Abstract
Introduction
Different approaches for disc regeneration are currently under investigation. Beside gene therapy and tissue engineering techniques, the application of growth and differentiation factors own promising potential. Studies using reduced intervertebral disc models, such as cell or tissue fragment cultures, have limited validity and show controversial results depending on the employed experimental model. Therefore, the goal of the current study was to investigate the effect of BMP-2 and TGF-β3 on intervertebral disc degeneration using an in vitro full-organ disc/endplate culture system.
Materials and methods
Intervertebral rabbit disc explants were cultured in the presence of 1 μg/ml BMP-2 or TGF-β3 for 21 days in DMEM/F12 media. Nucleus and annulus were analyzed for gene expression of collagen type I and II (Col I/II), aggrecan, collagenases (MMP-1/MMP-13) with RT-qPCR, histological changes with bone and proteoglycan-specific staining (von Kossa, toluidine blue) and differences in cellularity (DNA) and proteoglycan content (alcian blue binding assay).
Results
The results demonstrate that disc proteoglycan concentration decreased with time in the TGF-β3 and BMP-2 groups. In the annulus fibrosus (AF), TGF-β3 and BMP-2 resulted in an up-regulation of Col I and type II, and of aggrecan gene expression. In contrast, MMP genes were inhibited. In the nucleus, the growth factors decreased gene expression of aggrecan and spontaneous Col I up-regulation was inhibited by TGF-β3, whereas expression of Col II was decreased with BMP-2. There was no effect on expression of MMP-1 and MMP-13 for most sampling points. However, TGF-β3 and BMP-2 induced ossification of the AF was demonstrated by histology.
Conclusion
It can be concluded that both growth factors, at the tested concentrations, may not be suitable to regenerate the whole intervertebral disc organ but they are interesting candidates for being injected alone or in combination into a painful intervertebral disc to induce osseous fusion (spondylodesis).
Keywords: Intervertebral disc degeneration, TGF-β3, BMP-2, In vitro model, Ossification
Introduction
Current treatment of symptomatic intervertebral disc degeneration consists of either conservative measures, such as the application of analgesics and physiotherapy or, if not helpful, surgery is performed [1]. These approaches are not proven to slow down the degeneration process and consequently relapses or other adverse sequelae of discectomy, dynamic stabilization techniques, total disc replacement, or fusion surgery can be expected [2–6]. Therefore, and as disc degeneration has a high prevalence and is associated with major socioeconomic costs, regeneration of the organ would be desirable [7–9].
Different approaches are currently under investigation: gene therapy including gene silencing [10, 11], cell-based tissue engineering strategies including the utilization of stem cells from different origin [12–15] or the application of growth factors [10, 16–18].
Growth factors are small signaling proteins, which direct the development of cells and play a role in embryogenesis, proliferation and differentiation, tissue homeostasis, immune response, wound healing and angiogenesis, [19–21]. The TGF-β (transforming growth factor) superfamily consists currently of four major subfamilies of dimeric polypeptides: the TGF-β subfamily (with three isotypes) [22], the DVR related subfamily including the bone morphogenic proteins (BMPs) [23, 24], the growth differentiation factor subfamily (GDF) and the activin/inhibin subfamily.
One of the best described factors is BMP-2, which has been tested in various in vivo or in vitro intervertebral disc models. The osteoinductive potential of BMP-2 and promotion of spinal fusion have initially been demonstrated in animal models [25, 26]. Later, the factor entered clinical usage for anterior and posterior spondylodesis [27–30].
The capability to reverse intervertebral disc degeneration by up-regulating anabolic factors was described by Tim Yoon et al. [31] in 2003. In a rat model, BMP-2 increased gene expression of collagen type 2 and aggrecan in isolated annulus fibrosus (AF) cells. Similar results were demonstrated in a study employing alginate encapsulated nucleus pulposus (NP) cells. Here co-cultures with transduced bovine cartilage cells expressing BMP-2 resulted in cell proliferation and increased accumulation of proteoglycans and collagens [32]. However, there is some controversy with regard to the BMP-2 effect between these positive in vitro results and the later published animal studies. Animal studies have been conducted to reproduce the observed in vitro effects, using different kinds of disc degeneration models, such as the annular puncture method. In contrast to the former in vitro findings, when injected into the punctured intervertebral disc, BMP-2 provoked an acceleration of the degenerative process and even spontaneous osseous fusion occurred, as demonstrated on radiography and histology in rabbits [33]. In rabbit disc puncture models, the injection of transfected disc cells expressing BMP-2 did not show any regenerative potential [34] while the intradiscal injection of BMP-7 (OP-1) was able to restore biomechanical function [35]. Using human intervertebral disc cells encapsulated in alginate beads, BMP-7 has been shown to stimulate cell proliferation and proteoglycan synthesis [36].
Different tissue-specific isoforms of TGF-β with a homology of 70–80 % and its three receptors are produced by practically all mammalian cells. TGF-β1 is expressed primarily by endothelial, connective tissue and hematopoietic cells, TGF-β2 by epithelial and neuronal cells and TGF-β3 mRNA is found mainly in mesenchymal cells [19, 20].
The gene expression of all three TGF beta isoforms and type I and type II TGF receptors has been shown to decrease in cervical intervertebral discs in a senescence-accelerated mouse model [37]. The potential therapeutic effect of TGF-β on degenerated intervertebral discs has been studied in different systems. Gruber et al. [38] stimulated annular cells embedded in alginate or agarose beads with TGF-β1 and observed an increased cell proliferation in vitro. In a rabbit adenoviral vector transduction model, TGF-β1 secretion resulted in an increase of proteoglycan production in NP cells [39]. Similarly, the injection of TGF-β1 and GDF-5 into degenerated murine intervertebral discs resulted in an increase of collagen type II and aggrecan gene expression [40].
Unlike TGF-β1, the TGF-β3 isotype is currently sparsely investigated in disc degeneration or other spinal models. Steffen et al. [41] demonstrated a massive bone formation with β-tricalcium phosphate (TCP) cylinders impregnated with TGF-β3 in an anterior fusion baboon model. In another study, differences in gene expression patterns between TGF-β1 and TGF-β3 stimulation of rat intervertebral discs cells were demonstrated in vitro under different osmotic conditions [42].
As indicated above, the effects of BMP-2 and TGF-β strongly depend on the application and the model used. Our previous studies with full organ rabbit disc cultures have elicited a spontaneous degeneration of the organ in vitro over extended culture periods, while basic properties such as cell viability and differentiation status remained intact. The model has been validated and shown to be versatile for studying various pathological conditions [43–45]. The aim of the study was to investigate if BMP-2 or TGF-β3 are able to modify the spontaneous degenerative process of rabbit intervertebral discs in vitro.
Materials and methods
Preparation and culture of intervertebral disc specimens
Intervertebral discs from T7/8 to L6/S1 (12/animal) with adjacent vertebral endplates were isolated under sterile conditions within 12 h after sacrifice from six adult female Burgundy Rabbits (4–5 kg, 4–6 months old) as previously described [43, 45]. Specimens were assigned to three groups and cultured for 3 weeks in 6-well plates with sampling points at days 1, 3, 14 and 21. Standard media (9 ml DMEM/F12, 10 % FCS, 25 μg/ml l-ascorbate, 50 μg/ml penicillin, 100 U/ml streptomycin) was supplemented with BMP-2 (Medtronic, Inc., Minneapolis, MN, USA) or TGF-β3 solution (both dissolved in CaCl and acetate 0.002 %) (Produced by Novartis, kindly donated by Prof. T. Arvinte, Therapeomics AG, Basel, Switzerland), both factors with a final concentration of 1 μg/ml. The control group received CaCl/acetate solution without protein. Media and growth factors changes were performed three times/week.
Quantification of proteoglycans
Disc specimens (n = 3/group) were digested overnight with papain (Sigma, 60 °C, 125 μg/ml dissolved in 5 mM l-cysteine HCl, 5 mM Na-citrate, 150 mM NaCl, 5 mM EDTA, pH 6.0). Equal volume of 8 M guanidine HCl was added. For quantification of proteoglycans, the Alcian blue binding assay was applied [46] after a two-step precipitation with H2SO4 and Triton X-100; Alcian blue (2 %, 8GX, Fluka), Triton X-100 (0.25 %, Sigma) and guanidine-HCI (0.5 M) were added to the samples and left to precipitate overnight at 5 °C. Samples were washed with 40 % DMSO and the precipitate was dissociated with guanidine-HCI (4 M) and propanol (33 %). Bovine tracheal chondroitin sulphate (Sigma) was used as a standard. Optical density was determined photometrically (600 nm). The measures were normalized to DNA.
Quantification of DNA
DNA content was quantified with bisbenzimidol fluorescent dye (Hoechst 33258, Sigma). Specimens (n = 3/group) were prepared as for proteoglycan measurements. Known concentrations of calf thymus DNA (Sigma) were used as standard. Samples were diluted 1:50 with tris (hydroxymethyl)aminomethane (10 mM, Fluka), Na2EDTA dihydrate (1 mM) and NaCl (100 mM). Fluorescence was detected with Hoefer DyNAQuant (Amersham Biosciences, San Francisco, CA, USA).
Quantitative real time PCR
The disc material (n = 45) was dissected and collected in RNAlater at the specified sampling point (n = 3/group and sampling point) and stored at −80 °C until further processing. For RNA isolation, the sample was covered with liquid nitrogen and pulverized with RNase free mortar and pestle. The samples were resuspended with 1 ml of peqGOLD TriFast reagent (Peqlab) followed by further homogenization with a Polytron mixer (Kinematica, Newark, NJ, USA). RNA was further processed following the reagent manufacturer’s instructions.
One microgram of total RNA was used for the synthesis of the cDNA (iScript cDNA Synthesis Kit, BioRad). The complementary DNA template (5 μl) was mixed with the RT-PCR mix solution (iQ SYBR Green Supermix, Bio-Rad), which contained 5 μM specific primers as follows:
House keeping gene: GAPDH Forward: AAGGCCATCACCATCTTCCA Reverse: GGATGCGTTGCTGACAATCT, Metalloproteinases: MMP-1 Forward: ATACCTGGAAAACTACTACAATCTG Reverse: TCTTCAGGGTTTCAGCATCT, MMP-13 Forward: TGCCCCTCCTCAACAGTAAC Reverse: GAGCCCGCTGCATTCTTCTT Collagen I Forward: TTCTTGGTGCTGCTGGCATTC Reverse: GCAATCCGTTGTGTCCCTTTATG, Collagen II Forward: GACCCCATGCAGTACATG Reverse: GACGGTCTTGCCCCACTT, Aggrecan Forward: GAGGTCGTGAAAGGTGT Reverse: GTGTGGATGGGGTACCTGAC.
Quantitative real time RT-PCR (IQ5, Biorad) was conducted with the following settings: denaturation 95 °C-10 s (1 cycle), 40 amplification cycles: 95 °C-5 s, 64 °C-40 s; followed by melting curve analysis.
Histology
After tissue fixation in 4 % buffered p-formaldehyde, specimens were washed, dehydrated and embedded in PMMA (Sigma). Samples (n = 3) were subsequently cut in 6 μm slices with a microtome (HM360, Microm International AG, Switzerland). Specimens were stained with von Kossa (Sigma) and counterstained with toluidine blue (Sigma) dye to demonstrate bone formation as well as proteoglycan deposits.
Statistics
For statistical analysis the non-parametric ANOVA by ranks test (Kruskal–Wallis) was used with Statistica 7.1 software (StatSoft, Inc., Tulsa, OK, USA). A significance value of p < 0.05 was specified. For PCR experiments a difference of at least one PCR cycle was considered significant, based on serial dilution standard curves in quintuplicates (not shown).
Results
Gene Expression
Collagen type I/II
Collagen type I (Col I) mRNA was not detectable in the NP in any group until day 7 except one sample in the control group at a very low concentration (delta CT value of 17.08). In the BMP-2 treated group, Col I gene first appeared in NP on day seven while it was not expressed in the controls or in the TGF-β3 group. From day 14 on there was an increasing expression of Col I in the controls (data not shown). TGF-β3 strongly inhibited Col I gene expression from day 14 over the whole period (NP day 14 TGF-β3 0.17 ± 0.05 fold) while BMP-2 did not have any significant effect on the increasingly expressed Col I (NP day 14 BMP-2 2.02 ± 1.4 fold). On day 21, in the TGF-β3 group, Col I mRNA was not detectable anymore, while Col I gene expression further increased in the controls (NP day 21 approx. 1,800 fold compared to day 1 and 4.3 fold compared to day 14) (data not shown).
In contrast, collagen type II (Col II) gene expression was decreased by BMP-2 in the beginning from day 1 until day 3 (NP day 1 Col II 0.13 ± 0.19 fold, day 3 0.24 ± 0.13 fold) with no significant difference compared to the untreated controls in the subsequent course. TGF-β3 did not have any influence on Col II expression over the entire observation period in the nucleus (data not shown).
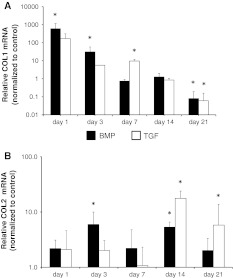
In the AF both BMP-2 and TGF-β3 strongly induced Col I gene expression on day 1 (AF Col I BMP-2 604 ± 567 fold, TGF-β3 171 ± 140 fold) with a subsequent decline (Fig. 1a). There was no qualitative difference between both growth factors except the kinetics of decline.
Fig. 1.
Effect of BMP-2 (black bars) and TGF-β3 (white bars) on gene expression of collagen type I (a) and collagen type II (b) in the annulus fibrosus. Gene expression of both collagens were increased with both growth factors. Tissue was analyzed with RT-qPCR at indicated time points. Data are presented as relative gene expression to the untreated control and normalized to GAPDH. Values represent the mean ± SD from triplicates. Asterisk sign. versus ctrl (see “Method” section)
Collagen type II gene expression in the annulus was constantly increased by both growth factors. This however was statistically significant only for day 14 (AF Col II BMP-2 5.3 ± 1.2 fold, TGF-β3 17.7 ± 6.2 fold) (Fig. 1b).
Aggrecan
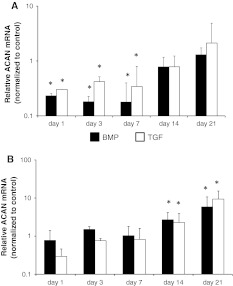
Both, BMP-2 and TGF-β3, had an inhibitory effect on gene expression of aggrecan in the NP in the first week (NP day 1 BMP-2 0.23 ± 0.02 fold, TGF-β3 0.3 ± 0.002). However, after 14 days and further after 21 days, BMP-2 and TGF-β3 did not show any significant difference compared to the controls (NP day 21 BMP-2 1.3 ± 0.4 fold, TGF-β3 2.1 ± 2.8) (Fig. 2a).
Fig. 2.
Effect of BMP-2 (black bars) and TGF-β3 (white bars) on gene expression of aggrecan in nucleus (a) and annulus (b) disc tissue. Gene expression of aggrecan was inhibited by both growth factors in the nucleus pulposus. In the annulus fibrosus a late minimal up-regulation of aggrecan could be observed. Nucleus and annulus tissue was analyzed separately with RT-qPCR at indicated time points. Data are presented as relative gene expression to the untreated control and normalized to GAPDH. Values represent the mean ± SD from triplicates. Asterisk sign. versus ctrl (see “Method” section)
In contrast, BMP-2 and TGF-β3 slightly increased aggrecan gene expression in the annulus from day 14 until day 21 (AF BMP-2 5.9 ± 4.8 fold, TGF-β3 9.4 ± 5.9 fold) (Fig. 2b).
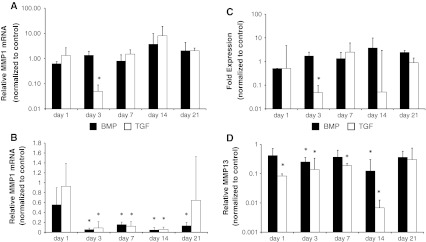
Collagenases MMP-1, MMP-13
In the annulus, as demonstrated in Fig. 3a, gene expressions of both tested collagenases, MMP-1 and MMP-13, were markedly suppressed by the growth factors over the whole observation period (AF maximum day 14 MMP-1 BMP-2 0.13 ± 0.18 fold, TGF-β3 0.007 ± 0.006 fold).
Fig. 3.
Effect of BMP-2 (black bars) and TGF-β3 (white bars) on gene expression of collagenases MMP-1 (a, b) and MMP-13 (c, d) in nucleus (a, c) and annulus disc tissue (b, d). Gene expression of both collagenases were suppressed with both growth factors in the annulus fibrosus. In the nucleus pulposus there was no effect of both growth factors except on day three. Nucleus and annulus tissue was analyzed separately with RT-qPCR at indicated time points. Data are presented as relative gene expression to the untreated control and normalized to GAPDH. Values represent the mean ± SD from triplicates. Asterisk sign. versus ctrl (see “Method” section)
In contrast, in the nucleus, TGF-β3 and BMP-2 did not have any significant effect on MMP-1 and MMP-13 gene expression after 21 days (NP MMP-1 BMP-2 2.0 ± 2.3 fold, TGF-β3 2.0 ± 0.6 fold, MMP-13 BMP-2 2.4 ± 0.5 fold, TGF-β3 0.9 ± 0.5 fold). Interestingly TGF-β3 suppressed gene expression of MMP-1 and MMP-13 only at day 3 in all tested nuclei (NP day 3 MMP-1 0.05 ± 0.04 fold, MMP-13 0.05 ± 0.05 fold). The other samples did not show any significant difference compared to the untreated controls (Fig. 3b).
Proteoglycan and DNA measures
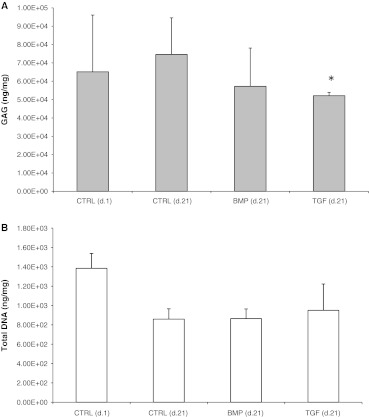
The quantification of the glycosaminoglycan content within the intervertebral disc after 21 days in culture demonstrated that both factors with BMP-2 or TGF-β3 resulted in a reduction of proteoglycan content compared to the control (Fig. 4a, day 21 BMP-2 65.8 ± 21.7 mg GAG/mg DNA, TGF-β3 57.4 ± 13.3 mg/mg, control 101.01 ± 23.18 mg/mg, p = 0.125 and p = 0.047).
Fig. 4.
a, b Effect of BMP-2 and TGF-β3 on GAG and DNA concentration in full organ disc cultures. A decline of proteoglycan content was detectable for both growth factors (BMP-2 p = 0.125, TGF-β3 p = 0.048) after 21 days compared to the control group which showed an increase of the protein concentration with culture time. No differences were found for DNA content (BMP-2 p = 0.94, TGF-β3 p = 0.62). Proteoglycans were quantified using an Alcian blue-based colorimetric binding assay. DNA amount was determined with bisbenzimide (Hoechst). GAG content was normalized to DNA content and DNA was normalized organ weight. Values at indicated time points represent the mean ± SD from double measures of triplicates. Asterisk sign. versus ctrl p < 0.05
In contrast the quantification of DNA content did not show any significant difference between the groups at day 21 (Fig. 4b, day 21 BMP-2 865 ± 100 ng/mg, TGF-β3 950 ± 272 ng/mg, control 859 ± 107 ng/mg, p = 0.94, p = 0.62).
Histology
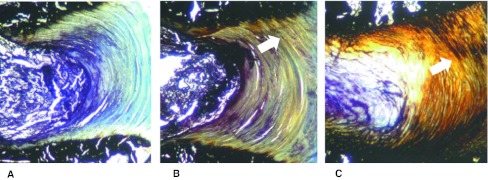
Histological analysis demonstrated the ossification of the AF in the TGF-β3 and the BMP-2 treated groups. Bone formation was more pronounced with BMP-2 than with TGF-β3 and first noticed at the anchorage of the annulus fibers with the endplate. An ossification of the NP was not observed. Semi-quantitative analysis indicated that proteoglycan contents in the annulus were reduced in the factor-treated groups (Fig. 5).
Fig. 5.
Effect of BMP-2 and TGF-β3 on disc histology. Disc specimens were embedded into PMMA and histological section were stained with von Kossa (bone specific, black stain) and toluidine blue (GAG specific, blue stain). Unlike the untreated control (left side) an ossification of the annulus (arrows) with a decrease of GAG concentration could be observed with BMP-2 (right side) and to a lesser extent with TGF-β3 (middle) after 21 days (12.5x)
Discussion
The objective of the study was to investigate the in vitro effects of BMP-2 and TGF-β3 on disc degeneration in the AF and NP compartment, as indicated by changes of anabolic/catabolic gene expression, alterations of proteoglycan content and histological alterations, in a full organ disc/endplate rabbit model. Spontaneous degenerative changes of disc explants with culture time has been observed in a previous study, as demonstrated by an up-regulation of collagen I and a decrease of aggrecan and type II collagen gene transcription in the nucleus and annulus compartment [43]. The hypothesis of the study was that BMP-2 and TGF-β3 may counteract disc degeneration with anabolic genes being increasingly expressed and the catabolic pathway blocked.
Both employed growth factors were administered with the culture media for 21 days in the concentration of 1 μg/ml. We decided in favour of a comparatively high concentration because of the potentially limited bio-availability and diffusion capacity of each factor within the relatively large disc organ. Furthermore, at neutral pH TGF-β3 tends to form inactive large precipitating aggregates [47]. Risbud et al. [42] employed the same factor at low concentrations of 10 ng/ml in a disc organ explant rat model. In other studies using cell cultures, BMP-2 factor concentrations ranged from 10 to 1,000 ng [31, 48]. However, for in vivo and clinical application, BMP-2 was administered in high concentrations such as 1.5 mg/ml as in the rhesus macaque study or 6 mg/cage in an interbody fusion study in humans [26, 29].
Our results demonstrate that TGF-β3 and BMP-2 show very similar characteristics in the defined outcome parameters in the AF and a few divergent responses in the nucleus pulposus.
In the gene expression studies with the AF, the qualitative results are uniform: both factors strongly continuously increased Col I mRNA at day 1 while Col II expression was only slightly elevated and aggrecan expression was mostly unchanged with a slight marginal late increase at day 21. In parallel the expression of the catabolic collagenases MMP-1 and MMP-13 were suppressed.
In the nucleus, however, gene expression of Col I and type II was differently influenced by both growth factors. Physiologically, collagen I gene expression is seen only in the inner annulus but not in the nucleus [49]. With culture time type I collagen starts to be increasingly expressed in the NP, which has also been demonstrated in an earlier study using the same in vitro model [43]. This was observed by other groups as well [50–52]. TGF-β3 inhibited this gene up-regulation while BMP-2 tended to promote type I collagen expression in the nucleus similar to the annulus. In contrast, for Col II, BMP-2 inhibited its expression while TGF-β3 was ineffective.
The expression of the collagenases MMP-1 and MMP-13 were unaffected in the nucleus in almost all samples over the whole culture period, except that TGF-β3 strongly inhibited both genes exclusively at day three.
The literature regarding gene pattern responses towards both growth factors in annulus and nucleus tissues is not consistent. The outer AF mainly consists of type I collagen, similar to tendon tissue produced by fibrocartilagenous cells [49, 53]. Yoon et al. [31] could demonstrate that isolated AF cells in monolayers up-regulated type II collagen (4.6 fold) and aggrecan (11.5 fold) expression in response to 1 μg/ml BMP-2. In contrast to our results, in this study gene expression of type I collagen was unaffected. Similarly, Li et al., demonstrated an increase of aggrecan and type II collagen gene expression employing a substance concentration of 200 ng/ml BMP-2 in cell culture media on rat annulus cells. Again type I collagen mRNA concentration did not respond to the growth factor application [48]. In contrast, in the cell culture studies by Kuh et al. [54] using AF cells BMP-2 stimulated the expression of both collagen types.
In the annulus, both applied growth factors induced a strong gene response of Col I expression in the first week and secondarily, when collagen I declined, an increase of Col II gene and to a smaller extent aggrecan in the second and third week.
In the NP we found that BMP-2 tended to increase type I collagen expression. This effect has also been demonstrated using 3D alginate bead cultures from isolated human nucleus (NP)/transition zone cells by Kim et al. [55]. Beside type I collagen, the expression of aggrecan and type II collagen but not of the osteocalcin gene were increased using various concentrations of BMP-2 (up to 2 μg/ml). This finding was confirmed by Gilbertson et al. [56] using human NP monolayer cultures and by Zhang et al. [57] with the quantification of the gene products (collagen and proteoglycan content). In contrast to this, in the current study we observed a down regulation of aggrecan and type II collagen gene expression in the first week in the NP with BMP-2. Conflicting Col I, Col II and aggrecan gene pattern in annulus and nucleus samples may be due to the organ culture characteristics, with resident growth factors embedded in the retained physiological extracellular matrix and the present cell-matrix interaction and/or to the high concentration of growth factors employed.
The effects of most growth and differentiation factors are studied predominantly in the context of cell differentiation using undifferentiated stem cells of different origin or using isolated organ cells like cartilage/connective tissue cells to study gene expression and matrix production under different environmental conditions such as in scaffolds for tissue engineering [58]. Consequently, data on the effects of the TGF beta isoforms on differentiated organs such as the intervertebral disc are sparse. Haberstroh et al. [59] studied human cell cultures using isolated NP cells from surgical specimens obtained from routine microdiscectomy. TGF-β3 (10 ng/ml) resulted in gene up-regulation of collagen types I, II, III, IX and aggrecan as well as matrix production. In NP cell cultures from volunteers, which were stimulated with platelet-rich plasma and 1 ng/ml TGF-β1, type II collagen, aggrecan as well as sox 9 were significantly up-regulated as demonstrated with real time PCR [60]. In a murine model encompassing the induction of disc degeneration with static compression 200 ng/ml TGF-β1 caused an expansion of fibrocartilage cells into the nucleus which were positive for Col II and aggrecan mRNA as demonstrated by in situ hybridization [40].
The histological results indicated a decrease of proteoglycan content in the annulus which can be explained by a slow loss via diffusion into the media and/or activation of gelatinases with time, which was not compensated by synthesis. The last finding could be confirmed by our measures of proteoglycan content, which, however, were taken from the entire intervertebral disc. Here a decrease of proteoglycan amount could be observed in both factor-treated groups after 21 days with a significance level of p < 0.05 only for TGF-β3. The decrease of the extracellular matrix macromolecule is in accordance with our PCR results, which demonstrated that aggrecan gene expression was down-regulated in the nucleus pulposus, the major localization of this protein and a mostly unchanged gene expression in the AF.
Histological analysis using von Kossa stain, furthermore, clearly demonstrated that BMP-2 and TGF-β3 induced ossification of the AF, which was pronounced at the anchorage of the annulus fibers with the vertebral endplate. Ossification encompassed the mineralization of the annular fibers with deposition of calcium salts. Similar to annulus tissue, a major compound of the organic bone matrix is Col I.
Several different spinal pathologies involve dystrophic ossification of the annulus and/or the longitudinal ligaments such as ankylosing spondylitis, ossification of the posterior longitudinal ligament (OPLL), diffuse idiopathic skeletal hyperostosis (DISH) or fibrodysplasia ossificans progressiva (FOP) [61, 62]. The latter is caused by a single nucleotide missense mutation of the bone morphogenic protein type I receptor (ACVR1/ALK2) [63]. Experimentally-induced ossification of the intervertebral discs by injection of BMP-2 (1 mg/ml) with or without coral was demonstrated by Huang et al. [33] in an annular stab incision rabbit model. Similarly, in a baboon model when using a TGF-β3/beta-TCP compound to fill vertebral bone defects, a massive regional bone formation around the bone implicating soft tissue ossification could be observed [41].
Taken together, the data demonstrated that the administration of BMP-2 or TGF-β3 to the intervertebral disc with its naturally retained microenvironment caused ossification of the AF within 3 weeks with an increased Col I gene expression (and to a lesser extent also Col II expression) and inhibition of catabolic collagenases. This may be regarded as an osseous anabolic process but involves changes of the original disc architecture and is commonly observed in late state disc degeneration. However, this conclusion must be qualified by our selection of a relatively high, yet clinically relevant, concentration of 1 μg/ml in the culture media.
In the nucleus pulposus, anabolic genes such as aggrecan and Col II were inhibited by BMP-2 and catabolic collagenases were practically unaffected by both factors. Interestingly, unlike in the annulus compartment, TGF-β3 inhibited the expression of Col I. Collagen type I expression in the NP is an indicator of disc degeneration. The relevance of these findings remains unclear as well as a possible impact on an eventual later ossification of the nucleus, which was so far not observed. This, however, may be a question of culture time. The employed protein concentration, intra-organ concentration differences and the way of growth factor administration are key points and have to be considered cautiously. Although both factors at the tested concentrations may not be suitable to regenerate the whole intervertebral disc organ, they are interesting candidates for being injected alone or in combination into a painful intervertebral disc to induce osseous fusion (spondylodesis). Whether the in vivo application of TGF-β3 would be devoid of MMP-2 typical adverse effects, which have been lately reviewed by Carragee [64] such as ectopic bone formation [65], radiculopathy [66], wound complications [67], implant migration [68] and even carcinogenesis [64] as well as the observed divergent effects of both factors and the analysis of the involved bone-related genes need further investigations.
Acknowledgments
The authors thank AO Foundation, Davos, Switzerland for funding, Prof. Tudor Arvinte (Therapeomics AG, Basel, Switzerland) for providing TGF-β3, Medtronic Inc. for providing BMP-2 and Ladina Ettinger Ferguson for excellent technical assistance.
Conflict of interest
None.
Contributor Information
Daniel Haschtmann, Phone: +44-38-57210, FAX: +44-38-57211, Email: daniel.haschtmann@kws.ch, Email: daniel.haschtmann@istb.unibe.ch.
Stephen J. Ferguson, Phone: +41-31-6315925, FAX: +41-31-6315960, Email: sferguson@ethz.ch
Jivko V. Stoyanov, Phone: +41-41-9396635, FAX: +41-41-9396640, Email: jivko.stoyanov@paranet.ch
References
- 1.Aebi M, Arlet V, Webb J, editors. AOspine manual. Stuttgart and New York: Georg Thieme Verlag; 2007. [Google Scholar]
- 2.McGirt MJ, Ambrossi GL, Datoo G, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Bydon A. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64:338–344. doi: 10.1227/01.NEU.0000337574.58662.E2. [DOI] [PubMed] [Google Scholar]
- 3.Glassman SD, Carreon LY, Djurasovic M, Dimar JR, Johnson JR, Puno RM, Campbell MJ. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9:13–21. doi: 10.1016/j.spinee.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32:816–823. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 5.Galbusera F, Bellini CM, Zweig T, Ferguson S, Raimondi MT, Lamartina C, Brayda-Bruno M, Fornari M. Design concepts in lumbar total disc arthroplasty. Eur Spine J. 2008;17:1635–1650. doi: 10.1007/s00586-008-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bothmann M, Kast E, Boldt GJ, Oberle J. Dynesys fixation for lumbar spine degeneration. Neurosurg Rev. 2008;31:189–196. doi: 10.1007/s10143-007-0101-9. [DOI] [PubMed] [Google Scholar]
- 7.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenig CM, Schmidt CO, Kohlmann T, Schweikert B. Costs of back pain in Germany. Eur J Pain. 2009;13:280–286. doi: 10.1016/j.ejpain.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Gandjour A, Telzerow A, Lauterbach KW. European comparison of costs and quality in the treatment of acute back pain. Spine. 2005;30:969–975. doi: 10.1097/01.brs.0000158944.54033.60. [DOI] [PubMed] [Google Scholar]
- 10.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(4):441–451. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Nishida K, Kakutani K, Maeno K, Yurube T, Takada T, Kurosaka M, Doita M. Sustained long-term RNA interference in nucleus pulposus cells in vivo mediated by unmodified small interfering RNA. Eur Spine J. 2009;18:263–270. doi: 10.1007/s00586-008-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh H, Zakharian K, Torre RP, Facek C, Vasquez A, Chaudhry GR, Svinarich D, Perez-Cruet MJ. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10:265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 13.Richardson SM, Hoyland JA. Stem cell regeneration of degenerated intervertebral discs: current status. Curr Pain Headache Rep. 2008;12:83–88. doi: 10.1007/s11916-008-0016-3. [DOI] [PubMed] [Google Scholar]
- 14.Maitre CL, Baird P, Freemont AJ, Hoyland JA. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthr Res Ther. 2009;11:R20. doi: 10.1186/ar2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiyama A, Mochida J, Sakai D. Stem cell applications in intervertebral disc repair. Cell Mol Biol (Noisy-le-grand) 2008;54:24–32. [PubMed] [Google Scholar]
- 16.Pratsinis H, Kletsas D. Growth factors in intervertebral disc homeostasis. Connect Tissue Res. 2008;49:273–276. doi: 10.1080/03008200802147951. [DOI] [PubMed] [Google Scholar]
- 17.Buttle DJ. Factors controlling matrix turnover in health and disease. Biochem Soc Trans. 2007;35:643–646. doi: 10.1042/BST0350643. [DOI] [PubMed] [Google Scholar]
- 18.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 21.Herpin A, Lelong C, Favrel P. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol. 2004;28:461–485. doi: 10.1016/j.dci.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 23.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 24.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–280. doi: 10.1016/0955-2235(89)90015-X. [DOI] [PubMed] [Google Scholar]
- 25.Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995;20:1326–1337. [PubMed] [Google Scholar]
- 26.Hecht BP, Fischgrund JS, Herkowitz HN, Penman L, Toth JM, Shirkhoda A. The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine. 1999;24:629–636. doi: 10.1097/00007632-199904010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Burkus JK, Sandhu HS, Gornet MF, Longley MC. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205–1212. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 29.Meisel HJ, Schnoring M, Hohaus C, Minkus Y, Beier A, Ganey T, Mansmann U. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17:1735–1744. doi: 10.1007/s00586-008-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlisle E, Fischgrund JS. Bone morphogenetic proteins for spinal fusion. Spine J. 2005;5:240S–249S. doi: 10.1016/j.spinee.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, Hutton WC. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Li Z, Thonar EJ, An HS, He TC, Pietryla D, Phillips FM. Transduced bovine articular chondrocytes affect the metabolism of cocultured nucleus pulposus cells in vitro: implications for chondrocyte transplantation into the intervertebral disc. Spine. 2005;30:2601–2607. doi: 10.1097/01.brs.0000187880.39298.f0. [DOI] [PubMed] [Google Scholar]
- 33.Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32:1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 34.Kong MH, Do DH, Miyazaki M, Wei F, Yoon SH, Wang JC. Rabbit model for in vivo study of intervertebral disc degeneration and regeneration. J Korean Neurosurg Soc. 2008;44:327–333. doi: 10.3340/jkns.2008.44.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GB, An HS. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692–703. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Imai Y, Miyamoto K, An HS, Thonar EJ, Andersson GB, Masuda K. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine (Phila Pa 1976) 2007;32:1303–1309. doi: 10.1097/BRS.0b013e3180593238. [DOI] [PubMed] [Google Scholar]
- 37.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg. 2003;98:63–67. doi: 10.3171/spi.2003.98.1.0063. [DOI] [PubMed] [Google Scholar]
- 38.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 39.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 41.Steffen T, Stoll T, Arvinte T, Schenk RK. Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J. 2001;10(Suppl 2):S132–S140. doi: 10.1007/s005860100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risbud MV, Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ, Shapiro IM. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 43.Haschtmann D, Stoyanov JV, Ettinger L, Nolte LP, Ferguson SJ. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine. 2006;31:2918–2925. doi: 10.1097/01.brs.0000247954.69438.ae. [DOI] [PubMed] [Google Scholar]
- 44.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–1966. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 45.Haschtmann D, Stoyanov JV, Gedet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17:289–299. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjornsson S. Quantitation of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis. Anal Biochem. 1998;256:229–237. doi: 10.1006/abio.1997.2494. [DOI] [PubMed] [Google Scholar]
- 47.Pellaud J, Schote U, Arvinte T, Seelig J. Conformation and self-association of human recombinant transforming growth factor-beta3 in aqueous solutions. J Biol Chem. 1999;274:7699–7704. doi: 10.1074/jbc.274.12.7699. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 49.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 50.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 52.Hutton WC, Toribatake Y, Elmer WA, Ganey TM, Tomita K, Whitesides TE. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine. 1998;23:2524–2537. doi: 10.1097/00007632-199812010-00007. [DOI] [PubMed] [Google Scholar]
- 53.Lu Z, Hu Y, Feng C. Gene expression of fibrous main collagen in the lumbar disc. Zhonghua Wai Ke Za Zhi. 1998;36:68–71. [PubMed] [Google Scholar]
- 54.Kuh SU, Zhu Y, Li J, Tsai KJ, Fei Q, Hutton WC, Yoon TS. A comparison of three cell types as potential candidates for intervertebral disc therapy: annulus fibrosus cells, chondrocytes, and bone marrow derived cells. Joint Bone Spine. 2009;76:70–74. doi: 10.1016/j.jbspin.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, Hahn SB, Lee HM. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679–2684. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 56.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, An HS, Thonar EJ, Chubinskaya S, He TC, Phillips FM. Comparative effects of bone morphogenetic proteins and sox9 overexpression on extracellular matrix metabolism of bovine nucleus pulposus cells. Spine. 2006;31:2173–2179. doi: 10.1097/01.brs.0000232792.66632.d8. [DOI] [PubMed] [Google Scholar]
- 58.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 59.Haberstroh K, Enz A, Zenclussen ML, Hegewald AA, Neumann K, Abbushi A, Thome C, Sittinger M, Endres M, Kaps C (2009) Human intervertebral disc-derived cells are recruited by human serum and form nucleus pulposus-like tissue upon stimulation with TGF-beta3 or hyaluronan in vitro. Tissue Cell 41(6):414–420 [DOI] [PubMed]
- 60.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, Lin TW, Lin WC, Huang TY, Deng WP. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744–754. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 61.Jones MD, Pais MJ, Omiya B. Bony overgrowths and abnormal calcifications about the spine. Radiol Clin North Am. 1988;26:1213–1234. [PubMed] [Google Scholar]
- 62.Armas JB, Couto AR, Bettencourt BF. Spondyloarthritis, diffuse idiopathic skeletal hyperostosis (DISH) and chondrocalcinosis. Adv Exp Med Biol. 2009;649:37–56. doi: 10.1007/978-1-4419-0298-6_3. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40–46. doi: 10.3171/2009.4.SPINE0876. [DOI] [PubMed] [Google Scholar]
- 66.Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine (Phila Pa 1976) 2009;34:1480–1484. doi: 10.1097/BRS.0b013e3181a396a1. [DOI] [PubMed] [Google Scholar]
- 67.Crawford CH, 3rd, Carreon LY, McGinnis MD, Campbell MJ, Glassman SD. Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine (Phila Pa 1976) 2009;34:1390–1394. doi: 10.1097/BRS.0b013e3181a2da08. [DOI] [PubMed] [Google Scholar]
- 68.Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557–562. doi: 10.1097/BSD.0b013e31815ea897. [DOI] [PubMed] [Google Scholar]