Abstract
Background
Clinical and epidemiological studies suggest an association between cannabis use and psychosis, but this relationship remains controversial.
Methods
Clinical High-Risk (CHR) subjects (age 12–22) with attenuated positive symptoms of psychosis (CHR+, n=101) were compared to healthy controls (HC, n=59) on rates of substance use, including cannabis. CHR+ subjects with and without lifetime cannabis use (and abuse) were compared on prodromal symptoms and social/role functioning at baseline. Participants were followed an average of 2.97 years to determine psychosis conversion status and functional outcome.
Results
At baseline, CHR+ subjects had significantly higher rates of lifetime cannabis use than HC subjects. CHR+ lifetime cannabis users (N=35) were older (p=0.015, trend), more likely Caucasian (p=0.002), less socially anhedonic (p<0.001), and had higher Global Functioning (GF):Social scores (p<0.001) than non-users (N=61). CHR+ cannabis users continued to have higher social functioning than non-users at follow-up (p<0.001), but no differences on role functioning. A small sample of CHR+ cannabis abusers (N=10) showed similar results in that abusers were older (p=0.008), less socially anhedonic (p=0.017, trend), and had higher baseline GF:Social scores (p=0.006) than non-abusers. Logistic regression analyses revealed that conversion to psychosis in CHR+ subjects (N=15) was not related to lifetime cannabis use or abuse.
Conclusions
The current data do not support low to moderate lifetime cannabis use to be a major contributor to psychosis or poor social and role functioning in high-risk youth.
Keywords: Cannabis use, high-risk, prodrome, social functioning, conversion, psychosis
Introduction
Cannabis ranks first among illicit drug use in patients with schizophrenia (Martins & Gorelick, 2011). In a review of over 50 studies of cannabis misuse in patients with psychosis, Green et al. (2005) found combined prevalence rates of 42.2% for lifetime cannabis use and 22.5% for lifetime cannabis misuse. Given the high rates of lifetime cannabis use in populations with psychosis, many studies have examined the effects of cannabis use and misuse on symptom manifestation, course of illness, and outcome. Several studies have found that cannabis using (or abusing) patients with psychosis or schizophrenia have increased positive symptoms of psychosis (Negrete et al. 1986; Allebeck et al. 1993; Caspari, 1999; Bersani et al. 2002; Grech et al. 2005; Stirling et al. 2005, Mauri et al. 2006; Addington & Addington, 2007; Henquet et al. 2010) and fewer negative symptoms (Bersani et al. 2002; Dubertret et al. 2006; Compton et al. 2007) or, in some studies, no difference in negative symptoms (Allebeck et al. 1993; Caspari, 1999; Grech et al. 2005; Stirling et al. 2005, Addington & Addington 2007) compared to non-users. In some studies, cannabis using patients were more likely to have a younger age of onset of psychosis (Bersani et al. 2002; Van Mastrigt et al. 2004; Veen et al. 2004; Barnes et al. 2006; Mauri et al. 2006; Large et al. 2011), experience more psychotic relapses (Linszen et al. 1994; Martinez-Arevalo et al. 1994; Linszen et al. 1997; Hides et al. 2006) and have more hospital visits (Negrete et al. 1986; Caspari, 1999) than their counterparts who do not use cannabis.
Epidemiological studies have also supported an association between cannabis use and psychosis. Moore et al. (2007) conducted a meta-analysis of 11 population-based longitudinal studies from 7 countries and found that cannabis users had both an increased risk of psychotic symptoms (OR=1.41, 95% CI 1.20–1.65) and psychotic disorders (OR=2.58; 95% CI 1.08–6.13) compared to non-users. This relationship was noted to be dose-dependent with a two-fold increase in risk for high frequency users (OR=2.09; 95% CI 1.54–2.84), and subclinical psychotic experiences further modified the risk for psychosis in the context of cannabis use (Arsenault et al. 2002; Henquet et al. 2005). These findings were confirmed in a more recent epidemiologic sample of 1923 subjects aged 14–23 in whom both incident and continued cannabis use were associated with incident or persistent psychotic experiences (Kuepper et al. 2011). Based on these findings, several authors (e.g., Hall & Degenhardt, 2006; Moore et al. 2007; Kuepper et al. 2011; Large et al. 2011) suggested that the results are strong enough to support public education on the risks of cannabis use and accompanying policy changes, despite the fact that the gold standard for testing causality was not used in these studies.
Given suggestions that cannabis use is associated with psychosis onset, examining cannabis use in individuals who are at clinical high-risk for developing psychosis, has become of great interest. Clinical high-risk youth, who are characterized by attenuated positive symptoms of psychosis that are just emerging and by conversion rates to psychosis of approximately 20–30% (Cannon et al. 2008; Ruhrmann et al. 2010), represent a unique sample for examining the causal relationship between cannabis use and subsequent development of psychosis.
Phillips and colleagues (2002) in Melbourne, Australia were the first group to explore the connection between cannabis use and psychosis in their sample of 100 “ultra high-risk” subjects. The authors did not find a significant association between self-reported cannabis use or dependence in the year prior to study entry and risk for conversion to psychosis at a 12 month follow-up (37% conversion rate for cannabis use vs. 29% for no use; 39% conversion rate for cannabis dependence vs. 31% for no dependence). Cannabis use and dependence were included in a follow-up article on risk factors for psychosis, again with non-significant results (Yung et al. 2004).
A second study, by Kristensen and Cadenhead (2007) in California, did find a significant relationship between cannabis use and conversion at a one year follow-up but the sample size was very small (1 subject (3.1%) with no/low cannabis converted vs. 5 subjects (31.3%) with lifetime abuse/dependence; p=0.012). Alcohol or other illicit drug misuse was not found to be associated with conversion to psychosis, although a positive association was found between nicotine use and conversion in this sample.
A more recent study focused on the temporal patterns of cannabis use and prodromal symptoms in a sample of high-risk subjects in an urban area of New York City (Corcoran et al. 2008). Of the 32 participants, 13 were characterized as cannabis users/abusers. The authors reported that users and non-users did not differ significantly on positive and negative prodromal symptoms, affective symptoms, and level of functional impairment at baseline or rates of conversion to psychosis. Cannabis use was found to be temporally related to perceptual disturbances, but no other positive symptoms or total positive symptoms.
Furthermore, in a large prospective study of a clinical risk cohort for psychosis by the North American Prodrome Longitudinal Study (NAPLS) group, substance abuse in general was associated with conversion to psychosis (Cannon et al. 2008), but cannabis abuse was not mentioned. By contrast, in another large, European sample, alcohol or any substance abuse was not predictive of conversion to psychosis (Ruhrmann et al. 2010), but, again, cannabis abuse does not seem to have been investigated separately.
In light of suggestive findings in the general population and already psychotic individuals, but negative or mixed findings in clinical at-risk subjects, further clarification of the potentially mediating effects of cannabis on psychosis development in people considered to be prodromal for psychosis is needed. Thus, the current study aimed to further explore the relationship between cannabis use and abuse and the development of psychosis and to possibly clarify previous discrepant results through examination of a clinical high-risk longitudinal sample enrolled in the Recognition and Prevention (RAP) program in New York. The present study differs from the previous studies of the specific effects of cannabis on psychosis development in high-risk subjects in that it is a large sample and patients were followed for a longer period of time. Specific aims of the current study were to 1) characterize substance use rates, including cannabis use, in this high-risk sample; 2) determine if lifetime cannabis use (or abuse) in high-risk subjects is associated with increased prodromal symptoms at baseline and problems in functioning at baseline and follow-up; and 3) to determine if lifetime cannabis use (or abuse) is significantly related to psychosis conversion in this high-risk sample.
Methods
Participants
Participants in this study were selected from the larger RAP Program research program at The Zucker Hillside Hospital (ZHH) of the North Shore-Long Island Jewish Health System (NSLIJHS) in Glen Oaks, NY. Participants include subjects from Phase I of the project (2000 to 2006). Participants were referred to the program’s research clinic primarily from the inpatient and outpatient divisions of ZHH; in addition, referrals were received from community providers, school personnel, and participants’ family members. Healthy control (HC) subjects were recruited from the community through advertisements. All procedures were approved by the Institutional Review Board for ZHH. Written informed consent (with assent from participants under 18) was obtained from all participants.
Subjects include 101 Clinical High Risk - Positive (CHR+) adolescents and young adults between the ages of 12 and 22. High-risk status was defined by ratings on the positive symptom subscale of the Scale of Prodromal Symptoms (SOPS) developed by McGlashan and colleagues (Miller et al. 1999; McGlashan et al. 2001). The five positive symptom items: unusual ideas, suspiciousness, grandiosity, perceptual abnormalities, and disorganized communication, are rated on a 7 point scale (0 = not present to 6 = psychotic). A score of a 3 (moderate) to 5 (severe) on any one of these attenuated positive symptoms is required for inclusion in the CHR+ group. The mean total positive score was 8.75 (SD=4.0; range 3–21). The CHR+ group is closely aligned with the Attenuated Positive Syndrome group described in Miller et al. (1999) and used by many other high-risk programs. Further details on the CHR+ group and the RAP program working model have been described in previous publications (Cornblatt et al. 2003; Cornblatt & Auther, 2005). Conversion to psychosis is based on the development of a 6 level (psychotic) severity on any positive symptom item of the SOPS. The SOPS negative symptom subscale contains 6 items: social anhedonia, avolition, decreased expression of emotion, decreased experience of emotion, decreased ideational richness, and decline in occupational functioning. These negative symptom items are rated on a similar 7 point scale (0=not present to 6=extreme). Although there is no negative symptom score requirement for inclusion in the CHR+ group, the mean total negative score is 11.66 (SD 5.2; range 0–27).
The CHR+ participants described above were compared to 59 age matched HCs. Exclusionary criteria in the current study for both groups include Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, American Psychiatric Association, 1994) Axis I diagnoses of any psychotic disorder. Additional exclusion criteria for both groups included a history of neurological, neuroendocrine, or medical conditions known to affect brain functioning, IQ < 70, any current substance dependence (but not substance abuse), and lack of English fluency.
Measures
Estimated IQ was obtained by administering the Vocabulary and Block Design subtests of the WISC-III (Wechsler, 1991) for subjects under 16 years old and the WAIS-R (Wechsler, 1981) for those 16 years and older. The last subject enrolled received the WASI (Wechsler, 1999). Parental socioeconomic status (SES) was calculated according to the Hollingshead & Redlich (1958) two-factor classification system derived from highest parental education and occupation.
The Kiddie Schedule for Affective Disorders and Schizophrenia – Epidemiologic Version (KSADS-E; Orvaschel & Puig-Antich, 1994) was used to record lifetime alcohol and tobacco use, frequency, and quantity. For cannabis and other illicit substances, the KSADS-E queries for lifetime use, use in the six months prior to baseline, and frequency of lifetime use. This measure was also used to screen for lifetime substance use disorders (any dependency was exclusionary), to screen for psychotic disorders at baseline (also exclusionary), and to confirm psychosis conversion diagnoses at follow-up after a participant reached a 6 level (psychotic) positive symptom on the SOPS.
Two scales, one measuring social functioning and the other, role functioning, that were developed for the NIMH funded multi-site NAPLS project (Cornblatt et al, 2007) were used in the current study as additional outcome measures. The Global Functioning: Social Scale (GF:Social; Auther et al. 2006) is a 10 point scale (10=superior functioning to 1=extreme dysfunction) with anchors taking into account contact with friends, family, and age appropriate intimate relationships. The Global Functioning: Role Scale (GF:Role; Niendam et al. 2006) is rated on a similar 10 point scale taking into account type and quality of the role (generally school or work), amount of support needed, and the participant’s performance in the role.
Procedures
All assessments were conducted by trained masters or doctoral level psychologists or clinicians. The KSADS-E, SOPS, GF:Social, and GF:Role measures were administered at baseline and follow-up. For the purposes of the current analyses only follow-up data on functioning (based on the GF Scales) and conversion (based on the SOPS) are presented. Follow-up assessments were conducted at 6 month intervals or at any point when a conversion was thought to have occurred. A parent/guardian informant was interviewed about the patient on the clinical and functioning measures and the same clinician conducted a separate direct interview with the patient and then determined a composite rating. Consensus was obtained via review of all SOPS ratings and KSADS-E diagnoses by a senior clinician (currently AA) and at the weekly RAP team meeting. High interrater reliability for individual SOPS items and prodromal diagnosis has been previously reported (Lencz et al. 2004) for RAP interviewers.
Statistical Methods
All analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, Illinois). For demographic data and substance use rate comparisons between CHR+ and HC subjects, categorical variables were analyzed using Pearson chi-square tests and continuous variables were analyzed using ANOVA. Comparisons between CHR+ participants with and without lifetime cannabis use (and abuse) on the SOPS and GF Scales were analyzed using ANOVA. Significance was set at p=0.01 for all of the above comparison analyses.
Repeated measures ANOVAs were conducted to determine the impact of cannabis use (and abuse) on functioning over time (baseline to follow-up), with cannabis use (or abuse) as the between subjects factor and social and role functioning as within-subject factors. Given that length of follow-up significantly differed between cannabis users and non-users, this variable was added a covariate. Significance was set at p=0.05 for these analyses.
Logistic regression analysis was used to examine the impact of lifetime cannabis use/abuse on conversion to psychosis, after adjustment for potential confounding variables. A binomial logistic regression model (forced-entry) was built with lifetime cannabis use as an independent variable and conversion to psychosis as a dependent variable, adjusting for age at baseline, SOPS total positive symptoms, and SOPS total negative symptoms. An identical model was also built using cannabis abuse as the independent variable. The variables entered into the logistic regression models as confounders were those that were significantly associated with conversion at the p<0.05 level in univariate logistic regression analyses. Other explanatory or confounding variables that did not meet the univariate criteria for inclusion in the adjusted models were gender, race, parental SES, estimated IQ, and GF:Social and GF:Role scores. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) are reported. Statistical significance was set at p<0.05 for the lifetime cannabis use and abuse models.
Results
Demographics
There were no significant differences between the CHR+ and HC participants on age at baseline, gender, parental SES, or race (see Table 1). HC participants had significantly higher estimated IQ scores than CHR+ participants.
Table 1.
Demographic Characteristics
| CHR+ (N=101) | HC (N=59) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | F | p | |||
| Age At Baseline | 16.09 (2.15) | 16.15 (2.64) | 0.26 | 0.872 | ||
| Estimated IQa | 103.27 (16.38) | 110.28 (14.11) | 7.27 | 0.008 | ||
|
| ||||||
| N | % | N | % | χ2 | p | |
|
| ||||||
| Gender (Male) | 66 | 65.3% | 30 | 50.8% | 3.26 | 0.071 |
| Parental SESb | 3.64 | 0.056 | ||||
| Classes I & II | 62 | 63.3% | 43 | 78.2% | ||
| Classes III – V | 36 | 36.7% | 12 | 21.8% | ||
| Race | 2.23 | 0.135 | ||||
| Caucasian | 70 | 69.3% | 34 | 57.6% | ||
| Non-Caucasian | 31 | 30.7% | 25 | 42.2% | ||
6 subjects missing IQ estimates (4 CHR+, 2 HC); WISC-III (47 CHR+, 30 HC), WAIS-R (49 CHR+, 27 HC) and WASI (1 CHR+)
7 subjects missing Parental SES (3 CHR+,4 HC); Parental SES classifications: Class I represents the highest level and Class V the lowest level (Hollingshead & Redlich, 1958)
Rates and Frequency of Substance Use
Alcohol was the most frequently reported substance used in both groups (see Table 2). CHR+ and HC participants showed comparable lifetime alcohol usage (44% in each group) and the two groups did not significantly differ on rates of current alcohol use frequency and quantity. There was a low rate of lifetime tobacco use in the HC group, and this rate significantly differed from the CHR+ group (p=0.001). There was no difference between groups on current usual frequency or quantity of tobacco use. Half of the subjects in each group who reported lifetime tobacco use reported current daily use at baseline.
Table 2.
Rates of lifetime and current substance use reported on KSADS-E interview at baseline
| CHR+ N = 101 | HC N = 59 | |||
|---|---|---|---|---|
| Substance | N (%) | N (%) | χ 2 | p |
| Alcohol | ||||
| Lifetime Use | 43 (44.3) | 26 (44.1) | 0.001 | 0.975 |
| Current Usual Frequency | 4.320 | 0.504 | ||
| 1–2x/ever | 18 (43.9) | 8 (30.8) | ||
| <1x/month | 10 (24.4) | 7 (26.9) | ||
| 1x/month to <1x/wk | 6 (14.6) | 8 (30.8) | ||
| 1x/week | 4 (9.8) | 1 (3.8) | ||
| 2–4 days/wk | 2 (4.9) | 2 (7.7) | ||
| 5–7 days/wk | 1 (2.4) | 0 (0) | ||
| Current Usual Quantity | 7.62 | 0.178 | ||
| 1–2 drinks | 15 (48.4) | 4 (21.1) | ||
| 2–3 drinks | 5 (16.1) | 8 (42.1) | ||
| 3–4 drinks | 2 (6.5) | 3 (15.8) | ||
| 4–5 drinks | 2 (6.5) | 2 (10.5) | ||
| 5–6 drinks | 3 (9.7) | 1 (5.3) | ||
| 6+ drinks | 4 (12.9) | 1 (5.3) | ||
| Tobacco | ||||
| Lifetime Use | 31 (34.4) | 4 (8.2) | 11.63 | 0.001 |
| Current Usual Frequency | 7.37 | 0.061 | ||
| Not at all currently | 11(36.7) | 0 (0) | ||
| 1–2x/week | 2 (6.7) | 2 (50) | ||
| 3–6x/week | 2 (6.7) | 0 (0) | ||
| Daily | 15 (50) | 2 (50) | ||
| Current Usual Quantity | 2.75 | 0.431 | ||
| 1–9 cigarettes | 11 (64.7) | 2 (50) | ||
| 10–20 cigarettes | 5 (29.4) | 2 (50) | ||
| 21–40 cigarettes | 1 (5.8) | 0 (0) | ||
| Cannabis | ||||
| Lifetime Use | 35 (36.5) | 7 (11.9) | 11.19 | 0.001 |
| Use in Past 6 months | 22 (62.8) | 3 (42.8) | 9.88 | 0.002 |
| Lifetime Frequency | 4.38 | 0.223 | ||
| 1–4 times | 14 (40) | 4 (57) | ||
| 5–9 times | 2 (5.7) | 1 (14.3) | ||
| 10–19 times | 1 (2.9) | 1 (14.3) | ||
| 20+ times | 18 (51.4) | 1 (14.3) | ||
| Amphetamines | ||||
| Lifetime Use | 4 (4.2) | 0 (0.0) | 2.52 | 0.112 |
| Use in Past 6 months | 1 (25) | 0 (0.0) | 0.64 | 0.424 |
| Lifetime Frequency | -- | -- | ||
| 1–4 times | 3 (75) | 0 (0.0) | ||
| 5–9 times | 0 (0.0) | 0 (0.0) | ||
| 10–19 times | 0 (0.0) | 0 (0.0) | ||
| 20+ times | 1 (25) | 0 (0.0) | ||
| Barbiturates | ||||
| Lifetime Use | 3 (3.1) | 0 (0.0) | 1.88 | 0.170 |
| Use in Past 6 months | 1 (33) | 0 (0.0) | 0.63 | 0.427 |
| Lifetime Frequency | -- | -- | ||
| 1–4 times | 2 (66.7) | 0 (0.0) | ||
| 5–9 times | 0 (0.0) | 0 (0.0) | ||
| 10–19 times | 0 (0.0) | 0 (0.0) | ||
| 20+ times | 1 (33.3) | 0 (0.0) | ||
| Cocaine | ||||
| Lifetime Use | 6 (6.2) | 2 (3.4) | 0.61 | 0.435 |
| Use in Past 6 months | 1 (16.7) | 1 (50) | 0.11 | 0.746 |
| Lifetime Frequency | 3.56 | 0.169 | ||
| 1–4 times | 5 (83.3) | 1 (50) | ||
| 5–9 times | 0 (0.0) | 1 (50) | ||
| 10–19 times | 0 (0.0) | 0 (0.0) | ||
| 20+ times | 1 (16.7) | 0 (0.0) | ||
| Opioids | ||||
| Lifetime Use | 8 (8.3) | 0 (0.0) | 5.18 | 0.023 |
| Use in Past 6 months | 4 (50) | 0 (0.0) | 2.64 | 0.105 |
| Lifetime Frequency | -- | -- | ||
| 1–4 times | 6 (75) | 0 (0.0) | ||
| 5–9 times | 1 (12.5) | 0 (0.0) | ||
| 10–19 times | 1 (12.5) | 0 (0.0) | ||
| 20+ times | 0 (0.0) | 0 (0.0) | ||
| PCP | ||||
| Lifetime Use | 1 (1.0) | 0 (0.0) | 0.62 | 0.432 |
| Use in Past 6 months | 0 (0.0) | 0 (0.0) | -- | -- |
| Lifetime Frequency | -- | -- | ||
| 1–4 times | 1 (100) | 0 (0.0) | ||
| 5–9 times | 0 (0.0) | 0 (0.0) | ||
| 10–19 times | 0 (0.0) | 0 (0.0) | ||
| 20+ times | 0 (0.0) | 0 (0.0) | ||
| Hallucinogens | ||||
| Lifetime Use | 11 (11.5) | 1 (1.7) | 4.88 | 0.027 |
| Use in Past 6 months | 6 (54.5) | 0 (0.0) | 3.99 | 0.046 |
| Lifetime Frequency | 0.55 | 0.76 | ||
| 1–4 times | 7 (63.3) | 1 (100) | ||
| 5–9 times | 2 (18.2) | 0 (0.0) | ||
| 10–19 times | 2 (18.2) | 0 (0.0) | ||
| 20+ times | 0 (0.0) | 0 (0.0) | ||
| Solvents/Inhalants | ||||
| Lifetime Use | 3 (3.1) | 0 (0.0) | 1.88 | 0.170 |
| Use in Past 6 months | 0 (0.0) | 0 (0.0) | -- | -- |
| Lifetime Frequency | -- | -- | ||
| 1–4 times | 3 (100) | 0 (0.0) | ||
| 5–9 times | 0 (0.0) | 0 (0.0) | ||
| 10–19 times | 0 (0.0) | 0 (0.0) | ||
| 20+ times | 0 (0.0) | 0 (0.0) | ||
| Ecstasy | ||||
| Lifetime Use | 3 (3.1) | 1 (1.7) | 0.30 | 0.586 |
| Use in Past 6 months | 0 (0.0) | 0 (0.0) | -- | -- |
| Lifetime Frequency | 0.444 | 0.505 | ||
| 1–4 times | 2 (66.7) | 1 (100) | ||
| 5–9 times | 0 (0.0) | 0 (0.0) | ||
| 10–19 times | 1 (33.3) | 0 (0.0) | ||
| 20+ times | 0 (0.0) | 0 (0.0) | ||
Note: Alcohol: 4 CHR+ missing lifetime data; 2 CHR+ alcohol users missing frequency; 10 CHR+ and 7 HC alcohol users missing quantity. Tobacco: 11 CHR+ and 10 HC missing lifetime data; 1 CHR+ smoker missing current frequency; 2 CHR+ smokers missing current quantity. Drugs: 5 CHR+ subjects missing data on drug use.
In terms of illicit substances, cannabis was the most widely used drug in this sample and all participants who reported drug use, with one exception, also reported cannabis use. CHR+ participants reported significantly higher rates of lifetime cannabis use than HC participants (p=0.001) and were also more likely to have used cannabis in the past 6 months (p=0.002). CHR+ participants who reported lifetime cannabis use (n=35) were more likely to be Caucasian (88.6% vs. 59.0%; χ2=9.21, p=0.002) and older in age at baseline (16.74±1.96 vs. 15.65±2.12; F(1,95)=6.18, p=0.015, trend) than CHR+ participants who did not report lifetime cannabis use. There were no differences between CHR+ subjects with or without lifetime cannabis use on gender, parental SES, or estimated IQ. Of the 35 CHR+ lifetime cannabis users, 17 (48.6%) could be characterized as low lifetime users (1–19 times) and 18 (51.4%) as high lifetime users (20+ times). There was no difference in frequency of cannabis use between the CHR+ and HC groups (see Table 2).
Rates of lifetime drug use other than cannabis were minimal in the CHR+ subjects and often absent in the HC subjects, limiting further analysis. However, CHR+ participants evidenced higher rates of lifetime opioid use (χ2=5.18, p=0.023, trend) and lifetime hallucinogen use (χ2=4.88, p=0.027, trend) compared to HC subjects.
Clinical Characteristics and Functioning in Cannabis Users
As reported in Table 3, CHR+ participants who reported lifetime cannabis use (n=35) were significantly less socially anhedonic (p<0.001) and had lower SOPS total negative symptom scores (p=0.033, trend level) than those who did not report lifetime cannabis use (n=61). Lifetime cannabis users had trend-level lower scores on grandiosity although the means for both groups were very low and not clinically meaningful. There was no difference between users and non-users on other SOPS positive symptoms or total positive symptoms score.
Table 3.
Baseline Prodromal Symptoms for CHR+ Subjects with Cannabis Use (N=35) vs. CHR+ Subjects without Cannabis Use (N=61)
| No Cannabis Use | Any Cannabis Use | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | F | p | |
| SOPS Positive Symptoms
| ||||
| Unusual Thoughts | 2.31 (1.86) | 1.80 (1.75) | 1.76 | 0.188 |
| Suspiciousness | 3.03 (1.59) | 2.54 (1.44) | 2.25 | 0.137 |
| Grandiosity | 0.80 (1.46) | 0.23 (0.77) | 4.67 | 0.033 |
| Hallucinations | 1.44 (1.60) | 2.06 (2.03) | 2.70 | 0.104 |
| Disorganized Communication | 1.69 (1.59) | 1.26 (1.42) | 1.77 | 0.187 |
| Total Positive Symptoms | 9.28 (4.28) | 7.89 (3.47) | 2.69 | 0.104 |
| SOPS Negative Symptoms
| ||||
| Social Anhedonia | 3.77 (1.57) | 1.83 (1.93) | 24.44 | <0.001 |
| Avolition | 2.65 (1.75) | 2.40 (1.75) | 0.40 | 0.529 |
| Expression of Emotion | 1.63 (1.73) | 1.20 (1.35) | 1.35 | 0.250 |
| Experience of Emotion | 1.12 (1.68) | 1.67 (1.92) | 1.85 | 0.178 |
| Ideational Richness | 0.90 (1.46) | 0.73 (1.08) | 0.31 | 0.579 |
| Occupational Functioning | 3.62 (1.74) | 3.83 (1.23) | 0.36 | 0.548 |
| Total Negative Symptoms | 12.56 (5.27) | 10.00 (4.86) | 4.74 | 0.033 |
Note: 14 subjects (5 cannabis users) missing all individual negative symptom ratings and total negative score; 1 subject (non cannabis user) missing only Experience of Emotion
In terms of functioning, CHR+ lifetime cannabis users had significantly higher GF:Social scores at baseline than CHR+ subjects who never used cannabis (6.91±1.40 vs. 5.51±1.21; F(1,95)=26.84, p<0.001), although there was not a significant difference for GF:Role scores (5.49±1.82 vs. 5.62±2.13; p=0.75).
Out of the 101 CHR+ participants, 92 (91.1%) had at least one follow-up. The mean follow-up period was 2.97 years (SD=1.63 years; range 0.11 to 7.19 years). Participants who were not followed up did not differ significantly from those who were in terms of demographic variables or rates of lifetime cannabis use or abuse at baseline. However, length of follow-up significantly differed for lifetime cannabis users vs. non-users (F(1,88)=6.64, p=0.012) and cannabis abusers vs. non-abusers (F(1,88)=3.85, p=0.053).
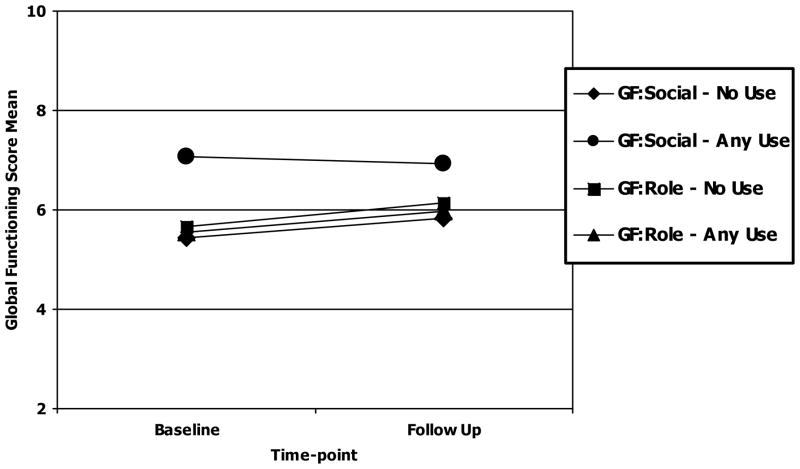
Of the 92 CHR+ subjects who had at least one follow-up timepoint, 86 subjects had GF:Social and GF:Role scores at both baseline and follow-up. For GF:Social, baseline and follow-up scores did not significantly differ, although there was a significant difference between groups in that lifetime cannabis users had significantly higher social functioning at both baseline and follow-up than non-users (F(1,83)=26.48, p<0.001; see Figure 1). The Time x Group interaction was not significant for the GF:Social scale. Length of follow-up was a significant covariate (F(1,83)=5.32, p=0.024) with lifetime cannabis users having approximately one year shorter follow-up than non-users (2.44±1.45 vs. 3.34±1.64 years, respectively). For GF:Role scores and lifetime cannabis use, there were no significant differences for Time, Group, or the Time x Group interaction. Length of follow-up was not a significant covariate for GF:Role.
Figure 1.
Cannabis Use and Functional Outcome from Baseline to Follow-Up in CHR+ subjects (N=86)
Note:
GF:Social - No Use: n = 55, Baseline: X= 5.45 (SD=1.25) Follow Up: X= 5.82 (SD=1.45)
GF:Social - Any Use: n = 31, Baseline: X= 7.06 (SD=1.34) Follow Up: X=6.94 (SD=1.59)
GF:Role - No Use: n = 55, Baseline: X= 5.65 (SD=2.21) Follow Up: X=6.15 (SD=2.42)
GF:Role - Any Use: n = 31, Baseline: X= 5.55 (SD=1.71) Follow Up: X=5.97 (SD=2.52)
Clinical Characteristics and Functioning in Cannabis Abusers
A small sample of ten CHR+ subjects (10.4%) met the full DSM-IV criteria for Cannabis Abuse at baseline according to the KSADS-E interview. These subgroups (cannabis use and cannabis abuse) have been analyzed separately to determine any dose-response effects. Consistent for the findings reported above for users, CHR+ subjects with Cannabis Abuse were significantly older at baseline (17.70±1.60 vs. 15.86±2.09, F(1,95)=7.26, p=0.008) and had higher estimated IQ scores (115.60±19.0 vs. 102.00±15.82, F(1,91)=6.31, p=0.014, trend) than CHR+ subjects who were not cannabis abusers. There were no significant gender, race, or parental SES differences between the two groups.
CHR+ participants who met the criteria for Cannabis Abuse (n=10) were less socially anhedonic (F(1,81)=5.95, p=0.017, trend) than non-abusers, but there were no differences in terms of other SOPS symptoms or SOPS total positive and negative symptoms.
In terms of functioning, CHR+ lifetime cannabis abusers had significantly higher GF:Social scores at baseline than CHR+ subjects who never abused cannabis (7.20±1.48 vs. 5.88±1.38; F(1,95)=7.99, p=0.006), although there was not a significant difference for GF:Role scores (5.60±1.96 vs. 5.57±2.03).
When examining functioning over time, baseline and follow-up scores did not significantly differ for the GF:Social scale, although there was a significant main effect for Group (F(1,83)=4.44, p=0.04) with abusers displaying better social functioning at both baseline and follow-up assessments (7.20±1.48 vs. 5.88±1.43 at baseline and 6.80±1.75 vs. 6.14±1.56 at follow-up). There was no Time x Group interaction. Length of follow-up was a significant covariate (F(1,83)=5.65, p=0.02) with cannabis abusers having approximately one year less follow-up than non-abusers (2.02±1.21 vs. 3.15±1.63 years, respectively). For GF:Role and Cannabis Abuse, there were no significant main effects for Time or Group, or Time x Group interactions. Length of follow-up was not a significant covariate for GF:Role.
Cannabis Use/Abuse and Conversion to Psychosis
Logistic regression was used to examine the impact of lifetime cannabis use/abuse on conversion to psychosis. In univariate regression analyses, lifetime cannabis use (OR=0.56, 95% CI=0.16–1.94, p=0.36) and cannabis abuse (OR=1.27, 95% CI=0.24–6.67, p=0.78) were not significant predictors of conversion.
The lack of association between cannabis use and cannabis abuse and conversion to psychosis was confirmed after adjusting for age at baseline, SOPS total positive symptoms, and SOPS total negative symptoms (see Table 4). The only variable that was significantly related to conversion in the cannabis use and abuse adjusted models (p<0.05) was SOPS total positive symptoms score, with higher scores representing increased risk of conversion. Age at baseline and SOPS total negative symptoms score were not significant predictors of conversion.
Table 4.
Adjusted Odds Ratios for Prediction of Psychosis in High-Risk Participants with Cannabis Use or Cannabis Abuse
| Predictors | p | OR | 95% C.I. | Predictors | p | OR | 95% C.I. | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Lifetime Cannabis Use | 0.836 | 0.834 | 0.150 | 4.652 | Lifetime Cannabis Abuse | 0.918 | 1.111 | 0.148 | 8.343 |
| Agea | 0.098 | 1.425 | 0.937 | 2.167 | Agea | 0.111 | 1.386 | 0.927 | 2.071 |
| SOPS Total Positive Syma | 0.027 | 1.206 | 1.022 | 1.422 | SOPS Total Positive Syma | 0.022 | 1.211 | 1.028 | 1.427 |
| SOPS Total Negative Syma | 0.082 | 1.133 | 0.984 | 1.303 | SOPS Total Negative Syma | 0.065 | 1.138 | 0.992 | 1.305 |
Variables adjusted for in the models were significant at the univariate level (p<0.05).
Note - For both models, N=77 due to missing data. For categorical predictors: Lifetime Cannabis Use (0=no use or 1=use); Lifetime Cannabis Abuse (0=no abuse or 1=abuse); For categorical outcome: Conversion (0=no conversion or 1=conversion).
Discussion
Cannabis and Prodromal Symptoms/Conversion
The major finding of this paper is that neither lifetime cannabis use nor abuse in this sizable sample of high-risk adolescents predicted conversion to psychosis. In addition, lifetime cannabis use was not associated with increased attenuated positive symptoms of psychosis at baseline in high-risk subjects. These results are in accordance with the Phillips et al. (2002) and Corcoran et al. (2008) findings where cannabis use or abuse was not related to psychotic conversion. This suggests that cannabis may not be a significant risk factor for conversion to psychosis in help-seeking high-risk samples. However, these findings are discrepant with the study by Kristensen and Cadenhead (2007), which targeted similar patients and did find a relationship between cannabis and psychosis conversion. The small sample size and difference in use patterns in that study may explain the discrepant results. The current high-risk subjects and Phillips et al. (2002) participants evidenced relatively low rates of cannabis abuse, unlike the Kristensen and Cadenhead (2007) sample where 33% of participants had cannabis abuse/dependence. It is possible that a dose-dependent relationship influenced the current results where participants may not have reached a certain required threshold. The discrepancy may also be related to the older mean age of participants in the Kristensen and Cadenhead program (18.6 years) versus the current study (16 years). However, the Phillips et al. (2002) and Corcoran et al. (2008) participants were also older in age on average (19.3 and 20.9 years, respectively), but similar negative results were found.
The current finding appears to be at odds with clinical and epidemiologic studies showing an increased risk of psychotic symptoms and psychotic disorders in those subjects who report cannabis use (Linszen et al. 1997; Semple et al. 2005). These relationships were noted to be dose related (Andreasson et al. 1988; van Os et al. 2002; Zammit et al. 2002; Henquet et al. 2005). However, one study (Arseneault et al. 2002) showed that using cannabis just three or more times during the teenage years was associated with increased psychotic symptoms at follow-up a decade or more later, although those who used cannabis by age 15 continued use at age 18, suggesting more frequent and longer duration of use. In the high-risk participants in the current study, lifetime frequency of use was almost evenly split between those who rarely used cannabis and those with frequent use. It is possible that the risk associated with asymptomatic persons who use cannabis and later develop psychosis is different from the risk of help-seeking individuals with attenuated symptoms moving to full psychosis. For example, patients followed at specialized prodromal clinics might have a number of additional risk factors which overpower any potential, residual risk that might be operant in general population samples (Arseneault et al. 2002; Henquet et al. 2005; Kuepper et al. 2011). In our analyses, the contribution of cannabis use was inconsequential compared to the direct association of emerging positive symptoms to the onset of psychosis. In other populations, there may be a small subgroup of individuals who have a particularly high predisposition (e.g., COMT Val/Val genotype, Caspi et al. 2005) and in whom, cannabis use does impact psychosis onset. However, for the more general population of adolescents participating in this study, who are being treated for subtle (i.e. attenuated) positive symptoms, there is no evidence to suggest that cannabis use, frequent or infrequent, is causally related to the onset of psychosis.
Cannabis Use and Social and Role Functioning
CHR+ lifetime cannabis users and abusers demonstrated higher social functioning at baseline and at follow-up in comparison to non-users/non-abusers and these scores were stable across time. Additionally, CHR+ lifetime cannabis users had lower scores on social anhedonia and total negative symptoms on the SOPS, both of which may be seen as proxies for better social functioning. Higher social functioning is indicative of having better social skills and may lead to more exposure to substances (i.e., one must acquire substances to use them) and peer group influences for this behavior. However, it was not possible to determine from the existing data the rates of cannabis use in social groups versus those who used alone. One interpretation of the current findings is that cannabis using subjects with good social integration might represent a higher functioning group in general that is less likely to have adverse outcomes such as psychosis conversion.
To our knowledge, no other study of high-risk patients has examined the issue of social functioning in relation to cannabis use. The current finding of better social functioning in lifetime cannabis users and abusers is consistent with literature involving substance abusing chronic and first-episode patients with schizophrenia-spectrum disorders, although the comparison is hampered by some studies not isolating cannabis abusers from those who also abuse alcohol or other substances. Nevertheless, overall these studies have generally found that substance abusing patients have better social functioning (Salyers & Mueser, 2001; Larsen et al. 2006) and fewer negative symptoms (e.g., anhedonia, avolition; Salyers & Mueser, 2001; Bersani et al. 2002; Joyal et al. 2003; Dubertret et al. 2006; Compton et al. 2007), although not all studies have found these relationships (Carey et al. 2003; Van Mastrigt et al. 2004; Barnes et al. 2006; Mauri et al. 2006; Addington & Addington, 2007).
An association between cannabis use and role (primarily academic) functioning was not evident in this high-risk sample. Results show that cannabis users have academic problems in the seriously impaired range at baseline and display modest but non-significant improvement at follow-up. Birth cohort studies have demonstrated that regular cannabis use early in adolescence confers a 5 times greater risk of dropping out of secondary school prematurely (Fergusson et al. 2003; Lynskey et al. 2003). Although cannabis use did not appear to confer a greater risk of role functioning problems in this sample, clinical high-risk status itself was associated with poor role functioning.
Study Limitations
The sample size of the current study is large in comparison to previous reports that focused specifically on the relationship between cannabis use and psychosis in help-seeking, high-risk subjects. However, the overall rates of lifetime cannabis use (36.5%) and abuse (10.4%) are not as high as in the other high-risk samples mentioned. Nonetheless, compared to a larger population sample, the rates of cannabis use in the current study are representative of use patterns of high school students across the US (Johnston et al. 2010). Thus, these results may be most applicable to adolescents with typical patterns of use, rather than to adolescents displaying aberrant or excessive use. In addition, participants with substance dependence (including cannabis dependence) that was current at baseline were excluded from the study, limiting the sample to those with less severe use. Furthermore, this study focuses on lifetime cannabis use rather than current or continued use over follow-up, which may affect outcomes differentially (Kuepper, et al, 2011; Yucel et al, 2012). A related limitation is the lack of data on the quantity and type of cannabis used by subjects. For example, a recent study has suggested that varying potencies of delta 9-tetrahydrocannabinol (THC) can have a significant psychotogenic effects (Bhattacharyya et al. 2010).
Despite these limitations, this is one of the largest and the longest study to date that prospectively examined the specific relationship between lifetime cannabis use and prodromal symptoms, psychosis conversion and social and role functioning. Lifetime cannabis use was not associated with higher attenuated positive or negative symptoms at baseline or with conversion to psychosis in this carefully characterized and prospectively followed high-risk sample that demonstrated average rates of lifetime cannabis use.
Acknowledgments
This work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (AA) and by the National Institute of Mental Health (grant number R01 MH061523 to BC). The authors gratefully acknowledge the assistance of Ruth Olsen in the formatting of this manuscript.
Footnotes
Declaration of Interest: Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza; American Academy of Child and Adolescent Psychiatry, AstraZeneca, Biotis, Bristol-Myers Squibb, Cephalon, Desitin, Eli Lilly, Gerson Lehrman Group, GSK, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, National Institute of Mental Health, Novartis, Ortho-McNeill/Janssen/J&J, Otsuka, Pfizer, ProPhase, Sunovion, Takeda and Teva. He has received grant support from BMS, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health (NIMH), National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka. None of the other authors have any conflicts to report.
References
- Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatrica Scandinavica. 2007;115(4):304–309. doi: 10.1111/j.1600-0447.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Allebeck P, Adamsson C, Engstrom A, Rydberg U. Cannabis and schizophrenia: a longitudinal study of cases treated in Stockholm County. Acta Psychiatrica Scandinavica. 1993;88(1):21–24. doi: 10.1111/j.1600-0447.1993.tb03408.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, D.C: 1994. [Google Scholar]
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. Lancet. 1988;1:1000–1001. doi: 10.1016/s0140-6736(88)91823-5. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt T. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. British Medical Journal. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auther A, Smith C, Cornblatt B. Global Functioning: Social Scale (GF: Social) The Zucker Hillside Hospital; Glen Oaks, NY: 2006. [Google Scholar]
- Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. British Journal of Psychiatry. 2006;188:237–242. doi: 10.1192/bjp.bp.104.007237. [DOI] [PubMed] [Google Scholar]
- Bersani G, Orlandi V, Kotzalidis GD, Pancheri P. Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. European Archives of Psychiatry and Clinical Neuroscience. 2002;252(2):86–92. doi: 10.1007/s00406-002-0366-5. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O’Carroll CM, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite Effects of Δ-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Carey MP, Simons JS. Correlates of substance use disorder among psychiatric outpatients: focus on cognition, social role functioning, and psychiatric status. Journal of Nervous and Mental Disease. 2003;191(5):300–308. doi: 10.1097/01.NMD.0000066152.87832.A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari D. Cannabis and schizophrenia: results of a follow-up study. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(1):45–49. doi: 10.1007/s004060050064. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the Catechol-O-Methyltransferase gene: Longitudinal evidence of a gene x environment interaction. Biological Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Compton MT, Whicker NE, Hochman KM. Alcohol and cannabis use in Urban, African American, first-episode schizophrenia-spectrum patients: associations with positive and negative symptoms. Journal of Clinical Psychiatry. 2007;68(12):1939–1945. doi: 10.4088/jcp.v68n1215. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, Schobel S, Harkavy-Friedman J, Goetz R, Colibazzi T, Cressman V, Malaspina D. Temporal association of cannabis use with symptoms in individuals at clinical high-risk for psychosis. Schizophrenia Research. 2008;106:286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM. Treating early psychosis: who, what, and when? Dialogues in Clinical Neuroscience. 2005;7(1):39–49. doi: 10.31887/DCNS.2005.7.1/bcornblatt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophrenia Bulletin. 2003;29(4):633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Dubertret C, Bidard I, Ades J, Gorwood P. Lifetime positive symptoms in patients with schizophrenia and cannabis abuse are partially explained by co-morbid addiction. Schizophrenia Research. 2006;86:284–290. doi: 10.1016/j.schres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction. 2003;98:1681–1692. doi: 10.1111/j.1360-0443.2003.00573.x. [DOI] [PubMed] [Google Scholar]
- Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. European Psychiatry. 2005;20(4):349–353. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. British Journal of Psychiatry. 2005;187:306–313. doi: 10.1192/bjp.187.4.306. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. What are the policy implications of the evidence on cannabis and psychosis? Canadian Journal of Psychiatry. 2006;51(9):566–574. doi: 10.1177/070674370605100904. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van Os J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. British Medical Journal. 2005;330(7481):11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, van Os J, Kuepper R, Delespaul P, Smits M, Campo JA, Myin-Germeys I. Psychosis reactivity to cannabis use in daily life: an experience sampling study. British Journal of Psychiatry. 2010;196(6):447–453. doi: 10.1192/bjp.bp.109.072249. [DOI] [PubMed] [Google Scholar]
- Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis: prospective study. British Journal of Psychiatry. 2006;189:137–143. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social class and mental illness. Wiley; New York, NY: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No 10-7583. National Institute on Drug Abuse; Bethesda, MD: 2010. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. [Google Scholar]
- Joyal CC, Halle P, Lapierre D, Hodgins S. Drug abuse and/or dependence and better neuropsychological performance in patients with schizophrenia. Schizophrenia Research. 2003;63(3):297–299. doi: 10.1016/s0920-9964(02)00387-0. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Research. 2007;151:151–154. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper R, van Os J, Lieb R, Wittchen HU, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. British Medical Journal. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Archives of General Psychiatry. 2011;68 (6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Larsen TK, Melle I, Auestad B, Friis S, Haahr U, Johannessen JO, Opjordsmoen S, Rund BR, Simonsen E, Vaglum P, McGlashan TH. Substance abuse in first-episode non-affective psychosis. Schizophrenia Research. 2006;88(1–3):55–62. doi: 10.1016/j.schres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophrenia Research. 2004;68(1):37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Archives of General Psychiatry. 1994;51(4):273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Nugter MA, Van der Does AJ, Scholte WF, Lenior MA. Patient attributes and expressed emotion as risk factors for psychotic relapse. Schizophrenia Bulletin. 1997;23(1):119–130. doi: 10.1093/schbul/23.1.119. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction. 2003;98(5):685–692. doi: 10.1046/j.1360-0443.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Arevalo MJ, Calcedo-Ordonez A, Varo-Prieto JR. Cannabis consumption as a prognostic factor in schizophrenia. British Journal of Psychiatry. 1994;164(5):679–681. doi: 10.1192/bjp.164.5.679. [DOI] [PubMed] [Google Scholar]
- Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug and Alcohol Dependence. 2011;119:28–36. doi: 10.1016/j.drugalcdep.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Volonteri LS, De Gaspari IF, Colasanti A, Brambilla MA, Cerruti L. Substance abuse in first-episode schizophrenic patients: a retrospective study. Clinical Practice and Epidemiology in Mental Health. 2006;2:4. doi: 10.1186/1745-0179-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller T, Mednick SA, McGlashan TH, Libiger J, Johannessen JO, editors. Early intervention in psychotic disorders. Kluwer Academic Publishers; Netherlands: 2001. pp. 135–149. [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Briesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychological Medicine. 1986;16(3):515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, Cannon TD. Global Functioning: Role Scale (GF: Role) University of California, Los Angeles; Los Angeles, CA: 2006. [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children - Epidemiologic Version. Center for Psychological Studies, Nova Southeastern University; Fort Lauderdale, FL: 1994. [Google Scholar]
- Phillips LJ, Curry C, Yung AR, Yuen HP, Adlard S, McGorry PD. Cannabis use is not associated with the development of psychosis in an ‘ultra’ high-risk group. Australian and New Zealand Journal of Psychiatry. 2002;36(6):800–806. doi: 10.1046/j.1440-1614.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkötter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Archives of General Psychiatry. 2010;67(3):241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Salyers MP, Mueser KT. Social functioning, psychopa thology, and medication side effects in relation to substance use and abuse in schizophrenia. Schizophrenia Research. 2001;48(1):109–123. doi: 10.1016/s0920-9964(00)00063-3. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. Journal of Psychopharmacology. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophrenia Research. 2005;75:135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- van Mastrigt S, Addington J, Addington D. Substance misuse at presentation to an early psychosis program. Social Psychiatry and Psychiatric Epidemiology. 2004;39(1):69–72. doi: 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. American Journal of Epidemiology. 2002;156(4):319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. American Journal of Psychiatry. 2004;161(3):501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale, Revised (WAIS-R) The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. The Psychological Corporation; San Antonio, TX: 1991. (WISC-III) [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale for Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C. The impact of cannabis use on cognitive functioning in patients with schizophrenia: A meta-analysis of existing findings and new data in a first-episode sample. Schizophrenia Bulletin. 2012;38(2):316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophrenia Research. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self-reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: Historical cohort study. British Medical Journal. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]