Abstract
Purpose
Intervertebral disc degeneration is considered to be a major feature of low back pain. Furthermore, oxidative stress has been shown to be an important factor in degenerative diseases such as osteoarthritis and is considered a cause of intervertebral disc degeneration. The purpose of this study was to clarify the correlation between oxidative stress and intervertebral disc degeneration using Broad complex-Tramtrack-Bric-a-brac and cap‘n’collar homology 1 deficient (Bach 1−/−) mice which highly express heme oxygenase-1 (HO-1). HO-1 protects cells from oxidative stress.
Methods
Caudal discs of 12-week-old and 1-year-old mice were evaluated as age-related models. Each group and period, 5 mice (a total of 20 mice, a total of 20 discs) were evaluated as age-related model. C9–C10 caudal discs in 12-week-old Bach 1−/− and wild-type mice were punctured using a 29-gauge needle as annulus puncture model. Each group and period, 5 mice (a total of 60 mice, a total of 60 discs) were evaluated. The progress of disc degeneration was evaluated at pre-puncture, 1, 2, 4, 8 and 12 weeks post-puncture. Radiographic, histologic and immunohistologic analysis were performed to compare between Bach 1−/− and wild-type mice.
Results
In the age-related model, there were no significant differences between Bach 1−/− and wild-type mice radiologically and histologically. However, in the annulus puncture model, histological scoring revealed significant difference at 8 and 12 weeks post-puncture. The number of HO-1 positive cells was significantly greater in Bach 1−/− mice at every period. The apoptosis rate was significantly lower at 1 and 2 weeks post-puncture in Bach 1−/− mice.
Conclusions
Oxidative stress prevention may avoid the degenerative process of the intervertebral disc after puncture, reducing the number of apoptosis cells. High HO-1 expression may also inhibit oxidative stress and delay the process of intervertebral disc degeneration.
Keywords: Intervertebral disc, Oxidative stress, Heme oxygenase-1, Intervertebral disc degeneration
Introduction
The intervertebral disc is one the most important structures for stabilizing the spinal column [1]. But several degenerative factors cause it to easily lose its function [2, 3]. Intervertebral disc degeneration is now thought to be an important trigger of low back pain [2]. Some of the main culprits of intervertebral disc degeneration include aging, smoking, mechanical stress, and genetic components [4, 5]. Oxidative stress has been shown to be an important underlying factor of these degenerative causes, such as inflammation and aging in living organisms [6, 7]. However, the precise pathogenesis of intervertebral disc degeneration is unclear, and its prevention and treatment remain impracticable. Recent basic studies about oxidative stress have focused on heme oxygenase-1 (HO-1), Broad complex-Tramtrack-Bric-a-brac and cap‘n’collar homology 1 (Bach 1) [8, 9].
HO-1 is a stress-responsive enzyme that makes free hemes, which in turn produce some products. Gas carbon monoxide and iron are produced by HO-1. They induce expression of heavy-chain ferritin and biliverdin, which are then converted to bilirubin by biliverdin reductase. The main biologic function of HO-1 is to avoid the accumulation of harmful free hemes. The effect of HO-1 is to protect against many diseases and to stabilize homeostasis [8].
In addition, expression of HO-1 is strongly induced in animal cells by various chemical stimulants such as cytokines, heavy metals, heat shock and oxidants that induce inflammatory damage [10, 11]. HO-1 is the rate-limiting enzyme in heme degradation, generating ferrous iron, carbon monoxide and biliverdin, which is rapidly reduced to bilirubin. Bilirubin, carbon monoxide and biliverdin have antioxidant and anti-inflammatory activities in vivo [12]. Thus, HO-1 is an antioxidant defense enzyme that converts toxic hemes into antioxidants and is essential for many animals to cope with various aspects of cellular stress and to regulate cellular iron metabolism [13]. In clinical conditions, the level of HO-1 expression has been associated with resistance to tissue injury [14, 15].
The expression of HO-1, which protects cells from various insults including oxidative stress, is highly upregulated in Bach 1−/− mice. Bach 1 is a transcriptional repressor of the HO-1 genes, and Bach 1−/− mice express high levels of HO-1 mRNA and protein in various organs [12, 16]. Especially in meniscus, inhibition of Bach-1 induced HO-1 suggested that meniscal degeneration could be prevented [17]. This result suggested that the degeneration of hypovascular tissue such as intervertebral disc can be prevented by induced HO-1.
The Bach1−/− mice were fertile and appeared grossly normal in size and morphology. HO-1 mRNA was expressed at much higher levels in thymus, heart, lung and liver in the Bach1−/− mice as compared with organs from the wild-type or heterozygous mutant mice. The differential levels varied among the tissues. There were >tenfold differences in the thymus and heart between the mutant and wild-type animals, whereas there were smaller but significant differences in the liver and lung [12]. The purpose of this study was to clarify the correlation between oxidative stress and intervertebral disc degeneration and the effect of HO-1 by investigating the process of intervertebral disc degeneration in Bach 1−/− mice.
Materials and methods
Experimental animals
The experimental animals were Bach 1−/− C57BL/6J mice and wild-type C57BL/6J mice. A total of 70 mice were used in this study. In total, 60 mice were 12 weeks old and 10 mice were 1 year old. The ethics committee which reviewed animal experiments at the Hiroshima University School of Medicine (Hiroshima City, Japan) approved this study.
Study 1: Age-related model
In this study, a total of 20 mice were used (5 mice each group, each period). One disc per one mouse was analyzed in this study. To confirm the effect of oxidative stress to a natural course of degeneration of the intervertebral disc, 12-week-old and 1-year-old Bach 1−/− and wild-type mice were used without any additional procedures. After an intraperitoneal anesthetic injection of 2,2,2-tribromoethanol (125 mg/kg), lateral radiographs of the tail were taken under sedation, using microfocus μFX-1000 (40 kV, 100 mA, 15 s at 1.5× magnification; Fuji film, Tokyo, Japan).
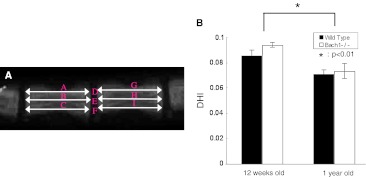
The lengths of the C9–C10 disc and adjacent vertebral bodies were measured and the disc height index (DHI), based on the method of Lu et al. [18] with a slight modification, was calculated (Fig. 1a). The intervertebral disc height was calculated by averaging the measurements obtained from the anterior, middle, and posterior portions of the intervertebral disc and dividing that by the average of adjacent vertebral body heights. After taking radiographs, the mice were killed and C9–C10 caudal discs were harvested and sectioned for histological evaluation. The tissues were fixed in 4 % paraformaldehyde in phosphate-buffered saline (pH 7.4), decalcified in EDTA for 2 weeks, paraffin-embedded, and sectioned to a 5-μm thickness. The sections were stained with hematoxylin and eosin for cellular constituents and Safranin O for proteoglycans. Sections were evaluated for histologic changes in the intervertebral disc and end plate regions using a semiquantitative grading scheme (Table 1) which was used for evaluation of intervertebral disc degeneration by aging [19]. This grading scheme is for evaluation of the intervertebral disc (range from 0 to 22 points, where 0 means no degeneration and 22 means most degeneration) and the end plate (range from 0 to 18 points, where 0 means no degeneration and 18 means most degenerated), and allows for broad evaluation of the whole intervertebral disc, including the annulus fibrosus and nucleus pulposus regions. The scoring was assessed by two independent observers. To confirm that 1-year-old mice were affected by aging, sections were stained with CML and the number of CML positive cells was counted in the center of the intervertebral disc at 400× magnification. Immunohistochemistry at 12-week-old and 1-year-old Bach 1−/− and wild-type mice was examined for HO-1 expression.
Fig. 1.
On lateral radiographs, the vertebral body height and intervertebral disc height were measured. Disc height index (DHI) was calculated by averaging the height of the anterior, middle, and posterior portions of the intervertebral disc and dividing that by the average of adjacent vertebral body heights (a). DHI = 2 × (D + E + F)/(A + B + C + G + H + I). Change of DHI of age-related model (b). At the age of 1 year, the DHI significantly decreased compared with that at 12 weeks. No significant difference was observed between Bach 1−/− mice and wild-type mice in both periods
Table 1.
Grading for age-related model in Bach1−/− and wild-type mouse
| Criteria (range) | Bach 1−/− | Wild type | ||
|---|---|---|---|---|
| 12 week | 1 year | 12 week | 1 year | |
| Intervertebral disc region | ||||
| Cells (0–6) | 1.0 ± 0.0 | 1.0 ± 0.7 | 1.0 ± 0.0 | 1.4 ± 0.5 |
| Mucous degeneration (0–4) | 0.2 ± 0.4 | 1.6 ± 0.5 | 0.0 ± 0.0 | 1.2 ± 0.4 |
| Cell death (0–4) | 0.4 ± 0.5 | 1.0 ± 0.0 | 0.4 ± 0.5 | 1.2 ± 0.4 |
| Tear and cleft formation (0–4) | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 0.4 |
| Granular changes (0–4) | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.4 |
| Endplate | ||||
| Cells (0–4) | 1.0 ± 0.7 | 1.6 ± 0.5 | 1.2 ± 0.4 | 1.4 ± 0.5 |
| Cartilage disorganization (0–4) | 1.0 ± 0.0 | 1.6 ± 0.5 | 1.0 ± 0.0 | 2.0 ± 0.7 |
| Cartilage cracks (0–4) | 0.0 ± 0.0 | 1.6 ± 0.5 | 0.0 ± 0.0 | 1.6 ± 0.5 |
| Microfracture (0–2) | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 |
| New bone formation (0–2) | 0.2 ± 0.4 | 1.0 ± 0.0 | 0.4 ± 0.5 | 1.0 ± 0.0 |
| Bony sclerosis (0–2) | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 |
| N = 5 | ||||
Study 2: Annulus needle puncture model
In this study, a total of 60 mice were used (5 mice each group, each period). One disc per one mouse was used and analyzed in this study. After an intraperitoneal anesthetic injection of 2,2,2-tribromoethanol (125 mg/kg), C9–C10 caudal discs of 12-week-old Bach 1−/− and wild-type murine tails were punctured using a 29-gauge needle at a controlled depth following the previous method [20]. The progress of the disc degeneration was evaluated by radiographic analysis of disc height at pre-operation and at 1, 2, 4, 8 and 12 weeks post-puncture. After radiographs were taken, the mice were killed and C9–C10 caudal discs were harvested. To assess the condition of degeneration, DHI was calculated from radiographs, and changes of the DHI were expressed as %DHI (postoperative DHI/preoperative DHI) from radiographs. Sagittal sections were stained with hematoxylin and eosin and Safranin O. Intervertebral disc degeneration was investigated using another semiquantitative grading scheme (Table 2) [21]. This grading scheme is used for evaluation of intervertebral disc injury model including puncture model. In this grading scheme, the cellularity and morphology of the AF and NP as well as the border between the two structures were assessed. Grades ranged from 5 to 15, with a normal disc receiving one point from each category and disc representative of severe degeneration scoring 3 points from each category. The scoring was assessed by two independent observers. Immunohistochemistry at 1, 2, 4, 8 and 12 weeks post-puncture was examined for HO-1 expression. The number of HO-1 positive cells was counted in the center of the intervertebral disc at 400× magnification. Each measurement included 5 discs (a total of 50 were used).
Table 2.
Histological grading scheme for intervertebral degeneration model
| I. Cellularity of the annulus fibrosus |
| Grade |
| Fibroblasts comprise more than 75 % of cells |
| Neither fibroblasts nor chondrocytes comprise more than 75 % of cells |
| Chondrocytes comprise more than 75 % of cells |
| II. Morphology of the annulus fibrosus |
| Grade |
| Well-organized collagen lamellae without ruptures or serpentine |
| Inward bulging, ruptured or serpentine fibers in less than one-third of the anulus |
| Inward bulging, ruptured or serpentine fibers in more than one-third of the anulus |
| III. Border between the annulus fibrosus and nucleus pulposus |
| Grade |
| Normal, without any interruption |
| Minimal interruption |
| Moderate or severe interruption |
| IV. Cellularity of the nucleus pulposus |
| Grade |
| Normal cellularity with stellar-shaped nuclear cells evenly distributed throughout the nucleus |
| Slight decrease in the no. of cells with some clustering |
| Moderate or severe decrease (>50 %) in the number of cells with all the remaining cells clustered and separated by dense area of proteoglycans |
| V. Morphology of the nucleus pulposus |
| Grade |
| Round, comprising at least half of the disc area in midsagittal sections |
| Round or irregularly shaped, comprising less than one quarter to half of the disc area in midsagittal sections |
| Irregularly shaped, comprising less than one quarter of the disc area in midsagittal sections |
The scale is based on 5 categories of degenerative change, with scores ranging from 5 points (1 in each category) for a normal disc to 15 points (3 in each category) for a severely degenerated disc
The number of apoptotic cells induced by oxidative stress was scored in each TUNEL-stained section (prepared using an in situ Apoptosis Detection Kit, Trevigen, USA) under a light microscope, and the apoptosis rate was determined by dividing the number of apoptotic cells by the total number of cells in the same section. Each measurement included 6 discs (a total of 60 discs were used).
In order to confirm that increased vascularization may provide heme into the disc, immunohistochemistry was examined for CD31 expression.
Statistical analysis
Values are presented as the means ± SDs. A Mann–Whitney U test was used to assess the significance of differences between the two groups at each time point, and analysis of variance (ANOVA) and Tukey–Kramer was used as a post- hoc test to assess the chronological changes in each group. All the statistical tests were performed using the Statview program package (version 5.0, SAS Institute Inc., Cary, NC, USA). Significance was considered as a probability value <0.05.
Results
Age-related model
Radiographs of murine tails showed bone growth and retained the height of intervertebral disc between 12-week-old and 1-year-old mice. The DHI of 1-year-old mice were significantly less compared with that of 12-week-old mice, but no significant differences were observed between Bach 1−/− mice and wild-type mice at each time point (Fig. 1b).
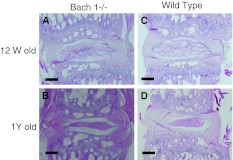
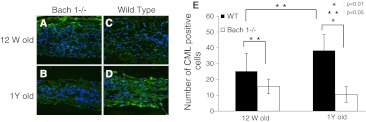
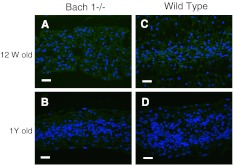
Histological changes were observed with aging. At 12 weeks, many notochordal cells were uniformly spread in the nucleus pulposus and there were well-organized lamellar components in annulus fibrosus. The cells and structure of the endplate were also well organized. At 1 year, notochordal cells gathered at the center of the nucleus pulposus, and the extracellular matrix area is spread around the nucleus pulposus. Cracks, microfractures, and other degenerative components increase in the endplate (Fig. 2). The histological score also progressed significantly, but no significant differences were observed between Bach 1−/− and wild-type mice at each time point (Table 1). Immunohistochemistry for CML showed significant difference of numbers of CML-positive cells in the nucleus pulposus between Bach 1−/− and wild-type mice at each point (Fig. 3a–d). With aging, CML positive cells were significantly increased in wild-type mice (Fig. 3e), but not in Bach 1−/− mice. Immunohistochemistry showed no HO-1 positive cells in the nucleus pulposus in both groups and both period (Fig. 4).
Fig. 2.
Histological changes of the age-related model. Overview of histological changes in the intervertebral discs of 12-week-old mice (a, c). The notochordal cells were uniformly spread in the nucleus pulposus, and the notochordal cells gathered at the center of the nucleus pulposus at 1 year (b, d). Scale bar = 200 μm (a–d)
Fig. 3.
Distribution of CML in the center of the intervertebral disc. Typical examples showing immunofluorescence of CML (green) and nucleus (blue) in sagittal sections of intervertebral discs from Bach 1−/− (a, b) and wild-type (c, d) mice. Scale bar = 40 μm. The number of CML positive cells in the center of the intervertebral disc in one visual field of ×400 (e). The CML-positive cells increased in wild-type mice, but in Bach 1−/− mice, CML positive cells were less than that in 12-week- and 1-year-old wild-type mice. CML-positive cells significantly increased in wild-type mice, but in Bach 1−/− mice, the CML-positive cells were small in number
Fig. 4.
Distribution of HO-1 protein in the center of the intervertebral disc. Typical examples showing immunofluorescence of HO-1 (green) and nucleus (blue) in sagittal sections of intervertebral discs from Bach 1−/− (a, b) and wild-type (c, d) mice. Scale bar = 40 μm
Annulus needle puncture model
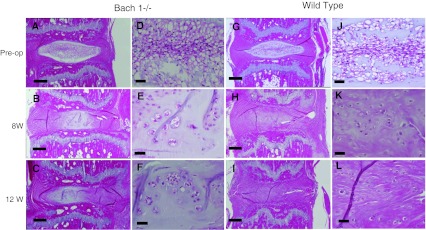
Radiographs of murine tails showed that the punctured disc decreased its height compared with the pre-puncture or control disc (Fig. 5a–f). The %DHI decreased significantly at 1 week compared with pre-operation and continued to decrease until 12 weeks post-puncture, but no significant differences were observed between Bach 1−/− and wild-type mice (Fig. 5g). The histological progression of degeneration is shown in Fig. 8. At pre-operation, almost no degenerative process can be observed (Fig. 6a, d, g, j). After puncture, the notochordal cells of the nucleus pulposus progressively decreased, and chondrocytes increased. The border of the nucleus pulposus and annulus fibrosus became interrupted. In wild-type mouse, the extracellular matrix in the nucleus pulposus had almost disappeared at 8 weeks after puncture (Fig. 6h, i, k, l), but the extracellular matrix remained at the same time point in Bach 1−/− mice (Fig. 6b, c, e, f). Statistical difference was observed at 8 and 12 weeks after puncture (Fig. 7).
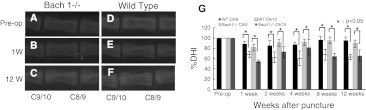
Fig. 5.
Lateral radiographs of annulus needle puncture model. The disc height of punctured intervertebral disc (C9/10) decreased at 1 week (b, e) to 12 weeks (c, f) compared with pre-operation (a, d) and control (C8/9). Change of % disc height index (%DHI) of the annulus needle puncture model (g). A significant decrease of %DHI was observed compared with the %DHI of the control disc (C8–C9). In the control intervertebral discs and punctured intervertebral disc, no significant difference was observed between Bach 1−/− mice and wild-type mice at every period
Fig. 8.
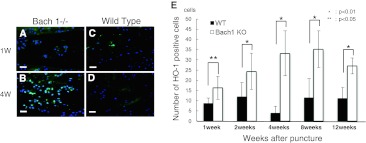
Distribution of HO-1 protein in the center of the intervertebral disc. Typical examples showing immunofluorescence of HO-1 (green) and nucleus (blue) in sagittal sections of intervertebral discs from Bach 1−/− (a, b) and wild-type (c, d) mice. Scale bar = 40 μm. The number of HO-1 positive cells in the center of the intervertebral disc in one visual field of ×400 (e). From 1 week to 12 weeks, the number of HO-1 positive cells in Bach 1−/− mice was significantly greater than that of wild-type mice at any period of time
Fig. 6.
Histological changes of the annulus needle puncture model. Overview of histological changes in the intervertebral discs by pre-operative Hematoxylin and eosin staining, 8 and 12 weeks after puncture in Bach 1−/− mice (a–f) and wild-type mice (g–l). The histological changes are clear in both groups, and less degenerative change can be observed in Bach 1−/− mice at 8 and 12 weeks after puncture. Scale bar = 200 μm (a–c, g–i) and Scale bar = 40 μm (d–f, j–l)
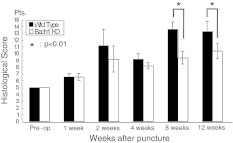
Fig. 7.
Changes in the histological score of the punctured disc. The histological score increased from pre-operation to 12 weeks after puncture. The histological score of Bach 1−/− mice at 8 and 12 weeks after puncture was significantly lower than that of wild-type mice (p < 0.01)
Immunohistochemistry revealed expression of HO-1 in the nucleus pulposus of Bach 1−/− mice, and there were fewer HO-1 positive cells in the center of the intervertebral disc of wild-type mice (Fig. 8a–d). At every period after puncture, the numbers of HO-1 positive cells in the center of the intervertebral disc of Bach 1−/− mice were significantly greater than in wild-type mice (Fig. 8e).
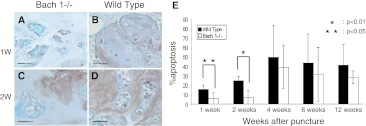
The proportion of apoptosis cells per total nucleus pulposus cells increased after puncture. The cells whose nuclei are stained with blue are normal cells, and the cells whose nuclei are stained with brown are apoptosis cells (Fig. 9a–d). And the proportion of apoptosis cells significantly increased in wild-type mice in the early periods (Fig. 9e). At 1 and 2 weeks after puncture, the proportion of apoptosis cells was significantly lower in Bach 1−/− mice than in wild-type mice. At 4–12 weeks after puncture, there was no significant difference in the proportion of apoptosis cells between Bach 1−/− and wild-type mice.
Fig. 9.
Detection of apoptosis cells. TUNEL-stained sections at 1 and 2 weeks after puncture (a–d). Apoptosis cells were stained brown and non-apoptosis cells were stained blue. The number of apoptosis cells increased after puncture in wild-type mice more than in Bach 1−/− mice. Scale bar = 40 μm. The apoptosis rate of Bach 1−/− mice was significantly lower than that of wild-type mice at 1 week (p < 0.01) and 2 weeks (p < 0.05) (e). No significant difference was observed from 4 to 12 weeks after puncture between Bach 1−/− mice and wild-type mice
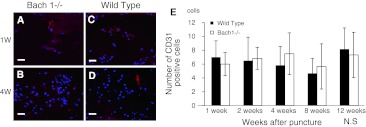
After puncture, CD31 positive cells were observed in the nucleus pulposus of Bach 1−/− and wild-type mice at each period (Fig. 10a–d). The number of CD31-positive cells showed no significant difference between Bach 1−/− and wild-type mice (Fig. 10e).
Fig. 10.
Distribution of CD31 in the center of the intervertebral disc at 1 and 4 weeks after puncture. Typical examples showing immunofluorescence of CD31 (red) and nucleus (blue) in sagittal sections of intervertebral discs from Bach 1−/− (a, b) and wild-type (c, d) mice. Scale bar = 40 μm. The number of CD31 positive cells in the center of the intervertebral disc in one visual field of ×400 (e). From 1 to 12 weeks, the number of CD31-positive cells in Bach 1−/− mice did not change significantly, and no significant difference was observed between Bach 1−/− mice and wild-type mice
Discussion
The present study showed that high expression of HO-1 in Bach 1−/− mice after annulus puncture had favorable effects on intervertebral disc degeneration.
In the present study, the degenerative changes with age showed no significant difference between Bach 1−/− and wild- type mice. However, in annulus puncture model, the histological score of Bach 1−/− mice was lower than that of wild-type mice at 8 and 12 weeks after puncture. The extracellular matrix gradually decreased after puncture. In wild-type mice, scar-like fibrous tissue increased in the nucleus pulposus after puncture.
Radiologically, the disc height decreased gradually with age. By 1 week after annulus puncture, the height of the disc had decreased. Previous studies have reported that the disc height decreases rapidly after annulus puncture and the degree of degenerative process changes according to the diameter of the needles [22]. In the present study, the disc height between Bach 1−/− mice and wild-type mice after puncture did not show any significant difference, and there seemed to be little relationship between the histological degree of degeneration and disc height. However, both aging and annulus puncture caused the DHI and %DHI to decrease significantly, so the results suggest that a decrease of DHI and %DHI proves the existence of disc degeneration but do not accurately reflect the degree of disc degeneration. Previous studies using rats, rabbits and other large animals have reported that histological progression of degeneration in intervertebral discs depends on needle size, and disc height shows the same tendency [22]. However, no studies using mice have declared that there is strong correlation between histological degeneration and disc height after annulus puncture, despite a decrease in disc height being statistically significant after puncture. According to these results, the disc height measurement may be useful to judge whether degeneration exists or not, but it may not accurately reflect the degree of degeneration.
Previous studies have shown that degeneration of the intervertebral disc progressively increases after puncture [20, 22], and the results of this current study have followed previous results. However, the histological score of Bach 1−/− mice was significantly lower than that of wild-type mice at 8 and 12 weeks after puncture. The mechanism of the difference can be explained by the fact that progress of intervertebral disc degeneration in Bach 1−/− mice is repressed as a result of oxidative stress prevention by HO-1. HO-1 protects the cells in the intervertebral disc from oxidative stress, causing cell damage to be reduced, which enables the cells to avoid apoptosis. Oxidative stress has been reported to have a bearing on intervertebral disc degeneration, secondary injury after spinal cord injury and other inflammatory joint diseases [6, 7, 19, 23, 24], but this study is of particular significance, because it has shown that inhibiting oxidative stress can repress the process of intervertebral disc degeneration.
In the preoperative intervertebral disc, HO-1 positive cells were found at very low levels. After puncture, expression of HO-1 was observed in both groups. But in Bach 1−/− mice, HO-1 expression was significantly higher than in wild-type mice. This result suggests that the intervertebral disc, which is hypovascular tissue in normal condition, is vascularized after puncture, and heme is driven into the intervertebral disc. HO-1 may confer protection from oxidative stress by metabolizing heme to form biliverdin and bilirubin, which are the antioxidants [12]. The reason why HO-1 positive cells were not observed before puncture and age-related model was suspected that heme, the material of HO-1, does not exist in the nucleus pulposus of non-punctured discs because of their hypovascularity. After puncture, increased vascularization might provide heme into nucleus pulposus and HO-1 expression was promoted. Discs of mice showed mild degenerative changes with normal aging, and therefore the aging model was inadequate to examine the influence of oxidative stress because in normal discs without heme show little expression of HO-1 and little antioxidant protection. With aging, human discs actually show microfractures, tears and vessel ingrowth which can also be observed in punctured discs of mice. In the puncture model, vessel ingrowth might introduce heme into discs and HO-1 might protect cells in the nucleus pulposus from degeneration.
There are some limitations of this study that should be taken into consideration. The nucleus pulposus of mice are populated with notochordal cells which are not observed in human intervertebral disc. However, the animal intervertebral disc degeneration model has been commonly used in many basic studies [25]. Another limitation is that the degenerative induction in this study is not physiologic, because the intervertebral discs of the mouse are not affected with weight bearing. However, surgical injury is a simple and technically easy method of inducing intervertebral disc degeneration. This method is frequently used in many studies [20, 26] and can lead to intervertebral disc degeneration [25].
Mice have been used as models in the study of intervertebral disc degeneration, in particular the mechanics of the intervertebral disc in relation to human intervertebral disc degeneration [27, 28]. Previous studies have shown that annulus injury could induce significant and progressive biochemical and molecular changes in the murine intervertebral disc [20]. These studies have implied that the process of intervertebral disc degeneration induced by annulus puncture shares some similarities with human intervertebral disc degeneration.
Apoptosis is considered to be an important component of intervertebral disc degeneration. Several studies have reported the relationship between apoptosis and intervertebral disc degeneration [4]. In this study, the apoptosis rate was lower in the early period of post-puncture in Bach 1−/− mice. However, histological degeneration was significantly different between the two groups later, at 8 and 12 weeks after puncture. This result may suggest that the repression of apoptosis in the early periods affects histological progression of degeneration and finally results in an intervertebral disc less affected by degeneration. The repression of apoptosis in Bach 1−/− mice appears in early period because the progression of intervertebral disc degeneration after puncture can be observed in early period and it caused the production of extracellular matrix gradually, and the histological differences appears after some time course. This relationship between apoptosis and disc degeneration has been reported in previous study [29]. To protect cells from apoptosis is one way to repress the progression of intervertebral disc degeneration. Indeed, the results of this study have followed and consolidated those of previous studies.
Conclusion
The results of this study suggest that HO-1 modulates intervertebral disc degeneration after puncture. The exact mechanism of modulation is not yet clear, but we do know that prevention of oxidative stress can repress the degenerative process of the intervertebral disc, reducing the number of apoptosis cells. And high HO-1 expression can inhibit oxidative stress and modulate the process of intervertebral disc degeneration.
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology-Japan (No. 20591743). The manuscript submitted does not contain information about medical device(s)/drug(s). Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflict of interest
None.
References
- 1.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–123. [PubMed] [Google Scholar]
- 2.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka N, An HS, Lim TH, et al. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1:47–56. doi: 10.1016/S1529-9430(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 4.An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11:541–552. doi: 10.1007/s00776-006-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seki S, Kawaguchi Y, Chiba K, et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607–612. doi: 10.1038/ng1557. [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Ha KY, Lee JS, et al. The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine. 2007;32:2443–2448. doi: 10.1097/BRS.0b013e318157395a. [DOI] [PubMed] [Google Scholar]
- 7.Poveda L, Hottiger M, Boos N, et al. Peroxynitrite induces gene expression in intervertebral disc cells. Spine. 2009;34:1127–1133. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Shan Y, Lambrecht RW, Ghaziani T, et al. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAS. J Biol Chem. 2004;279:51769–51774. doi: 10.1074/jbc.M409463200. [DOI] [PubMed] [Google Scholar]
- 10.Shibahara S, Muller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- 11.Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. [PubMed] [Google Scholar]
- 12.Sun J, Hoshino H, Takaku K, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock WW, Buelow R, Sayegh MH, et al. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 15.Odaka Y, Takahashi T, Yamasaki A, et al. Prevention of halothane-induced hepatotoxicity by hemin pretreatment: protective role of heme oxygenase-1 induction. Biochem Pharmacol. 2000;59:871–880. doi: 10.1016/S0006-2952(99)00386-X. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiai S, Mizuno T, Deie M, et al. Oxidative stress reaction in the meniscus of Bach 1 deficient mice: potential prevention of meniscal degeneration. J Orthop Res. 2008;26:894–898. doi: 10.1002/jor.20579. [DOI] [PubMed] [Google Scholar]
- 18.Lu DS, Shono Y, Oda I, et al. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine. 1997;22:1828–1834. doi: 10.1097/00007632-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 19.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Leung VY, Luk KD, et al. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–121. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- 21.Han B, Zhu K, Li FC, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33:1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 22.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Tanaka N, Nakanishi K, et al. Modulation of the secondary injury process after spinal cord injury in Bach1-deficient mice by heme oxygenase-1. J Neurosurg Spine. 2008;9:611–620. doi: 10.3171/SPI.2008.10.08488. [DOI] [PubMed] [Google Scholar]
- 24.Davies CM, Guilak F, Weinberg JB, et al. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–630. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 26.Olmarker K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine. 2008;33:850–855. doi: 10.1097/BRS.0b013e31816b46ca. [DOI] [PubMed] [Google Scholar]
- 27.Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–722. doi: 10.1097/01.BRS.0000116982.19331.EA. [DOI] [PubMed] [Google Scholar]
- 28.Sarver JJ, Elliott DM. Mechanical differences between lumbar and tail discs in the mouse. J Orthop Res. 2005;23:150–155. doi: 10.1016/j.orthres.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Ariga K, Miyamoto S, Nakase T, et al. The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine. 2001;26:2414–2420. doi: 10.1097/00007632-200111150-00004. [DOI] [PubMed] [Google Scholar]