Abstract
The attentional blink phenomenon is the reduced ability to report a second target (T2) after identifying a first target (T1) in a rapid serial visual presentation (RSVP) of stimuli (e.g., letters), which are presented at approximately 10 items per second. Several explanations have been proposed, which focus primarily on cognitive aspects, such as attentional filter-, capacity limitation- and retrieval failure‐processes.
Here, we focus on the hypothesis that an entrainment of alpha oscillations (with a frequency of about 10 Hz) is a critical factor for the attentional blink phenomenon. Our hypothesis is based on the fact that item presentation rate in the RSVP typically lies in the alpha frequency range and is motivated by theories assuming an inhibitory function for alpha. We predict that entrainment – during the time window of T2 presentation – is larger for attentional blink (AB) items (when T2 cannot be reported) than for NoAB trials (when T2 cannot be reported).
The results support our hypothesis and show that alpha entrainment as measured by the amplitude of the alpha evoked response and the extent of alpha phase concentration is larger for AB than for NoAB trials. Together with the lack of differences in alpha power these findings demonstrate that the differences between AB and NoAB trials – during presentation onset of T2 – are due to an entrainment of alpha phase and not due to an amplitude modulation. Thus, we conclude that alpha entrainment may be considered the critical factor underlying the attentional blink phenomenon.
Keywords: Attentional blink, Alpha entrainment, Phase concentration, Early categorization, P1
Highlights
► Alpha was entrained via 10 Hz stimulation rate with an attentional blink design. ► Attentional blink showed stronger alpha amplitude and alpha phase-locking. ► Besides entrainment, a phase concentration at the negative peak was crucial. ► Processes of P1 generation interfere with encoding processes. ► Early categorization processes are interrupted through alpha phase entrainment.
Introduction
The aim of the present study is to test the hypothesis that alpha entrainment is a critical factor for the occurrence of the attentional blink phenomenon, which is the reduced ability to report a second target after identifying a first target in a rapid serial visual presentation (RSVP) of stimuli (e.g., letters), presented at approximately 10 items per second. The structure of a single trial is illustrated in Fig. 1. Each item is displayed briefly with or without an interstimulus interval (ISI). In the case an ISI is used, item exposure time (of e.g., 25 ms) and ISI (of e.g., 75 ms) add up to a SOA of about 100 ms. Subjects have to search for two targets, T1 and T2. The critical result is that subjects fail to report T2 in about 50% of the cases when T1 is identified and when the two targets are separated by at least 1 item but not more than about 7 intervening items. This failure to report T2 – after a successful encoding of T1 – constitutes the attentional blink phenomenon. It can be observed in a time window of about 100 to 500 ms after T1 (Raymond et al., 1992; Shapiro et al., 1997) provided at least one non-target stimulus follows T1 and T2.
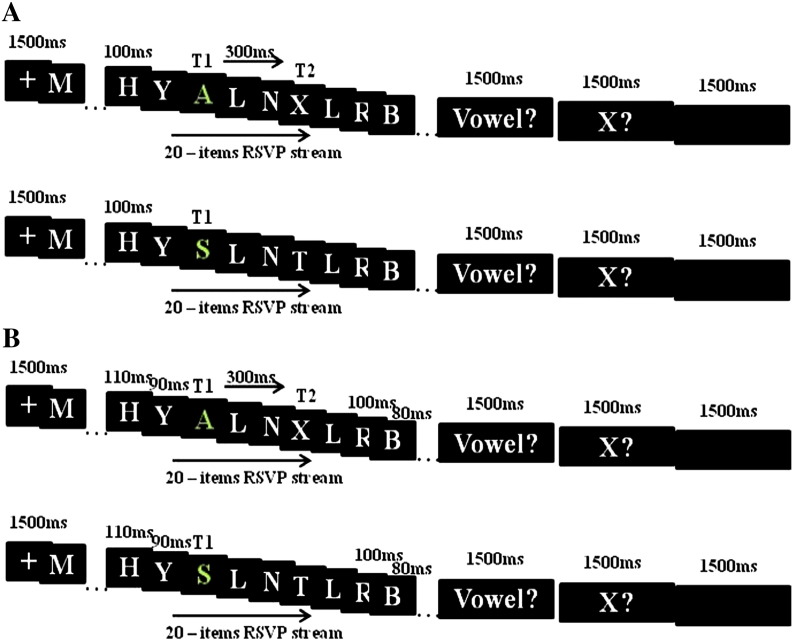
Fig. 1.
Illustration of a trial of the attentional blink paradigm. A) First a fixation cross was presented for 1500 ms, followed by a stream of 20 letters, which consisted of consonants and vowels. Each letter was presented for 100 ms. Target 1 (T1) always appeared at the 7th position and was characterized by a green letter. A second target (white X = T2) appeared after 300 ms (Lag 3, 10th position of stimulus stream) in 50 % of the trials. Instead of T2, distracters (consonants) were presented in control trials. After the stream of 20 letters, subjects had to decide whether T1 and T2 were presented. A time window of 1500 ms was given for each decision. A blank screen appeared for 1500 ms at the end of the trial. B) In the varied block stimulus presentation was jittered between 80 to 120 ms with +/− 10, 20 ms steps, whereby T2 always appeared 300 ms after T1 (Lag 3).
Our hypothesis is based on the fact that in attentional blink paradigms, items are presented with a stimulus onset asynchrony (SOA) of about 100 ms (which represents a stimulation frequency of around 10 Hz) and is motivated by theories which assume an inhibitory function for alpha oscillations (Foxe and Snyder, 2011; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Mathewson et al., 2011). In addition, there is evidence that alpha is actively and causally involved in shaping visual perception and responds with a phase specific entrainment as recent rhythmic transcranial magnetic stimulation studies have shown (Romei et al., 2010; Thut et al., 2011b). Here we focus on a specific aspect of the assumed inhibitory function of alpha, which was termed ‘P1 inhibition timing hypothesis’ (Klimesch, 2011). It states that the P1 of the visual ERP is generated at least in part by evoked alpha and reflects an inhibitory filter which controls the signal to noise ratio during early stimulus categorization (Klimesch, 2011, Klimesch et al., 2011).
Several explanations have been proposed, which can be categorized – according to Hanslmayr et al. (2011) – in three groups: ‘filter-’, ‘capacity limitation-’, and ‘retrieval failure’ theories. The central idea of filter theories is that the attentional focus (‘filter’) on T1 acts to suppress the processing of subsequent stimuli (cf. Olivers and Meeter, 2008 for a more recent model; Raymond et al., 1992). Related theories by Di Lollo et al. (2005) or Kawahara et al. (2006) assume that a ‘re-orientation’ of the attentional filter towards T2 is the critical factor underlying the attentional blink phenomenon. Capacity limitation theories proceed from the assumption that the establishment of an episodic code for T2 – which is a necessary precondition to report that target at the end of a trial – interferes with the episodic encoding of T1 (cf. Chun and Potter, 1995; Jolicoeur, 1999; Jolicoeur and Dell'Acqua, 1998). Finally, the third group of theories assumes that the failure to report T2 is due to the inability of retrieving T2 from working memory (cf. Duncan et al., 1994; Shapiro et al., 1994; Ward et al., 1996).
Only a few studies have focused on a relationship between alpha and the attentional blink. As an example, MacLean and colleagues have shown that frontal alpha power is correlated with attentional blink magnitude. Large resting alpha power and a large extent of event-related alpha desynchronization (i.e. ERD, reflecting suppression of alpha power) during task performance is associated with a large magnitude of attentional blink (MacLean and Arnell, 2011; MacLean et al., 2012). These findings are important because they suggest a direct link between alpha and the attentional blink phenomenon. Most interestingly, however, there is – to our knowledge – no research that has yet considered alpha entrainment (due to the stimulation frequency of the RSVP) as the cause for the failure to report T2, although it is a well established finding that flickering stimuli (such as the RSVP) evoke a steady state visually evoked potential (SSVEP) with a dominant frequency in the alpha band. The properties of driven EEG activity are well investigated (e.g. Herrmann, 2001; Kawaguchi et al., 1993; Lakie and Combes, 1999; Lazarev et al., 2001; Mast and Victor, 1991; Sakamoto et al., 1993; Shils et al., 1996; for a recent review see Thut et al., 2011a) and show in general that the alpha frequency range is most responsive. An important aspect thereby is that a stimulation in the broad alpha frequency range leads to a partial or full entrainment of the alpha rhythm. We proceed here from the assumption that alpha entrainment with minimal phase lag relative to stimulus onset of T2 is responsible for the attentional blink. In order to motivate our hypothesis, we give a brief review of the few studies that have investigated the relationship between the phase lag of the SSVEP and cognitive performance.
Because the SSVEP comprises sinusoidal components of the stimulus frequency it is easy to calculate the phase difference (also termed ‘phase lag’ or ‘SSVEP phase’; cf. Silberstein, 1995) between the flickering stimulus and the oscillatory EEG response. Silberstein and colleagues were able to demonstrate that with increasing task demands SSVEP phase lag (latency) tends to increase. As an example, in a visual search task, designed to test the influence of increased attentional demands, subjects had to view shapes of squares and circles either passively or under the instruction to detect a modified circle (Silberstein et al., 1990). Compared to passive viewing, the requirement to detect a modified circle was associated with a transient reduction of SSVEP amplitude at occipital sites and an increased phase lag. In another study cognitive processes during performance of the Wisconsin Card Sorting Test (WCST) were analyzed with the SSPT technique (Silberstein et al., 1995). This test consists of many cards containing objects that vary in color, number and shape. Subjects are presented several cards and asked to find some rule that is common for the presented cards. After subjects have found the sorting criterion, a new criterion is introduced by the experimenter in the next trial. This procedure is repeated several times. From neuropsychological evidence it is well known that particularly the shift to a new criterion is a specific task demand that is closely associated with the prefrontal cortex (cf. Gazzaniga, 1995). Most interestingly, it was found that during the introduction of a new sorting criterion a significant reduction in SSVEP amplitudes and an increased phase lag was observed at anterior recording sites (cf. Silberstein, 1995 for a review).
These findings suggest that a demanding cognitive task is associated with a pronounced phase lag, whereas minimally demanding tasks are associated with a small phase lag. With respect to our hypothesis, we assume that a large phase lag enables good performance whereas a small lag may reduce processing capacity. We assume here that phase locking of the SSVEP – in the alpha frequency range – with minimal phase lag is an important factor underlying the attentional blink. In addition, we expect that the absolute phase angle will also play an important role. The reason for this latter assumption is that the appearance of the P1 – which has a peak latency of about 100 ms – will be generated at exactly that time point where the next following stimulus is presented. If alpha is completely entrained with minimal phase lag, the interference between those processes that underlie the generation of the P1 and those that enable encoding of the next following stimulus will be particularly large. Because there is good evidence that the P1 not only has a frequency characteristic in the alpha band but also may be generated at least in part by alpha oscillations it is obvious to assume that the cognitive processes associated with the P1 will interfere with the encoding of the next following stimulus. Research by Klimesch et al. (2011) suggests that the P1 reflects early stimulus categorization which precedes stimulus identification (a process associated with the appearance of the N1 component).
Our hypothesis is that attentional blink may stem from an interference between early stimulus identification of the preceding target (T1) and the encoding of the subsequent target (T2). If the onset of these two processes overlap (i.e., are time locked with minimal delay or ‘lag’), they will interfere and result in a failure to encode T2. We, thus, predict a larger entrainment (as measured by alpha phase locking and phase concentration) during the stimulus onset of T2 in trials with attentional blink (AB trials) as compared to trials without attentional blink (NoAB trials). In order to investigate, whether entrainment decreases when the RSVP stream is not presented with an SOA of exactly the same length for all presentations but instead with a jittered SOA, we perform two experimental blocks, one with fixed and one with varied presentation times.
We want to emphasize that for our hypothesis alpha phase locking in relation to stimulation onset, reflecting entrainment, is the critical factor. This should not be confused with alpha phase coherence, which reflects the normalized degree of phase variability between electrode pairs. This coherence measure which sometimes is simply referred to as ‘alpha phase synchronization’ was already investigated in attentional blink tasks. As an example, Gross et al. (2004) made the important observation that beta phase coherence is reduced prior to T2 in NoAB trials only. This finding agrees with studies that have focused on the influence of prestimulus alpha phase coherence and target identification performance. Hanslmayr et al. (2007) have shown that reduced long-range synchrony in the prestimulus interval predicts successful stimulus identification (cf. also Kranczioch et al., 2007). These findings are consistent with but are indifferent for testing our hypothesis.
Specific evidence for our hypothesis comes from findings reported by Mathewson and colleagues. As an example, in a visual target detection task, Mathewson et al. (2009) found that for undetected trials, the phase at stimulus onset was different from that of detected trials (cf. Busch et al., 2009 for similar findings). Most importantly, when the target was not detected a prominent negative peak at stimulus onset was associated with significantly reduced P1 amplitude. This may suggest that in trials where the phase of alpha at stimulus onset interferes with the generation of the P1, the stimulus will not be detected. The idea is that an ongoing alpha with 10 Hz and a period of 100 ms which exhibits a negative peak at stimulus onset will develop a negative peak at 100 ms poststimulus which would lead to a suppression of the (positive) P1 amplitude.
The logic of our analyzing approach is the following. We use a standard attentional blink paradigm and in a first step, will measure the peak amplitude of the peristimulus ERP component in response to T2. In analogy to the findings of Mathewson et al. (2009), we expect a larger negative peak around the onset of T2 for AB as compared to NoAB trials. In a next step we will analyze the alpha filtered ERP in order to test, whether the expected findings are prominent for this frequency range. Then we will analyze phase locking of alpha (relative to T2 onset), in order to test, whether AB trials exhibit a larger extent of phase locking (entrainment) than NoAB trials. Finally, we will analyze absolute phase, in order to determine differences in the phase angle between AB and NoAB trials. We expect that only for AB trials, (absolute) alpha phase will coincide with the negative peak of the peristimulus ERP component (which is termed CT2 later in the text).
Method
Subjects
An original sample of 24 subjects was obtained on the basis of course requirements that subjects had to fulfill to finish their diploma study. All subjects participated in the experiment after giving informed consent. They filled out a short response sheet where they were asked for their age, psychoactive drug uses, and neurological disorders. All of the recordings were carefully supervised by the first author (A.Z.) and the EEG was checked for artifacts after the experiment was performed. 10 subjects could not be used for data analysis. This large rejection rate is partly due to the fact that the student experimenters were not that skilled as professional EEG assistants are and that subjects participated to receive their course credits. Out of the 10 rejected subjects, 4 subjects were lost due to electrodes losing the proper impedance during the experiment, 2 subjects reporting their intake of drugs to cure depression, and 4 subjects showing excessive movement artifacts. The final sample consisted of 14 subjects (7 males and 7 females). Mean age was 25.6 years (SD = 3.7 years). All subjects of the final sample reported no neurological disorders or psychological pathologies.
Stimuli and task
We used a traditional AB-paradigm, as e.g., described in the study by Kranczioch et al. (2003). Each trial consisted of a rapid serial visual presentation (RSVP) of 20 letters. Two types of trials were constructed, experimental trials containing two targets and control trials containing only one target. The two targets were a green letter (vowel or consonant) and the (white) letter X. The green letter was the first target (T1) which always appeared at the 7th position, whereas the letter X was the second target (T2) which always appeared at the 10th position. A set of 160 trials was used, comprising 80 experimental and 80 control trials. In half of the experimental trials, T1 was a vowel, in the other half of the trials a consonant. Likewise in control trials, in half of the cases T1 was a vowel, in the other half of the trials a consonant.
For the present study we used a subset of 21 letters of the alphabet, which was obtained by excluding vowel I and the consonants F, K, Q, and Z. This subset contains 4 vowels and 17 consonants. The construction of individual trials was done with the following restrictions. After the selection of target items (with 2 positions for experimental and 1 for control trials), there were 18 or 19 item positions for distracter items. Only consonants were used as distracters. Thus, a remaining set of 15 or 16 consonants (in experimental items T1 can also be a consonant, whereas T2 always consists of the consonant ‘x’) had to be used to fill 18 or 19 item positions. This means that item repetitions were necessary. Consonants were presented in random sequence but with the restriction that the same consonant would never occupy two adjacent positions.
Each trial started with the presentation of a fixation cross that appeared 1500 ms before (and remained on the screen until) the onset of the first letter of the entire 20-letter stream. Half of all trials were experimental trials, the other half control trials. They were presented in a random sequence. In control trials T2 was not presented. All letters were presented in white on black background (with the exception of T1 which was presented in green).
Two different experimental blocks were performed. In block A, each letter of the RSVP was exposed for 100 ms (‘fixed’ presentation block). In block B, the ‘varied’ presentation block, presentation time was ‘jittered’ pseudo-randomly between 80 and 120 ms with +/− 10, 20 ms steps, whereby T2 always appeared 300 ms after T1. As for block A, 160 trials (80 experimental, 80 control) were constructed for block B.
The subject's task was to report both targets. They were informed that T2 would be missing in only half of all trials. At the end of each trial (immediately after the presentation of the 20 letters) a visual response cue for T1 and then for T2 (each presented for 1500 ms) appeared on the screen. During the presentation of the response cue for T1, subjects had to report whether the item was a consonant or vowel. During the response cue for T2, subjects had to report whether they have seen an ‘X’. Responses were given by pressing a respective response key. The interstimulus interval between trials (between the offset of the response cue for T2 and the onset of the fixation cross) was 1500 ms. Task demands and the structure of a single trial is illustrated in Fig. 1.
EEG data acquisition
A Brain Vision Recorder (1000 Hz, 64 channels; BrainProducts, Inc.) was used for EEG recording. EEG-signals were referenced to a nose electrode and subsequently (off-line) re-referenced to digitally linked ((A1 + A2) / 2) ear lobe references. Band-pass filters were set from 0.5 to 100 Hz and a notch filter at 50 Hz. Signals were digitized at a sampling rate of 500 Hz. 60 Ag–AgCl-electrodes were mounted using an EasyCap on the following positions: Fp1, Fp2, Af7, Af3, Afz, Af4, Af8, F7, F5, F3, F1, Fz, F2, F4, F6, F8, Ft7, Fc5, Fc3, Fc1, Fcz, Fc2, Fc4, Fc6, Ft8, T7, C5, C3, C1, Cz, C2, C4, C6, T8, Tp7, Cp5, Cp3, Cp1, Cpz, Cp2, Cp4, Cp6, Tp8, P7, P5, P3, P1, Pz, P2, P4, P6, P8, Po7, Po3, Poz, Po4, Po8, O1, Oz, O2. Impedances were kept below 8 kΩ. To control for vertical and horizontal eye movements two bipolar EOG-channels were mounted. After re-referencing epochs containing eye or muscle artifacts were rejected. Data were segmented from 1000 to 1500 ms relative to T1. Data analyses were performed using BrainVision Analyzer (BrainProducts, Inc.) and Matlab® 7.9 (The MathWorks, Inc.).
Each session started with the recording of the resting EEG for 1 min. Subjects were asked to close their eyes.
EEG data analysis
All basic steps were done with Brain Vision Analyzer. Single-trial phase-angle analyses were done with custom-made Matlab-scripts. At first the data were re-referenced as mentioned above and then broadly filtered between 0.5 and 70 Hz. Then data were manually checked and corrected for muscle- and eye-blinked artifacts. Subsequently data were separately filtered between 1–30 Hz and 8–12 Hz and segmented according to target detection performance, resulting in NoAB trials (both targets detected), in AB trials (T1 detected, T2 missed) and control trials (only T1 presented and detected); the latter allowing a false alarm rate to be calculated.
Calculation of ERPs
Due to the short SOA (and repeated rhythmic stimulus presentation) which prevents the EEG to return to baseline, the obtained ERPs actually represent SSVEPs. Our analysis aims to analyze the dominant ERP response component to T1 and T2. Because the SSVEP cannot contain the well known P1 and N1—components which can be observed for long SOAs (of about 500 ms and longer), we term the dominant components to T1 and T2, component (C) elicited by T1 and T2 respectively (CT1 and CT2).
For the calculation of ERPs, single trial data were filtered between 1–30 Hz and 8–12 Hz. Visual inspection revealed a negative component around stimulation onset of T1 and T2. The mean peak amplitude and latency of CT2 were semi-automatically computed in a time window of 250 to 350 ms around T2 appearance. The peak detection was carried out over the ERP for each subject and each condition (experimental and control) separately for each ROI (see below).
Calculation of alpha power, phase locking and phase distribution
Power analysis
Whole power was calculated employing wavelet transformation for 1 to 20 Hz with the use of a 7-cycle complex Morlet wavelet to obtain an adequate time–frequency resolution. Time–frequency analyses were carried out for all trials in each condition. The frequency of interest was the 8–12 Hz alpha band.
Phase locking index
The phase-locking index (PLI) indicates the intertrial phase-variability for a given frequency across time (Schack and Klimesch, 2002; Tallon-Baudry et al., 1996). The PLI is a normalized value that ranges between 0 (reflecting a lack of phase locking) and 1 (reflecting perfect phase locking). For PLI analyses complex (single trial) wavelet coefficients were calculated for every time and frequency bin within a frequency range of 1–20 Hz. Statistical analyses focus on the broad alpha frequency band (8–12 Hz). In a control analysis to check for the influence of individual alpha frequency (IAF) we used a narrow band around 10 Hz (9.5–10.5 Hz).
Phase distribution
In order to investigate differences in the preferred phase angle between AB and NoAB and control trials, data were Hilbert transformed. The Kuipers uniform test was run to test for deviations of uniform distribution. Paired sample t-test controlled for equal set size of single trials (AB, NoAB) for fixed SOA (t (13) = −.359; p = .725) and varied SOA (t (13) = −.837; p = .418). For all cases with significant deviations from a uniform distribution, the mean phase vector, representing the preferred mean phase angle, was computed.
Statistical analyses
ROI analysis, fixed and varied presentations
Alpha usually is the largest at posterior (occipital and parietal) recording sites. In order to determine, whether the expected effects are indeed restricted to these sites, we performed paired sample t-test for all sites to test for significant differences between conditions in a time window around the presentation of T2. Because our central hypothesis is focusing on alpha phase locking relative to T2, we focus on a time interval preceding and following the onset of the second target by 50 ms each (i.e. an interval of 250 to 350 ms following the onset of T1). The mean ERP activity (in terms of the difference-area of the respective ERP-segments) between (1) AB and NoAB, (2) AB and control trials, (3) NoAB and control trials was calculated and statistically compared. Only those electrodes were selected at which all three comparisons yielded significant effects (p < . 05).
For the evaluation of statistical differences between conditions of the different EEG parameters (e.g. CT2 amplitude and latency, power and PLI), one-way ANOVAs for repeated measurements with 3 conditions (AB, NoAB and control) were run separately for each ROI. Greenhouse–Geisser correction was applied where necessary and the significance level was set to p < .05. Phase distribution was evaluated by the Kuipers test.
For the examination of the influence of individual alpha frequency (IAF) on behavior (AB magnitude) and phase locking at stimulation frequency (as measured by the PLI at 10 Hz) we performed 2-way ANOVAs with factor IAF (subjects with IAF around 10 Hz versus below and above 10 Hz) and factor task (fixed vs. varied). Dependent measures were either AB magnitude (percentage of trials with AB) or PLI. IAF was determined for the resting EEG at electrodes O1, Oz, and O2. IAF varied between 8.8 and 11.7 Hz. Factor IAF was obtained by grouping subjects in a group with an IAF around 10 Hz (9.0–10.7 Hz) and a group deviant from 10 Hz (below 9.0 and above 10.7 Hz).
Results
ROI analysis, fixed and varied presentations
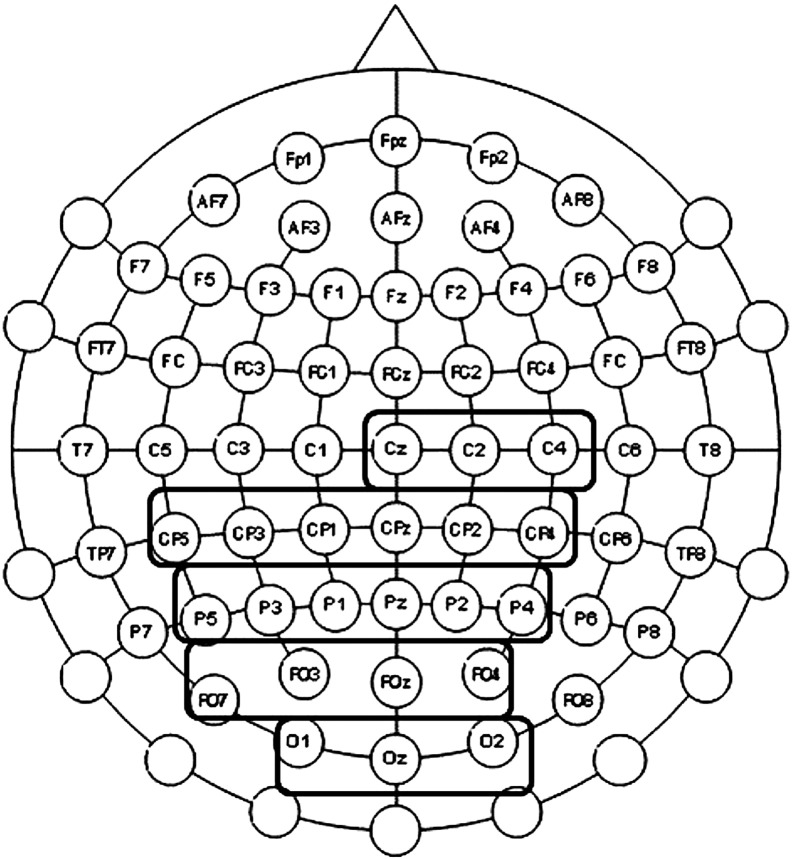
For the fixed presentation block, the analysis yielded 5 ROIs, which were termed (1) central ROI (z, C2, C4), (2) centro-parietal ROI (CP5, CP3, CP1, CPz, CP2, CP4), (3) parietal ROI (P5, P3, P1, Pz, P2, P4), (4) parieto-occipital ROI (Po7, Po3, Poz, Po4), and an (5) occipital ROI (O1, Oz, O2). An overview of the selected ROIs is given in Fig. 2.
Fig. 2.
Illustration of the results of the ROI analysis. Only these electrodes were selected for ROI at which AB significantly differed from both NOAB and control trials and in addition NOAB from control trials (p < .05).
For the varied presentation block no significant results were obtained. This shows that the ERP-segment around T2 does not differ significantly between conditions. Despite this lack of significant findings, the same ROIs – as obtained for the fixed representation block – were used to assess differences between conditions on selected parameters as reported below.
The direction of statistical differences is consistent for all ROIs and shows that mean polarity (in the time window of 250 to 350 ms relative to the onset of T1) is most negative for AB trials and least negative (or already positive) for NoAB, with control trials exhibiting an intermediate position (for an illustration of this finding see Fig. 3a).
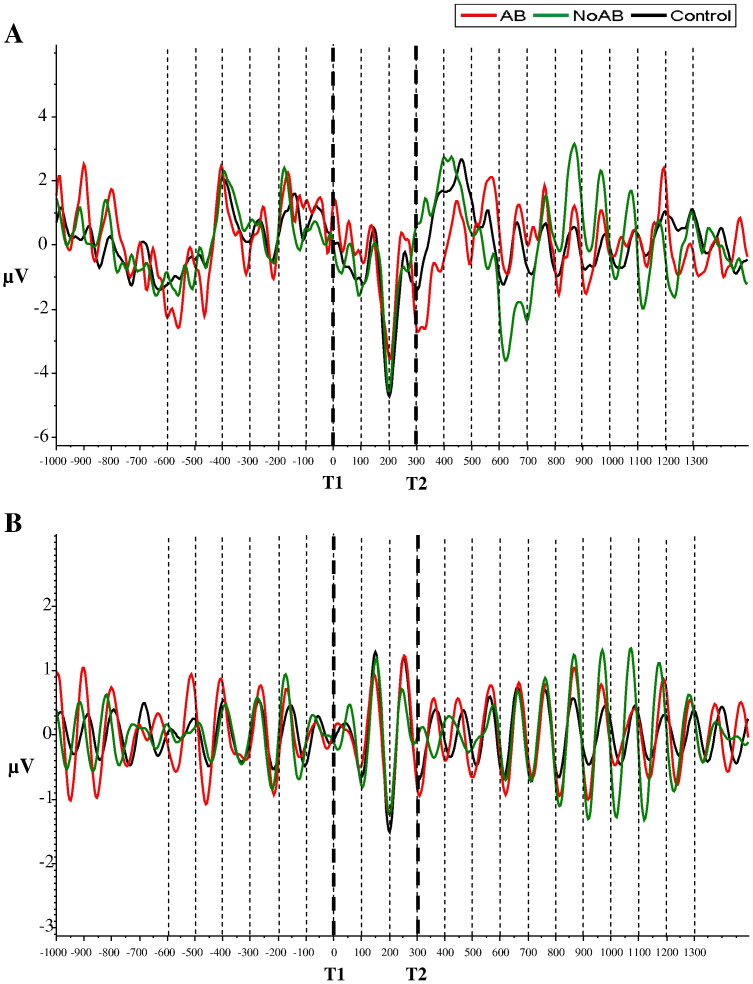
Fig. 3.
SSVEP's fixed SOA. A) Unfiltered ERP for parietal ROI. Bold vertical dashed lines indicate the onset of T1 and T2 (for experimental trials). Vertical dashed lines reflect the onset of distracters. Note the strong negative component around T2 appearance for AB (red) compared to NoAB (green) and control (black). B) 8–12 Hz filtered ERP for parietal ROI.
Behavioral data, fixed and varied presentations
An AB trial is defined by a correct report of T1 but a failure to report T2. NoAB trials consist of a correct report of both targets, T1 and T2. Thus, both AB and NoAB trials represent experimental trials, in which the first target (T1) was correctly reported. For the fixed presentation task, this set consisted of 76.7 trials (SD = 3.8) which is 95.9% of all experimental trials. This means that in 4.1% from the 80 experimental trials, T1 was not reported. From the remaining 76.7 trials in which T1 was reported, 50.2% were NoAB and 49.8% were AB trials. For control trials, the target (T1) could be reported in 90.3% of all cases. T1 was missed in 2.3% and T2 was erroneously reported, i.e., false alarms, in 7.4% of the cases (cf. a summary of the behavioral data is in Table 1).
Table 1.
Behavioral data.
| Fixed |
Varied |
|||
|---|---|---|---|---|
| Correct (%) | Incorrect (%) | Correct (%) | Incorrect (%) | |
| Experimental trials | ||||
| T1 | 95.9 | 4.1 | 93.6 | 6.4 |
| T2 | 50.2 | 49.8 | 52.2 | 47.8 |
| Control trials | ||||
| T1 | 90.3 | 2.3 | 90.9 | 2.4 |
| T2 false alarm rate | 7.4 | 6.7 | ||
In the varied presentation task, T1 was not reported in 6.4% of all trials. From the remaining 70.0 trials in which T1 was reported, 52.2% were NoAB and 47.8% were AB trials. For control trials, the T1 could be reported in 90.9% of all cases. T1 was missed in 2.4% and T2 was erroneously reported in 6.7% of the cases.
The results of a 2-way ANOVA with factor TASK (fixed, varied) and TARGET TYPE (T1, T2) and the percentage of correct responses showed – as expected – a significant effect for TARGET TYPE (F(1,13) = 136.8; p < .001). Most importantly, neither significant effects for factor TASK nor the interaction TASK × TARGET TYPE was obtained.
IAF and performance
The results of a 2-way ANOVA with factor IAF (subjects with IAF around 10 Hz vs. IAF deviant from 10 Hz), factor TASK (fixed vs. varied), and AB magnitude as dependent variable showed that individual alpha frequency had no impact on performance. Neither factor IAF nor the interaction reached or exceeded the 5%-level of significance. IAF varied between 8.8 and 11.7 Hz with a mean of 10.4 Hz (SD = 1.1 Hz).
ERPs
Steady state responses, fixed presentation
An example for an ERP of the entire stream of 20 item presentations for the parietal ROI is shown in Fig. 3a. Visual inspection reveals a negative component that appears around the onset of almost each stimulus. Most interestingly, immediately before the presentation of T2 (i.e., at stimulus onset of the letter preceding T2), this negative component reaches a maximum (relative to all 20 letter presentations). It is important to note that here the negative component is larger for NoAB and control trials as compared to AB trials. During the presentation of T2 – as the ROI analysis has already shown – this relationship reverses, with AB trials showing now the largest negative component relative to the other two trial types.
The band pass filtered ERPs (in the 8–12 Hz frequency range) – as depicted in Fig. 3b for the parietal ROI – show that the prestimulus negative component has a strong frequency characteristic in the alpha band. It is also interesting to note that after the appearance of T1, the evoked alpha response increases until the onset of T2 and then decreases for the next 2–3 item presentations.
Steady state responses, varied presentation
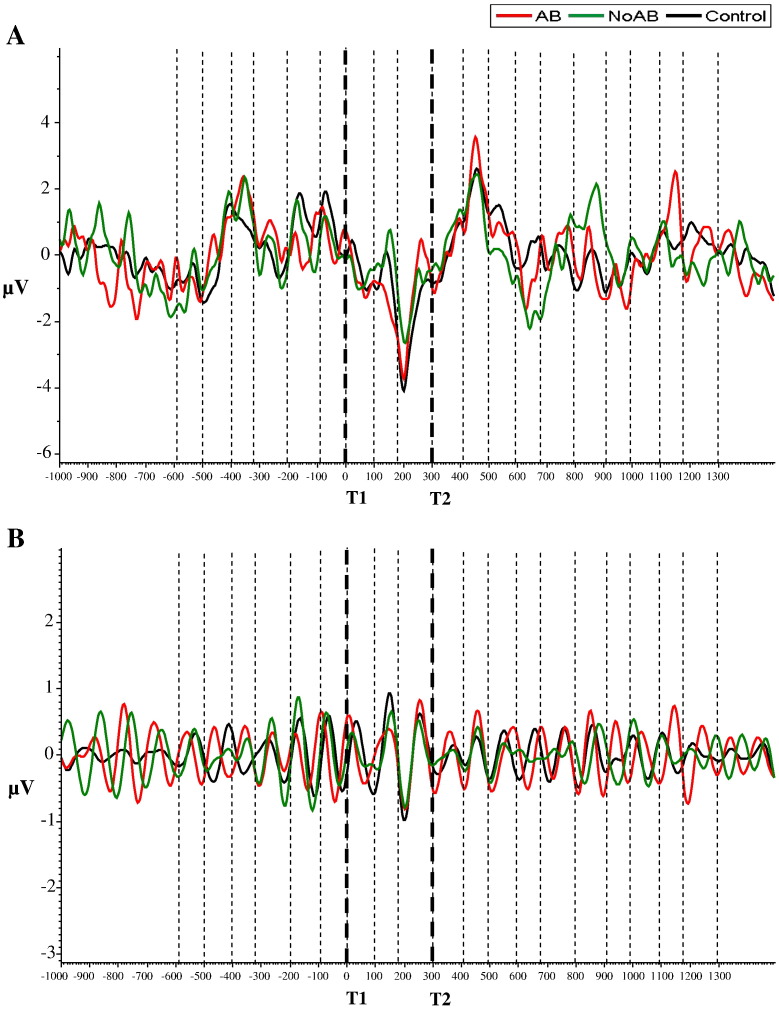
The ERPs of the varied presentation block are depicted in Fig. 4a. They show that the pronounced ERP differences between conditions (i.e., between AB, NoAB and control trials) during – and following – the onset of T2, which can be observed in the fixed task are absent in the varied task. Most interestingly, the alpha filtered ERPs are quite similar in the fixed and varied presentation blocks.
Fig. 4.
SSVEP's varied SOA. Data presentation is analogous to Fig. 3.
CT2 peak amplitude and latency, fixed presentation
The results of one-way ANOVAs for the CT2 peak amplitude show significant main effects between AB, NoAB and control trials for central (F2/26 = 10,6; p < .001), centro-parietal (F2/26 = 15,6; p < .001), parietal (F2/26 = 12,2; p < .001), parieto-occipital (F2/26 = 6,5; p < .01) but not for occipital ROIs. Pairwise comparisons showed significant (p < .05) larger amplitudes for AB as compared to NoAB or control trials. No significant differences were obtained between NoAB and control trials. No significant effects were observed for CT2 peak latencies.
CT2 peak amplitude and latency, varied presentation
The one-way ANOVAs for the data of the varied task did not yield significant effects for any of the ROIs. Thus, the amplitude of the CT2 component does not differ between conditions.
For peak latency of the CT2 component, a significant main effect (F2/26 = 3.9; p < .05) was observed at a parieto-occipital ROI. Inspection of the respective means indicates that the latency for NoAB trials is about 20 ms shorter than for AB and control trials.
Alpha band (8–12 Hz)
Whole power, fixed and varied presentations
None of the ANOVAs for whole power in the fixed and varied tasks reached significance. This indicates that there is no significant power differences between AB, NoAB and AB trials that are not time or phase locked.
Evoked alpha: CT2 peak amplitude and latency of the filtered ERPs, fixed presentation
The filtered ERPs' significant main effects between AB, NoAB and control trials were obtained for centro-parietal (F2/26 = 5,3; p < .05), parietal (F2/26 = 6.4; p < .01), parieto-occipital (F2/26 = 4,1; p < .05) but not for central and occipital ROIs. Pairwise comparisons yielded similar effects as for the unfiltered data. Again, the direction of the differences is that the CT2 amplitude is largest for AB as compared to NoAB and AB trials. No significant effects were observed for CT2 peak latencies.
Evoked alpha: CT2 peak amplitude and latency of the filtered ERPs, varied presentation
For the peak component, a similar pattern of results could be observed (cf. Figs. 3a and 4a), but the differences between AB, NoAB and control trials did not reach significance. For the centro-parietal ROI, the respective F-value (F2/26 = 5.3; p = .056) closely missed exceeding the 5%-level of significance. Again, no significant effects were observed for CT2 peak latencies.
Phase locking index (PLI), fixed presentation
Significant main effects were observed for central (F2/18,289 = 4.8; p < .05), for centro-parietal (F2/26 = 6.2; p < .01), parietal (F2/26 = 6.0; p < .01) and parieto-occipital (F2/26 = 4.2; p < .05), but not for occipital ROIs. For these ROIs with significant main effects, pairwise comparisons yielded significant differences between AB and control trials only. Inspection of the respective means shows that phase locking is largest for AB and smallest for control trials with NoAB trials exhibiting slightly larger values than control trials. The largest effects were observed for electrode P4.
Phase locking index (PLI), varied presentation
In a similar way as for the fixed presentation block, significant main effects were observed for central (F2/26 = 3.7; p < .05), and for centro-parietal (F2/26 = 3.4; p < .05) ROIs. No other ROIs reached significance.
Entrainment of phase, fixed presentation
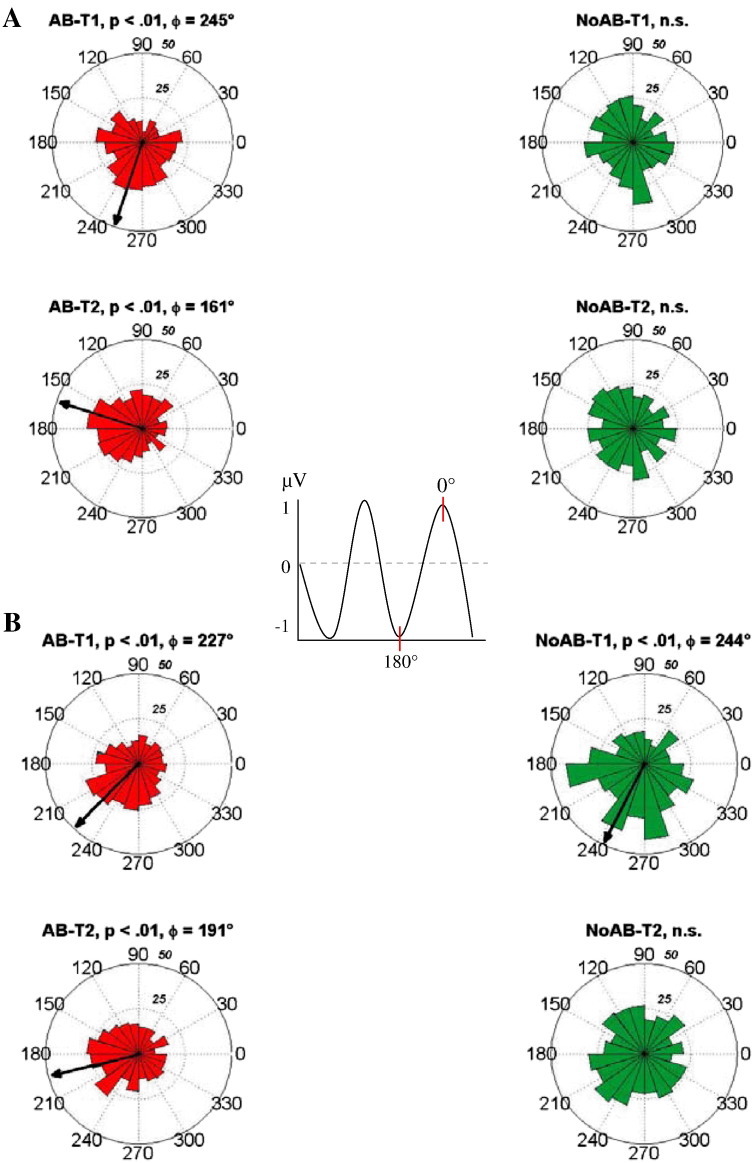
The findings for the phase locking index show that intertrial phase stability is larger for AB trials. This finding, however, gives no information about the preferred phase angle at which phase locking occurs. Thus, we calculated the phase distribution during the onset of T1 and T2 for electrode P4 which exhibited the largest PLI. Then, we used the Kuipers test, to examine, whether the observed phase distributions exhibit a significant phase concentration that differs from a random distribution. Finally, for cases with significant phase concentrations, we calculated the mean phase angle. The results, as depicted in Fig. 5a—show that a significant phase distribution could be observed for T1 and T2 (tαcrit = .01 in both cases) but only for AB and not for NoAB trials. For T1 the mean phase angle is at 245°, which is in the positive going slope, near the 0-crossing. For T2, however the mean angle is 161° which is close at the negative peak of the alpha wave.
Fig. 5.
Mean phase angle for the 8–12 Hz filtered data. A) For fixed SOA, AB (left column) single trials showed significant deviation from uniform distribution with a mean phase angle of 245° for T1 (top) and 161° for T2 (bottom) onset. NoAB trials were uniformly distributed (right column). B) For varied SOA, AB (left column) single trials showed a non-uniform distribution with a mean phase angle of 227° for T1 (top) and 191° for T2 (bottom) onset. NoAB (right column) single trials were non-uniformly distributed at T1 onset (top) with a mean phase angle of 244°.
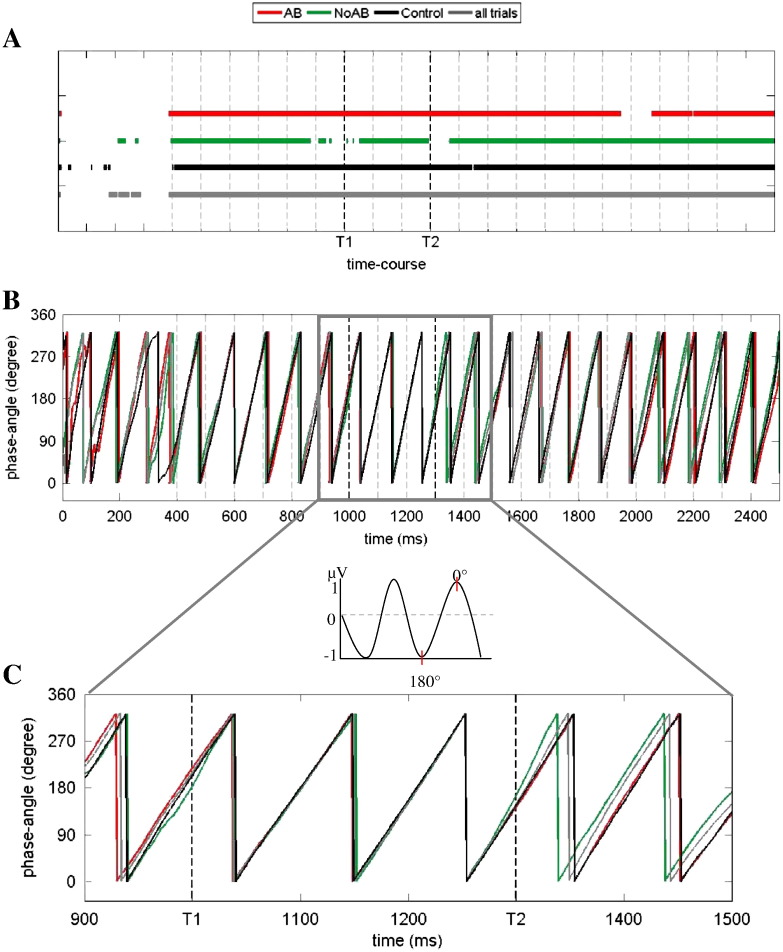
When the Kuipers test is calculated for any sample point of the entire series of 20 letters, the following two important findings emerge. As illustrated in Fig. 6a, a significant phase concentration is the dominant characteristic for almost the entire period of item presentations. However, in stark contrast to AB trials, a lack of phase concentration – reflecting a ‘release’ from entrainment – can be observed for NoAB trials.
Fig. 6.
Results of Kuiper's test for fixed SOA (8–12 Hz filtered data). A) AB (red), control (black) and all trials (grey) showed a non-uniform distribution over the time course (continuous lines). Interestingly, NoAB trials (green) were uniformly distributed around T1 and T2 onset (broken lines). B) The phase-angle is illustrated for each condition over the whole time course. C) Extract of phase-angle distribution for the time window around T1 and T2 appearance.
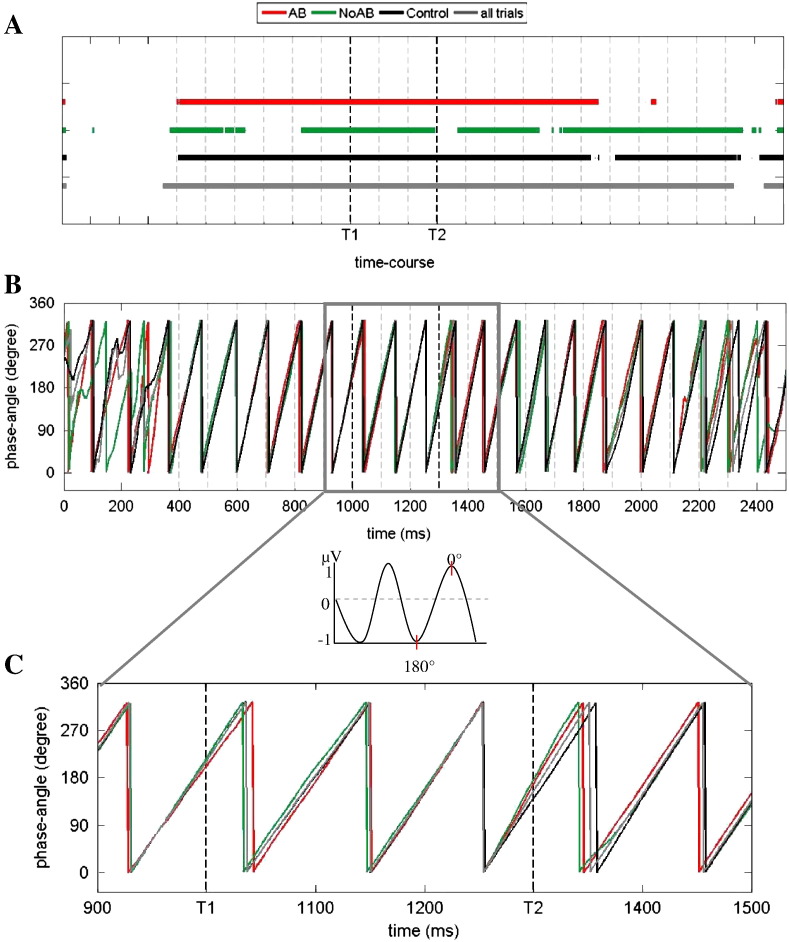
Entrainment of phase, varied presentation
The general pattern of results is similar for the varied presentation. A significant phase distribution could be observed for T1 and T2 (tαcrit = .01 in both cases) for AB trials. Phase angles also show similar values (227° for T1 and 191° for T2). However, a significant phase concentration with a mean phase angle of 244° can also be seen for NoAB trials during T1.
Comparable to the fixed presentation, a lack of phase concentration – reflecting a ‘release’ from entrainment – can also be observed for NoAB trials in the varied condition. However, a significant phase concentration can be seen also for T1.
Influence of IAF on entrainment
The results of a 2-way ANOVA with factor IAF (subjects with IAF around 10 Hz vs. IAF deviant from 10 Hz), factor TASK (fixed vs. varied), and PLI at 10 Hz on electrode P4 as dependent measure showed that individual alpha frequency had no significant influence on phase locking at stimulation frequency. Neither factor IAF nor the interaction reached or exceeded the 5%-level of significance.
Frequency range of phase-locking
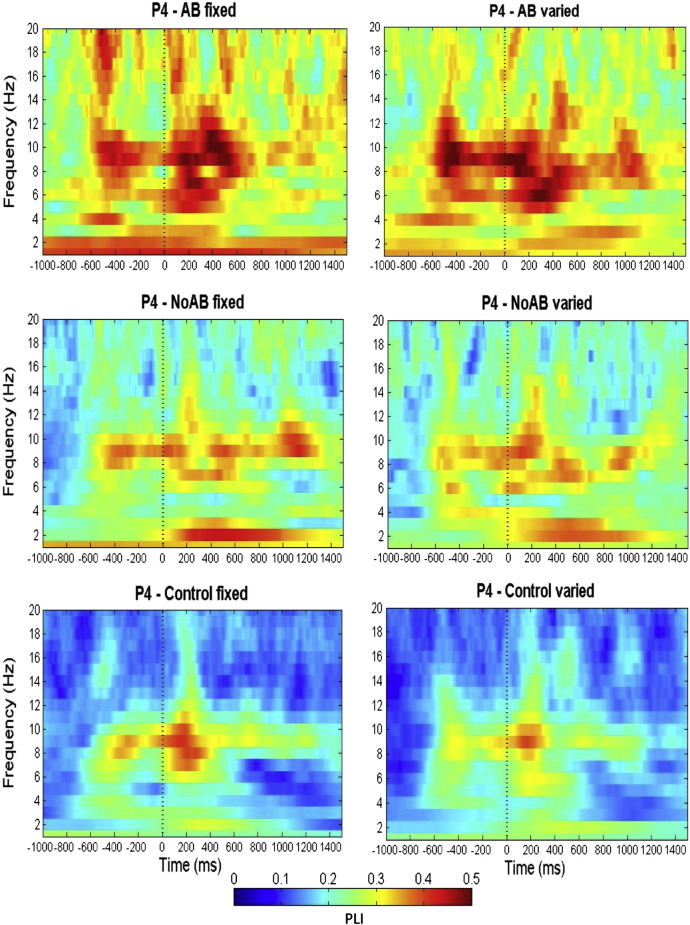
Our frequency analysis has focused on the broad alpha range of 8–12 Hz. In order to illustrate to what extent specific frequencies are involved in the attentional blink phenomenon, we refer to the time–frequency plots of the PLI as depicted in Fig. 8. The plots nicely demonstrate that phase locking around T2 is largest in the broad alpha band and also comprises the theta frequency range around 5 Hz but is not concentrated at stimulation frequency of 10 Hz (fixed condition).
Fig. 8.
Time-frequency plots for the phase locking index (PLI). Note the strong phase-locking in the broad alpha range (8–12) for AB fixed (left, top) and varied (right, top) SOA compared to NoAB (middle) and control (bottom).
The results of a 2-way ANOVA with factor TASK (fix, varied) and BAND (lower alpha 8–10 Hz vs. upper alpha 10–12 Hz) and PLI (peristimulus to T2, 250–350 ms) as dependent measure yielded no significant, neither for the main effects nor for the interaction. This suggests that phase locking around T2 is a broad-band phenomenon.
A comparison of phase locking between fixed and varied presentation
An interesting observation is that phase locking shows very similar results for the fixed and varied presentation blocks. For the alpha band the peristimulus PLI (for the time interval of +/− 50 ms preceding and following the onset of T2) does not differ significantly between the two blocks, as the results of a paired t-test (calculated separately for each of the 5 ROIs) indicate. This lack of differences most likely is due to the fact that T2 appears exactly 300 ms after T1 in both blocks.
Phase locking in control trials
The data show that phase locking is largely diminished in control trials. First of all, there is a methodological reason for this finding because we have about twice as much control trials than AB or NoAB trials and because the magnitude of the PLI is known to decrease with increasing sample size (Kutil, 2011; Vinck et al., 2010). But there is possibly, in addition, yet another reason. Let us proceed from the fact that subjects could not know whether T2 will be presented and let us assume that half of the control trials exhibits large phase locking whereas the other half shows only minor phase locking. In experimental trials the half of trials with large phase locking constitute the AB trials, the other half the NoAB trials. Exactly the same may hold true for control trials, with the only exception that a distinction between – and selective averaging for – AB and NoAB trials is not possible because T2 is missing. As phase locking is calculated over all of the control trials, the variance between all trials increases and the PLI decreases.
Discussion
The central hypothesis for this study is that alpha entrainment with minimal phase lag is a critical factor for the attentional blink phenomenon. Thus, we have predicted that entrainment – during the time window of T2 presentation – is larger for AB than NoAB trials. The extent and kind of alpha entrainment can be judged by at least three EEG parameters, the amplitude of the ERP (= SSVEP) in response to stimulus onset – particularly to T1 and T2 – the extent of alpha phase concentration (e.g., as measured by the PLI) during stimulus onset, and the absolute phase angle of alpha during stimulus onset.
As depicted in Fig. 3a the SSVEP consists of components with positive and negative peaks that have a pronounced frequency characteristic in the alpha range (cf. Fig. 3b). The interesting observation here is that in a time window of 100 ms preceding T2 a large negative peak can be observed. At the time point of stimulus onset of T2, this negative peak component (termed CT2) is much larger for AB as compared to NoAB and control trials. Statistical analyses clearly show that these differences are statistically significant for the CT2 peak amplitude of the ERP, for evoked alpha power, for alpha PLI but not for alpha power (whole power). These findings demonstrate that the differences in the CT2 amplitude of the SSVEP are due to the phase locking of the alpha rhythm and not due to an amplitude modulation as the lack of differences in alpha (whole) power indicates. Thus, we can conclude that at least part of the increase in the CT2 peak amplitude – which characterizes AB trials – is due to alpha phase locking. It should also be emphasized that the increased CT2 amplitude in AB trials is unlikely to be caused by a high proportion of unconsciously seen items that did not reach/exceed the ‘seen’‐threshold. The reason is that unconsciously seen items generally tend to lower ERP amplitude (e.g., Hillyard et al., 1971).
Another important aspect is the entrainment of alpha phase with the negative peak during stimulus onset of T2. Phase analysis revealed a significant phase concentration of alpha at the negative peak during presentation onset of T2 for AB trials only. This is an important observation that is central for the evaluation of the hypothesis that alpha phase entrainment – with minimal phase lag – leads to an interference of those processes that underlie the generation of the P1 with those underlying the encoding of the next following stimulus. The reasoning is the following: if the alpha rhythm is entrained with zero phase lag, as we observed for AB trials, the negative alpha peak (reflected by an phase angle of 180°; cf. Figs. 5–7) coincides exactly with the presentation onset of T2 and, as a consequence, the next negative peak coincides exactly with the ‘processing peak’ of early categorization of T2. Under normal conditions (i.e., in tasks with an SOA longer than about 500 ms) the P1 – reflecting early categorization (cf. Klimesch, 2011 for an extensive review) – would be generated in exactly that time window, where in AB trials the negative alpha peak is generated. The lack of a significant alpha entrainment in NoAB trials may provide the possibility to avoid this interference between early categorization of T2 and encoding of the next following, because these two processes would not be completely time locked to each other.
Fig. 7.
Results of Kuiper's test for varied SOA (8–12 Hz filtered data). A) AB (red), control (black) and all trials (grey) showed a non-uniform distribution over the time course (continuous lines). NoAB trials (green) were uniformly distributed around T2 onset (broken lines). B) The phase-angle is illustrated for each condition over the whole time course. C) Extract of phase-angle distribution for the time window around T1 and T2 appearance.
We conclude that alpha entrainment alone is not sufficient to generate the AB phenomenon. It is rather the combination between entrainment and phase concentration at the negative peak that is important. As illustrated in Figs. 6 and 7 entrainment can be observed in AB trials over almost the entire trial, including the presentation of T1. But despite of entrainment during the onset of T1, this target can be reported. The difference between T1 and T2 lies in the phase angle. During presentation onset of T1, alpha phase angle is close to 240°, which represents the positive going slope close to the zero crossing of the alpha oscillation, whereas during T2 the phase angle is 180°. Thus, the next negative peak of the alpha oscillation (relative to the presentation onset of T1) appears earlier at about 80 ms and, thus, does not overlap with the P1 time window as in the case for T2. The calculation is the following: the phase angle at T1 is 240°. Relative to this time point, the next following negative peak appears at 180° after the cycle is completed going from 240° to 360° (= 120°). Thus, the peak latency is 300° (180° + 120° = 300°) which represents 83 ms (considering that 1° = 0.278 ms when assuming a frequency of exactly 10 Hz).
We have to emphasize that the varied presentation block showed very similar results, behaviorally and electrophysiologically. Exactly the same pattern of results can be observed, also showing that entrainment with zero phase lag is the critical factor for the AB phenomenon. This is surprising, because we have assumed that the jittered presentation would lead to both, a reduction in the frequency of attentional blink trials and a reduction of phase entrainment. The key for understanding this finding may be seen in another surprising finding: phase locking in the fixed presentation block is not concentrated at stimulation frequency (of 10 Hz) but shows up in a broad frequency range as Fig. 8 demonstrates. This is unexpected because flicker studies usually show a specific response at the stimulation frequency (cf. e.g. Herrmann, 2001). However, studies examining the relationship between stimulation frequency and individual alpha frequency (IAF) have revealed that response frequency in the EEG may shift toward IAF. As an example, Gebber et al. (1999) reported that for a subject with IAF = 10.8 Hz and a stimulus frequency of 4.9 Hz, the response frequency was largest at 9.8 Hz. In addition to IAF, alpha entrainment also depends on the individual power of alpha (Sakamoto et al., 1993).
This phenomenon of ‘IAF-entrainment’, which means that the largest EEG response is not exactly at stimulation frequency (10 Hz in our case) but close to IAF, could be responsible for the observed broad response frequency in the alpha band, as it is well known that IAF exhibits a large interindividual variation. This interpretation, however, is not very likely, because we found that IAF neither had a significant influence on AB magnitude, nor on phase locking. We also have to emphasize that the variation in IAF was not very large. Thus, an evaluation of the ‘IAF-entrainment’ hypothesis is difficult on the basis of our data. An alternative interpretation is based on the idea that alpha is not a single rhythm with a frequency at IAF but consists of a population of rhythms that vary (possibly in a task dependent manner) around IAF. Accordingly, the responsiveness of alpha with respect to entrainment may be understood as a broad-band phenomenon. This interpretation could easily explain why alpha phase locking is not diminished in the varied presentation block. The jittered presentation reflects a variation in stimulation frequency, but due to ‘broad band’ or ‘population’ entrainment, the entrained frequencies between the two task blocks do not differ. It should also be mentioned that IAF-entrainment has primarily been studied in tasks where subjects were passively exposed to flicker stimulation without any additional task demands. The present attentional blink paradigm, however, combines rhythmic stimulation with an increase in cognitive demands.
‘Population’ entrainment may not be diminished as long as the jittered SOAs lie in the alpha frequency range, which is the case in our study. The jittered SOA varied between 80 and 120 ms. The short SOA with 80 ms represents a period of fast alpha at 12.5 Hz, whereas 120 ms represents a period of slow alpha at 8.3 Hz. Behavioral results with jittered SOAs show that the attentional blink can indeed be reduced (Martin et al., 2011) if the jitter is large, ranging from 34 ms to 170 ms (reflecting a frequency of about 30 Hz and 5 Hz respectively). Thus, it appears plausible to assume that jittered presentations reduce the attentional blink, but probably only if the corresponding stimulation frequencies are well outside the alpha frequency range, thereby avoiding alpha entrainment.
There may even be a third reason which may be responsible for the lack of behavioral and electrophysiological differences between the fixed and varied presentation. In both blocks, T2 appeared exactly at 300 ms poststimulus to T1. This enables subjects to use temporal expectation to improve their performance (cf. Mathewson et al., 2010), particularly if we would assume that temporal expectation has a preferential time span in the range of several hundred ms, which could be characteristic for a sensory buffer.
Finally, let us address the question whether other findings linking alpha and AB magnitude are consistent with our observations regarding entrainment. Of particular interest here is the work by MacLean and colleagues. In one experiment they observed that resting alpha power is positively associated with AB magnitude (MacLean et al., 2012). In another, they observed a complex interaction between (alpha) ERD, AB magnitude and lag (MacLean and Arnell, 2011). With regard to the earlier experiment, it should be noted that ERD was measured in a foreperiod of 2 s, i.e., before the RSVP started. At short lag (351 ms after T1) large ERD (reflecting large alpha power suppression) was associated with low T2 accuracy (high AB magnitude), but at long lag (936 ms after T1) was associated with high T2 accuracy. The authors interpreted this finding in terms of an attentional (over-)investment leading to an increase in ERD in a foreperiod preceding the RSVP which in turn leads to an increased AB magnitude at short lag. These findings are well in line with the hypothesis that alpha reflects an inhibitory filter (Klimesch et al., 2007; Klimesch, 2011).
It is worth emphasizing that in our study suppression of T2 is associated with a specific phase response but not with a change in power. This is well in line with the findings of other target detection tasks which are performed under difficult perceptual conditions. As an example, Hanslmayr et al. (2005) observed that good performers showed smaller power during a foreperiod (immediately preceding target detection) than bad performers. In addition, it was found that good performers showed a significantly larger phase response (in terms of phase locking) during task performance. This finding also suggests that attentional investment leads to a decrease in power in a foreperiod and to an increased phase response during target detection.
In summarizing, the present findings provide clear and strong support for the hypothesis that alpha entrainment is a critical factor for the attentional blink phenomenon. Further studies will be necessary to determine the exact role played by alpha and how oscillation in this particular frequency band relates to existing theories of the AB, which emphasize a strong role for the process of transferring perceptual input into short-term visual memory. For example, alpha may reflect a top-down process that controls access to a memory trace of an expected item (cf. Klimesch, 2011). This process may increase the likelihood of entrainment and the resulting interference on the processing of T2.
Acknowledgments
This research was supported by the Austrian Science Foundation (FWF Project P21503-B18). The first author, Andrea Zauner, of this article was financially supported by the Doctoral College "Imaging the Mind" of the Austrian Science Fund (FWF-W1233).
References
- Busch N.A., Dubois J., VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun M.M., Potter M.C. A two-stage model for multiple target detection in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Di Lollo V., Kawahara J., Shahab Ghorashi S.M., Enns J.T. The attentional blink: resource depletion or temporary loss of control? Psychol. Res. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Duncan J., Ward R., Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369:313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Foxe J.J., Snyder A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga M.S. MIT Press; Cambridge, MA: 1995. The Cognitive Neurosciences. [Google Scholar]
- Gebber G.L., Zhong S., Lewis C., Barman S.M. Human brain alpha rhythm: nonlinear oscillation or filtered noise? Brain Res. 1999;818:556–560. doi: 10.1016/s0006-8993(98)01303-1. [DOI] [PubMed] [Google Scholar]
- Gross J., Schmitz F., Schnitzler I., Kessler K., Shapiro K., Hommel B., Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. U. S. A. (PNAS) 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Klimesch W., Sauseng P., Gruber W., Doppelmayr M., Freunberger R., Pecherstorfer T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005;375:64–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Aslan A., Staudigl T., Klimesch W., Herrmann C.S., Bäuml K.H. Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Gross J., Klimesch W., Shapiro K.L. The role of alpha oscillations in temporal attention. Brain Res. Rev. 2011;67:331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp. Brain Res. 2001;137:346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Squires K.C., Bauer J.W., Lindsay P.H. Evoked potential correlates of auditory signal detection. Science. 1971;172:1357–1360. doi: 10.1126/science.172.3990.1357. [DOI] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:12. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. Concurrent response-selection demands modulate the attentional blink. J. Exp. Psychol. Hum. Percept. Perform. 1999;25:1097–1113. doi: 10.1037//0096-1523.25.6.1483. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Dell'Acqua R. The demonstration of short-term consolidation. Cogn. Psychol. 1998;36:138–202. doi: 10.1006/cogp.1998.0684. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Jijiwa H., Watanabe S. The dynamics of phase relationships of alpha waves during photic driving. Electroencephalogr. Clin. Neurophysiol. 1993;87:88–96. doi: 10.1016/0013-4694(93)90115-c. [DOI] [PubMed] [Google Scholar]
- Kawahara J., Enns J.T., Di Lollo V. The attentional blink is not a unitary phenomenon. Psychol. Res. 2006;70:405–413. doi: 10.1007/s00426-005-0007-5. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Evoked alpha and early access to the knowledge system: the P1 inhibition timing hypothesis. Brain Res. 2011;1408:52–71. doi: 10.1016/j.brainres.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Fellinger R., Freunberger R. Alpha oscillations and early stages of visual encoding. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranczioch C., Debener S., Engel A.K. Event-related potential correlates of the attentional blink phenomenon. Cogn. Brain Res. 2003;17:177–187. doi: 10.1016/s0926-6410(03)00092-2. [DOI] [PubMed] [Google Scholar]
- Kranczioch C., Debener S., Maye A., Engel A.K. Temporal dynamics of access to consciousness in the attentional blink. NeuroImage. 2007;37:947–955. doi: 10.1016/j.neuroimage.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Kutil R. Biased and unbiased estimation of the circular mean resultant length and its variance. Statistics. 2011:1–13. [Google Scholar]
- Lakie M., Combes N. The phase of postural hand tremor is not influenced by repetitive photic brain stimulation. Clin. Neurophysiol. 1999;110:2020–2025. doi: 10.1016/s1388-2457(99)00180-7. [DOI] [PubMed] [Google Scholar]
- Lazarev V.V., Simpson D.M., Schubsky B.M., Deazevedo L.C. Photic driving in the electroencephalogram of children and adolescents: harmonic structure and relation to the resting state. Braz. J. Med. Biol. Res. 2001;34:1573–1584. doi: 10.1590/s0100-879x2001001200010. [DOI] [PubMed] [Google Scholar]
- MacLean M.H., Arnell K.M. Greater attentional blink magnitude is associated with higher levels of anticipatory attention as measured by alpha event-related desynchronization (ERD) Brain Res. 2011;1387:99–107. doi: 10.1016/j.brainres.2011.02.069. [DOI] [PubMed] [Google Scholar]
- MacLean M.H., Arnell K.M., Cote K.A. Resting EEG in alpha and beta bands predicts individual differences in attentional blink magnitude. Brain Cogn. 2012;78:218–229. doi: 10.1016/j.bandc.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Martin E.W., Enns J.T., Shapiro K.L. Turning the attentional blink on and off: opposing effects of spatial and temporal noise. Psychon. Bull. Rev. 2011;18:295–301. doi: 10.3758/s13423-011-0056-2. [DOI] [PubMed] [Google Scholar]
- Mast J., Victor J.D. Fluctuations of steady-state VEPs: interaction of driven evoked potentials and the EEG. Electroencephalogr. Clin. Neurophysiol. 1991;78:389–401. doi: 10.1016/0013-4694(91)90100-i. [DOI] [PubMed] [Google Scholar]
- Mathewson K.E., Gratton G., Fabiani M., Beck D.M., Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J. Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K.E., Fabiani M., Gratton G., Beck D.M., Lleras A. Rescuing stimuli from invisibility: inducing a momentary release from visual masking with pre-target entrainment. Cognition. 2010;115:186–191. doi: 10.1016/j.cognition.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Mathewson K.E., Lleras A., Beck D.M., Fabiani M., Ro T., Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed inhibition of ongoing cortical processing. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers C.N., Meeter M. A boost and bounce theory of temporal attention. Psychol. Rev. 2008;115:836–863. doi: 10.1037/a0013395. [DOI] [PubMed] [Google Scholar]
- Raymond J.E., Shapiro K.L., Arnell K.M. Temporary suppression of visual processing in an RSVP task: an attentional blink? J. Exp. Psychol. Hum. Percept. Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Romei V., Gross J., Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J. Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Inouye T., Shinosaki K. Preservation of alpha rhythm shortly after photic driving. Int. J. Neurosci. 1993;73:227–233. doi: 10.3109/00207459308986673. [DOI] [PubMed] [Google Scholar]
- Schack B., Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci. Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Shapiro K.L., Raymond J.E., Arnell K.M. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1994;20:357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- Shapiro K.L., Raymond J.E., Arnell K.M. The attentional blink. Trends Cogn. Sci. 1997;1:291–296. doi: 10.1016/S1364-6613(97)01094-2. [DOI] [PubMed] [Google Scholar]
- Shils J.L., Litt M., Skolnick B.E., Stecker M.M. Bispectral analysis of visual interactions in humans. Electroencephalogr. Clin. Neurophysiol. 1996;98:113–125. doi: 10.1016/0013-4694(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Silberstein R.B. Steady-state visually evoked potentials, brain resonances, and cognitive processes. In: Nunez P., editor. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; New York: 1995. pp. 272–303. [Google Scholar]
- Silberstein R.B., Schier M.A., Pipingas A., Ciorciari J., Wood S.R., Simpson D.G. Steady-state visually evoked potential topography associated with a visual vigilance task. Brain Topogr. 1990;3:337–347. doi: 10.1007/BF01135443. [DOI] [PubMed] [Google Scholar]
- Silberstein R.B., Ciorciari J., Pipingas A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalogr. Clin. Neurophysiol. 1995;96:24–35. doi: 10.1016/0013-4694(94)00189-r. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Delpuech C., Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Schyns P.G., Gross J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol. 2011;2:170. doi: 10.3389/fpsyg.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Veniero D., Romei V., Miniussi C., Schyns P., Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M., van Wingerden M., Womelsdorf T., Fries P., Pennartz C.M. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. NeuroImage. 2010;51:112–122. doi: 10.1016/j.neuroimage.2010.01.073. [DOI] [PubMed] [Google Scholar]
- Ward R., Duncan J., Shapiro K. The slow time-course of visual attention. Cogn. Psychol. 1996;30:79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]