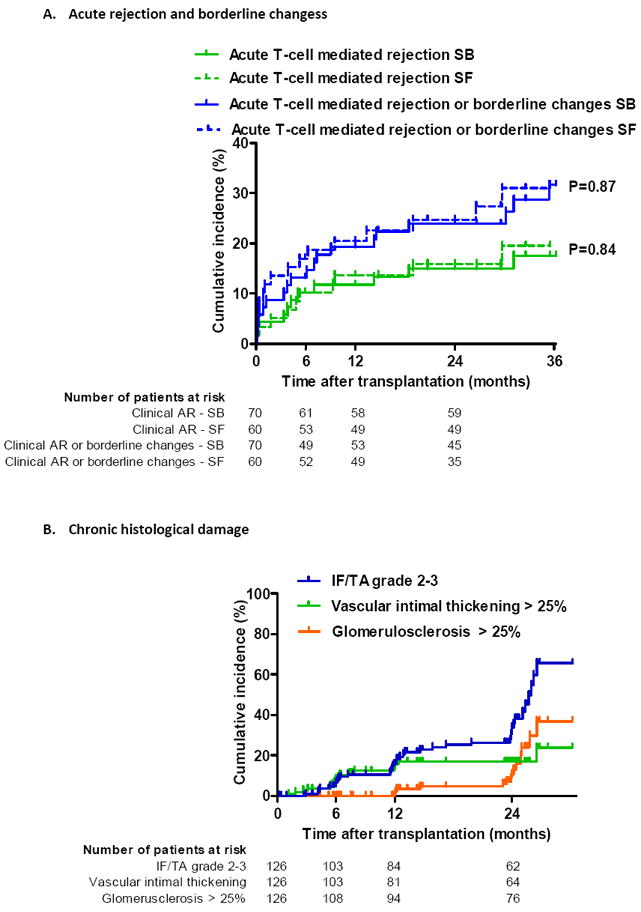
(A) Cumulative incidence of clinical (in biopsies for cause) acute T-cell mediated rejection or borderline changes according to treatment arm.
(B) Cumulative incidence of different grades of interstitial fibrosis/tubular atrophy (IFTA), vascular intimal thickening and global glomerulosclerosis by time after transplantation, expressed as 1-the Kaplan-Meier survival estimate.
(C) Cumulative incidence of different grades of interstitial fibrosis/tubular atrophy (IFTA) according to treatment arm. IFTA grade 1 is IFTA encompassing <25% of the biopsy core, while grade 2 corresponds to 25-50% and grade 3 to IFTA in >50% of the biopsy.
For the Kaplan–Meier estimates of event rates presented here, patients were censored at the time of their last biopsy or at 24 months if indication biopsies were performed later than 24 months. The histological lesions were scored according to the updated Banff classification on sequential biopsy specimens obtained on indication (graft dysfunction) and at prescheduled time points (protocol biopsies). P-values comparing survival distribution between SF and SB study group were obtained using the log-rank test.
When we examined protocol biopsies, performed in patients with stable graft function, there were no differences in subclinical acute T-cell mediated rejection episodes between both groups (Table 2 and Figure 3A). Cumulative acute cellular rejection incidence (including subclinical rejection in protocol biopsies) at 1 year was 20.0% in SF patients vs. 21.4% in SB patients (P=0.84) and 3 years after transplantation 23.3% in the SF patient group and 28.6% in the SB patient group by (P=0.50) (Table 2 and Figure 2A). Although borderline changes were seen numerically more frequently in the steroid-free patient group at both 12 and 24 months after transplantation (Table 2) there was no increased risk for borderline rejection changes in protocol biopsies between SF and SB patient groups. The incidence of subclinical antibody-mediated rejection was too low in this study to allow for any meaningful statistical analysis.
Figure 3. Prevalence of different histological lesions at each time point after transplantation.
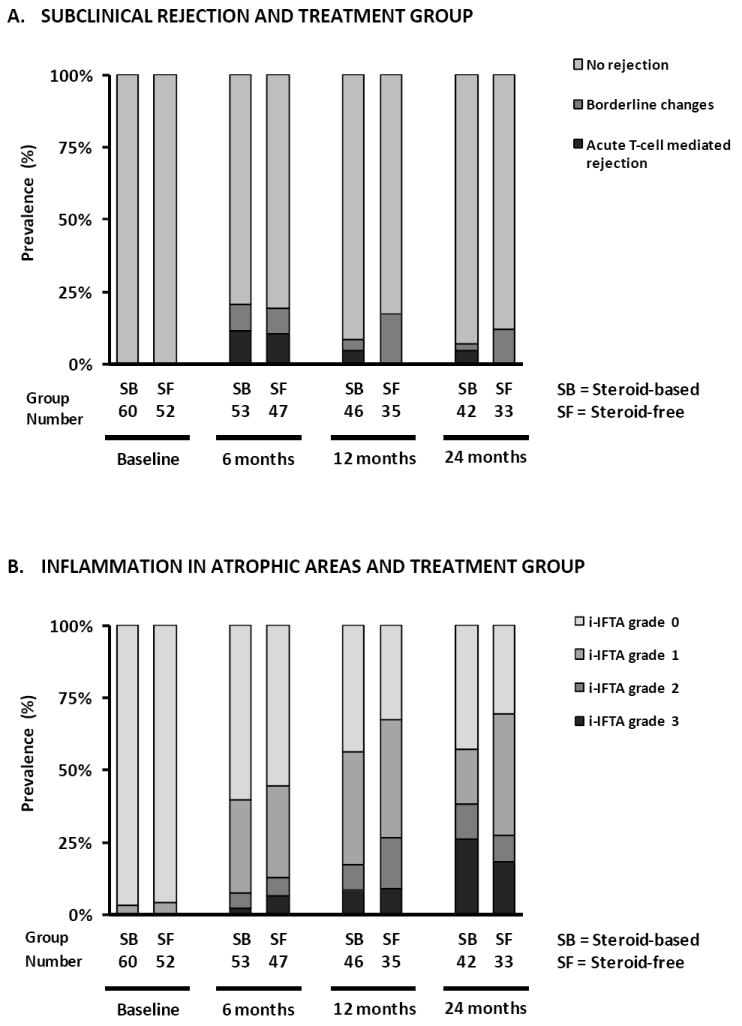
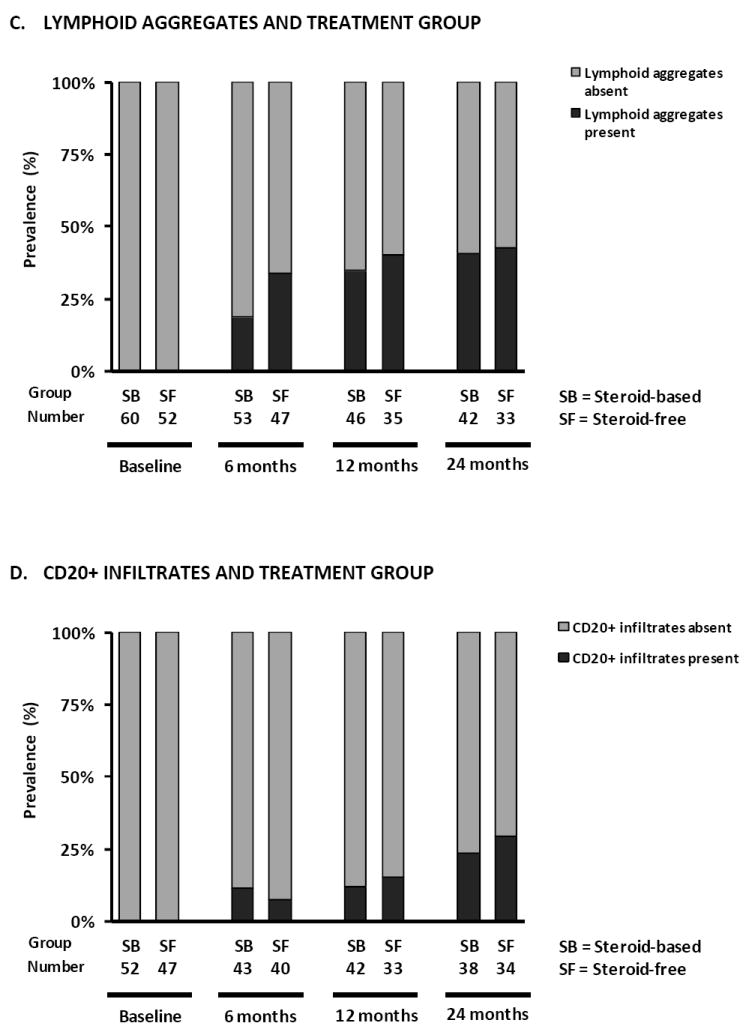
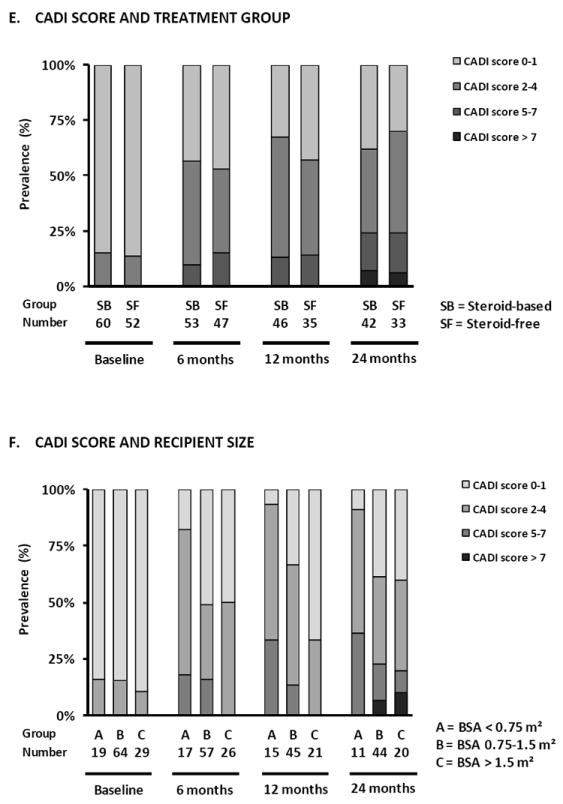
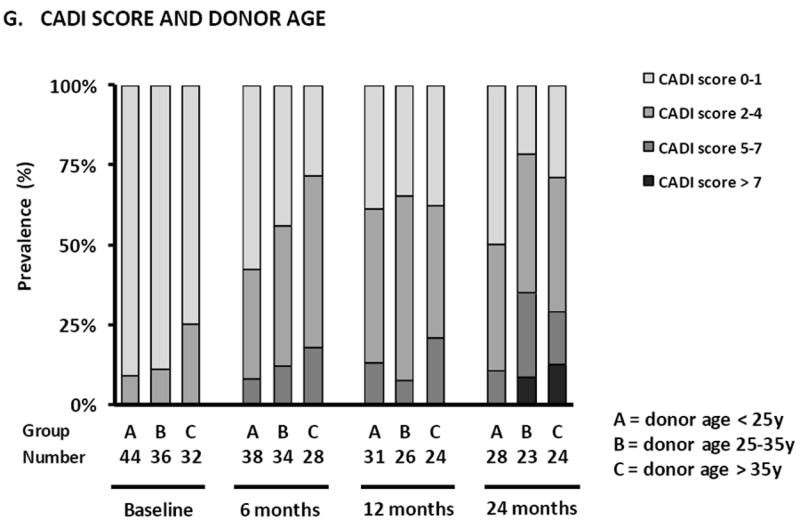
Prevalence of subclinical rejection (A), inflammation in atrophic areas (B), lymphoid aggregates (C) and CD20+ infiltrates (D) according to treatment arm in protocol biopsies. (E) Prevalence of different degrees of CADI score according to treatment arm, (F) recipient size and (G) donor age in protocol biopsies. There were no statistically significant differences between the treatment arms in terms of the different histological lesions in GEE analysis (Supplemental Table 3). Smaller recipient size (p<0.0001) and older donor age (p<0.05) were independently associated with higher degrees of CADI score in post-transplantation biopsies in GEE analysis.
Regarding more subtle features of immunologic activity (Figure 3B, 3C and 3D), we found no differences between both treatment groups in the prevalence of inflammation in atrophic areas or CD20+ (B-cell) infiltration at the different protocol biopsy time points. There was a non-significant trend towards more lymphoid aggregates in protocol biopsies of the SF group patients at 6 months (34.0% vs. 18.9%; p=0.08). This difference was no longer present by 12 months and 24 months after transplantation (40% vs. 35%, p=0.45 and 42% vs. 40%, p=0.87 respectively). The prevalence of histological lesions suggestive of antibody-mediated rejection (peritubular capillaritis, C4d positivity) was too low to allow for robust statistical analysis: 1.2 % at 6 months, 0% at 12 months and 2.7% at 24 months after transplantation (p=0.31; Supplemental Table 2).
In the first 2 years after transplantation, a gradual increase in IFTA, glomerulosclerosis, ischemic glomerular changes, vascular intimal thickening, tubular microcalcifications, mesangial matrix increase, the presence of lymphoid aggregates, CD20+ cell infiltration and interstitial inflammation in atrophic areas was observed in the overall group (Figure 2B and Supplemental Table 2). As a consequence, the CADI score and cCNIT score increased significantly in the first 2 years after transplantation: the number of patients with a CADI score of 0-1 decreased from 86% at implantation to 45%, 37% and 35% by 6, 12 and 24 months after transplantation (p<0.0001; Supplemental Table 2). The prevalence of arteriolar hyalinosis (3.6%, 8%, 7.4%, 6.7% at respectively 0, 6, 12 and 24 months; p=0.39) and transplant glomerulopathy (0%, 1%, 1.2%, 2.7% at respectively 0, 6, 12 and 24 months; p=0.09) did not increase during the first 2 years after transplantation (Supplemental Table 2). There was no association between C4d staining and CD20+ infiltration, but there was a significant association between the presence of lymphoid aggregates and B-cell infiltration: 87% of biopsies with CD20+ infiltration also had lymphoid aggregates, while only 19% of biopsies without CD20+ infiltration had lymphoid aggregates (P<0.0001).
Clinical determinants of chronic histological damage
When comparing chronic histological damage in protocol biopsies between the SF and the SB treatment arms, no significant difference in the prevalence or evolution of the different histological lesions (see above) or calculated scores was observed. Univariate Odds ratios for differences comparing steroid-free immunosuppression versus steroid-based immunosuppression were 1.14 (0.71-1.83) (p=0.60) for risk of higher IF/TA grade; 0.70 (0.38-1.28) (p=0.24) for presence of vascular intimal thickening; 1.94 (0.82-4.61) (0.13) for presence of arteriolar hyalinosis; 1.22 (0.77-1.93) (p=0.40) for presence of ischemic glomeruli; 0.98 (0.49-1.96) (p=0.95) for presence of tubular microcalcifications; 0.86 (0.51-1.46) (p=0.58) for risk of higher number of sclerosed glomeruli; 0.93 (0.58-1.49) (p=0.77) for risk of higher CADI scores (Figure 3E and Supplemental Table 3).
Small recipient size (closely correlating with recipient age) was an independent risk factor for a higher grade of IFTA (body surface area [BSA] <0.75 m2 vs. >1.5 m2, P=0.0002; 0.75-1.5 m2 vs. >1.5 m2, P=0.052), independent of time after transplantation (Figure 4F and Supplemental Table 3). Consequently, also the calculated CADI (BSA <0.75 m2 vs. >1.5 m2, P<0.0001; 0.75-1.5 m2 vs. >1.5 m2, p=0.02) and cCNIT (BSA <0.75 m2 vs. >1.5 m2, p=0.0002; 0.75-1.5 m2 vs. >1.5 m2, p=0.049) scores were significantly higher in post-transplantation biopsies from smaller pediatric recipients, independent of time after transplantation (Supplemental Table 3). Similar results were obtained when recipient size was replaced by recipient age.
Despite the pristine condition of the kidneys at implantation, there was a significant association between higher donor age and arteriolar hyalinosis in post-transplant protocol biopsies (Supplemental Table 3). Higher donor age was also independently associated with an increased risk for higher CADI score (>35 years vs. <25 years, p=0.0007; 25-35 years vs. <25 years, p=0.12) and cCNIT score (>35 years vs. <25 years, p=0.0001; 25-35 years vs. <25 years, p=0.01), independent of time after transplantation (Figure 3G and Supplemental Table 3). Higher donor age was also associated with vascular intimal thickening and glomerulosclerosis. Donor and recipient gender were not associated with renal allograft histology in the first 2 years after transplantation. The prevalence of antibody-mediated rejection was too low to allow for valid statistical analysis.
Graft function and survival
Graft function, calculated with the Schwartz formula (23), was similar for both the SF and SB group, at all time points in the first 3 years post transplantation. Overall renal allograft survival was 95% (57/60) and 90.0% (63/70) at 3 years post transplantation for the SF and the SB groups, respectively (p=0.30). Patient survival was 100% in both treatment arms.
There was a significant but weak correlation between the histological appearance of the grafts at 6 months and absolute graft function (mL/min) at 12 months: IFTA grade (p=0.0002; R=-0.38), inflammation in atrophic areas (p=0.004; r=-0.30), lymphoid aggregates (p=0.047; r=-0.21), the CADI score (p=0.0005; r=-0.35) and the cCNIT score (p=0.004; r=-0.30) all correlated significantly with absolute graft function at 1 year after transplantation. Similar correlations were observed between histological lesions at 6 months and absolute graft function at 24 months: IFTA grade (p=0.0006; r=-0.37), lymphoid aggregates (p=0.04; r=-0.23), the CADI score (p=0.002; r=-0.34) and the cCNIT score (p=0.003; r=-0.32). Graft function was not different between patients with or without subclinical acute T-cell mediated rejection.
DISCUSSION
In this serial histological analysis, embedded in a randomized multicenter clinical trial of steroid avoidance in pediatric renal allograft recipients treated with tacrolimus, MMF and prolonged induction with daclizumab, we found significant progression of chronic graft injury in the first 2 years post-transplantation in both study arms. Complete steroid avoidance with prolonged IL-2 receptor blockade was associated with neither a higher risk for clinical rejection nor with a higher risk for subclinical inflammation of renal allografts. There was no difference between the SB and the SF treatment arms with respect to chronic histological damage, graft function or graft survival. In addition, this study confirmed that in pediatric kidney transplant recipients, small recipient size and higher donor age are the primary risk factors for progressive chronic tubulo-interstitial damage of renal allografts. The histological appearance of the grafts in was significantly correlated to absolute graft function, although it should be noted that this correlation was rather weak.
The current study showed no increased risk of clinical acute T-cell mediated rejection with a steroid avoidance protocol in a pediatric population. Moreover, we demonstrated for the first time that a SF immunosuppressive regimen does not lead to higher prevalence of subclinical inflammation in this population, as detected in protocol biopsies (all with stable graft function). As both subclinical inflammation in non-atrophic areas and inflammation in atrophic areas have been associated with worse long-term renal allograft outcome (24;25), this is a reassuring finding that suggests relatively long-term safety of the steroid-free immunosuppressive protocol (Sarwal et al, submitted). No statistically significant differences were observed between the SF and SB patients in terms of antibody-mediated rejection, although the numerically higher prevalence of acute antibody-mediated rejection in the SF group is of potential clinical importance in recipients with an immunologically higher risk profile. Studies with longer follow-up are needed to elucidate whether this numeric difference translates in statistically significant differences on the long term. Studies in adult recipients have shown increased risk of graft rejection in steroid avoidance studies (26), which differs by the findings in the current pediatric study, and another recent multicenter, randomized trial in pediatric recipients (15). Whether this reflects the duration of daclizumab use, the pediatric population or other as yet undefined factors cannot be answered from the current study. Finally, as daclizumab has been withdrawn from the market, it will be necessary to separately evaluate the safety and efficacy of the IL-2 receptor blocker basiliximab for induction in steroid-free immunosuppressive regimens in pediatric kidney transplantation.
Importantly, the current study showed that donor age is a correlate of graft histology after transplantation and that progressive histological damage occurs even in kidneys from relatively young donors transplanted in young recipients, independent of the occurrence of acute rejection, these events being noted fairly soon after transplantation. The impact of older donor age and acceleration of chronic histological injury in the post-transplant allograft has been previously shown in published studies in adult recipients (2;27) and with single-center data in pediatric recipients (4;28).
The etiology of this histological damage is probably multifactorial (5), and the current study offers new insights into possible clinical determinants of this progressive injury. Importantly, we observed that there were no differences between the treatment arms in terms of immune injury. We also observed that steroids do not appear to have a protective role in the avoidance of chronic histological IF/TA damage after transplantation. Earlier animal model studies have suggested that steroids may have a protective role against calcineurin inhibitor induced nephrotoxicity (29), and one recent study suggested that acute calcineurin inhibitor nephrotoxicity is more prevalent in a steroid withdrawal group than in patients maintained on steroids, although the definition of acute CNIT was not detailed (16). In our current study, there was no significant difference in the incidence of chronic histological damage or histological lesions suggestive of calcineurin inhibitor nephrotoxicity between the SF and SB treatment groups, although we acknowledge that the power to detect minor histological effects of steroid avoidance is insufficient in the current study. This is related to attrition over time of the number of protocol biopsies in each treatment arm, a common phenomenon in protocol biopsy studies, even in adult recipients (2;27;30). In addition, lack of treatment blinding and lack of data on medication adherence and drug levels could have further decreased the study power, especially since medication adherence can be problematic in adolescents (31). The fact that graft loss was very low in the current study (in contrast to the protocol biopsy studies in adults), and the equal percentage of biopsies in each study arm, avoids any systematic bias that could arise from the declining proportion of biopsies over time. Larger protocol biopsy studies with lower attrition rates would be very welcome, but accrual and expense could compromise the feasibility of larger clinical trials of this nature in pediatric recipients.
Furthermore, we evaluated other clinical determinants of progressive chronic histological damage of renal allografts. We demonstrated that higher donor age is independently associated with increased chronic histological damage, despite the pristine nature of the kidneys at time of implantation. This suggests that the effects of higher donor age reach beyond the quality of the graft at implantation and continue to be important for the histological and functional evolution in the post-transplantation period (32;33). Some of these cellular and molecular mechanisms of aging include subcellular structural changes, DNA mutation accumulation, telomere shortening, oxidative stress, accumulation of advanced glycosylation end products etc resulting in distinct gene expression profiles. The functional limitations such as tubular dysfunction, increased susceptibility to ischemia and drug toxicity, likely persist even in the absence of overt structural changes in implantation biopsies.
Finally, in the current multicenter study, we independently validated the previously described association between smaller pediatric renal allograft recipient size and increased risk for chronic tubulo-interstitial injury (4;28). This finding could explain the absence of improvement of absolute GFR (mL/min) in association with growth of the smallest pediatric renal allograft recipients, in contrast to the findings in older children where the absolute GFR increases with growing (34-36). As we confirmed an association between small recipient size and chronic (irreversible) histological damage, the irreversibility of the functional adaptation of renal allograft function to the size of the pediatric recipients is likely caused by chronic renal graft ischemia, insufficient renal auto regulation and secondary chronic irreversible tubulo-interstitial damage associated with the donor-recipient size discrepancy. One could hypothesize that the aortic blood flow in smaller pediatric recipients is insufficient to provide adequate perfusion of the adult-sized kidney with adult-sized vasculature, as previously demonstrated in vivo (37;38). The independent effect of donor-recipient size discrepancy on both irreversible histological damage of the transplanted kidney and on its functional evolution, suggest that the current strategies to assure optimal intravascular volume (37;38) are not sufficient, and exploration of additional therapeutic approaches to increase allograft perfusion could further extend the graft survival benefit (39;40) of adult-sized kidneys transplanted into small children. It remains unclear how this accelerated progression of chronic histological damage in infants relates to the superb graft survival in this age group (39). It could be hypothesized that this apparent contradiction relates to the lower prevalence of late severe rejection phenomena in this age group compared to adolescent recipients, which is often associated with problems with medical adherence (31). Longer-time follow-up studies should be performed to unravel the impact of the progressive chronic histological damage on long-term graft outcome.
Supplementary Material
Acknowledgments
This study was supported by a grant AI-055795 to Dr. Salvatierra from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, made as a component of the Cooperative Clinical Trials in Pediatric Transplantation Consortium. RE was supported in part by the Casey Lee Ball Foundation. We are also grateful to Astellas and Roche Pharmaceuticals for their generous financial support for this trial.
We would like to acknowledge Dr. Neeraja Kambham for the systematic histological scoring of the biopsies and her pivotal role in this study. We also acknowledge the assistance and support of staff at the NIH- NIAID, including Nikki Williams, and Yvonne Morrison; staff at PPD for supporting the clinical database, specifically Jun Liu, Jason Berg, Brandon Graham; Janie Waskerwitz for study support; Sue Hsieh, Tara Sigdel and Li Li in the Sarwal Lab; and the willing participation of the patients and their families in the SNSO1 study.
MN and MMS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MMS, OS and MN were responsible for study design, data analysis, results interpretation and writing of the manuscript. MMS, MB, RE, VD, WH and RM were involved in patient inclusion, results interpretation and writing of the manuscript.
Footnotes
DISCLOSURE
None of the authors have any conflict of interest to declare in relation to this study.
References
- 1.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004 Jun 24;350(26):2654–62. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell B, Richard J, Borrows R, Fung CL-S, O’Connell PJ, Allen R, et al. The natural history of chronic allograft nephropathy. New Engl J Med. 2003;349(24):2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. American Journal of Transplantation. 2009 Mar;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 4.Naesens M, Kambham N, Concepcion W, Salvatierra O, Jr, Sarwal M. The evolution of non-immune histological injury and its clinical relevance in adult-sized kidney grafts in pediatric recipients. Am J Transplant. 2007;7(11):2504–14. doi: 10.1111/j.1600-6143.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JR, O’Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005 Oct 1;16(10):3015–26. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- 6.Hariharan S, Kasiske B, Matas A, Cohen A, Harmon W, Rabb H. Surrogate Markers for Long-Term Renal Allograft Survival. American Journal of Transplantation. 2004 Jul 1;4(7):1179–83. doi: 10.1111/j.1600-6143.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 7.Vidhun JR, Sarwal MM. Corticosteroid avoidance in pediatric renal transplantation. Pediatr Nephrol. 2005 Mar;20(3):418–26. doi: 10.1007/s00467-004-1786-4. [DOI] [PubMed] [Google Scholar]
- 8.Fine RN. Management of growth retardation in pediatric recipients of renal allografts. Nat Clin Pract Nephrol. 2007 Jun;3(6):318–24. doi: 10.1038/ncpneph0502. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas TR, Meier-Kriesche HU. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin J Am Soc Nephrol. 2008 Mar;3(Suppl 2):S101-16–S101-S116. doi: 10.2215/CJN.03510807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustine JJ, Hricik DE. Steroid sparing in kidney transplantation: changing paradigms, improving outcomes, and remaining questions. Clin J Am Soc Nephrol. 2006 Sep;1(5):1080–9. doi: 10.2215/CJN.01800506. [DOI] [PubMed] [Google Scholar]
- 11.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. American Journal of Transplantation. 2008 Feb;8(2):307–16. doi: 10.1111/j.1600-6143.2007.02057.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarwal MM, Vidhun JR, Alexander SR, Satterwhite T, Millan M, Salvatierra O., Jr Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation. 2003 Nov 15;76(9):1331–9. doi: 10.1097/01.TP.0000092950.54184.67. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Chang A, Naesens M, Kambham N, Waskerwitz J, Martin J, et al. Steroid-Free Immunosuppression Since 1999: 129 Pediatric Renal Transplants with Sustained Graft and Patient Benefits. American Journal of Transplantation. 2009 May 6;9(6):1362–72. doi: 10.1111/j.1600-6143.2009.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarwal MM, Benfield MR, Ettenger R, Dharnidharka V, Mathias R, Portale A, et al. Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with 3 year follow up. Submitted for publication. 2012 doi: 10.1111/j.1600-6143.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. American Journal of Transplantation. 2010 Apr;10(4):828–36. doi: 10.1111/j.1600-6143.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 16.Laftavi MR, Stephan R, Stefanick B, Kohli R, Dagher F, Applegate M, et al. Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery. 2005 Mar;137(3):364–71. doi: 10.1016/j.surg.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van VP. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008 Oct;248(4):564–77. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 18.Kumar MS, Xiao SG, Fyfe B, Sierka D, Heifets M, Moritz MJ, et al. Steroid avoidance in renal transplantation using basiliximab induction, cyclosporine-based immunosuppression and protocol biopsies. Clin Transplant. 2005 Feb;19(1):61–9. doi: 10.1111/j.1399-0012.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 19.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008 Apr;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 20.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006 Jan 26;354(4):343–52. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 21.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, et al. Molecular Heterogeneity in Acute Renal Allograft Rejection Identified by DNA Microarray Profiling. N Engl J Med. 2003 Jul 10;349(2):125–38. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz F, Paavonen T, Tornroth T, Koskinen P, Finne P, Salmela K, et al. Predictors of renal allograft histologic damage progression. J Am Soc Nephrol. 2005;16:817–24. doi: 10.1681/ASN.2004060475. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976 Aug;58(2):259–63. [PubMed] [Google Scholar]
- 24.Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. American Journal of Transplantation. 2005 Oct;5(10):2464–72. doi: 10.1111/j.1600-6143.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 25.Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, et al. Subclinical Rejection Associated with Chronic Allograft Nephropathy in Protocol Biopsies as a Risk Factor for Late Graft Loss. American Journal of Transplantation. 2006;6(4):747–52. doi: 10.1111/j.1600-6143.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 26.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis Transplantation. 2010 Jan 15;89(1):1–14. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 27.Naesens M, Lerut E, de JH, Van DB, Vanrenterghem Y, Kuypers DR. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009 Nov;20(11):2468–80. doi: 10.1681/ASN.2009020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dart AB, Schall A, Gibson IW, Blydt-Hansen TD, Birk PE. Patterns of chronic injury in pediatric renal allografts. Transplantation. 2010 Feb 15;89(3):334–40. doi: 10.1097/TP.0b013e3181bc5e49. [DOI] [PubMed] [Google Scholar]
- 29.Stillman IE, English J, Burdmann EA, Andoh TF, Franschini N, Bennett WM, et al. Prednisone alters the histopathology of chronic cyclosporine nephropathy. Exp Nephrol. 1997 Jan;5(1):61–8. [PubMed] [Google Scholar]
- 30.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, et al. The histology of solitary renal allografts at 1 and 5 years after transplantation. American Journal of Transplantation. 2011 Apr;11(4):698–707. doi: 10.1111/j.1600-6143.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 31.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant. 2005 Jun;9(3):381–90. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int. 2008 Sep;74(6):710–20. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 33.Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007 Jan;211(2):198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- 34.Gellert S, Devaux S, Schonberger B, May G. Donor age and graft function. Pediatr Nephrol. 1996 Dec;10(6):716–9. doi: 10.1007/s004670050197. [DOI] [PubMed] [Google Scholar]
- 35.Qvist E, Krogerus L, Ronnholm K, Laine J, Jalanko H, Holmberg C. Course of renal allograft histopathology after transplantation in early childhood. Transplantation. 2000 Aug 15;70(3):480–7. doi: 10.1097/00007890-200008150-00015. [DOI] [PubMed] [Google Scholar]
- 36.Dubourg L, Cochat P, Hadj-Aissa A, Tyden G, Berg UB. Better long-term functional adaptation to the child’s size with pediatric compared to adult kidney donors. Kidney Int. 2002 Oct;62(4):1454–60. doi: 10.1111/j.1523-1755.2002.kid576.x. [DOI] [PubMed] [Google Scholar]
- 37.Salvatierra O, Jr, Singh T, Shifrin R, Conley S, Alexander S, Tanney D, et al. Successful transplantation of adult-sized kidneys into infants requires maintenance of high aortic blood flow. Transplantation. 1998 Oct 15;66(7):819–23. doi: 10.1097/00007890-199810150-00001. [DOI] [PubMed] [Google Scholar]
- 38.Salvatierra O, Jr, Sarwal M. Renal perfusion in infant recipients of adult-sized kidneys is a critical risk factor. Transplantation. 2000 Aug 15;70(3):412–3. doi: 10.1097/00007890-200008150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Horslen S, Barr ML, Christensen LL, Ettenger R, Magee JC. Pediatric transplantation in the United States, 1996-2005. Am J Transplant. 2007 May;7(Suppl 1):1339–58. 1339–58. doi: 10.1111/j.1600-6143.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 40.Sarwal MM, Cecka JM, Millan MT, Salvatierra O., Jr Adult-size kidneys without acute tubular necrosis provide exceedingly superior long-term graft outcomes for infants and small children: a single center and UNOS analysis. United Network for Organ Sharing Transplantation. 2000 Dec 27;70(12):1728–36. doi: 10.1097/00007890-200012270-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


