Abstract
Introduction
Displaced spinopelvic dissociation with cauda equina syndrome is still unclear challenging problem with multiple instabilities. This retrospective study tried to evaluate and analyze the results of surgical decompression and lumbopelvic fixation of these injuries.
Methods
Twenty-eight polytrauma patients with displaced spinopelvic dissociation and cauda equina syndrome were included. Preoperatively, they had thorough clinical, neurological, and radiological evaluation and classification. Operatively, they underwent primary, secondary, or tertiary decompression then spinopelvic fixation. Postoperatively, they were followed up for an average of 26 months. Hannover pelvic scoring system was applied for outcome evaluation.
Results
The mean age was 33.7 years; 17 cases were males and 11 were females. According to Roy-Camille, 13 cases had type II and 15 cases had type III injuries; cauda equina syndrome was incomplete in 17 cases and complete in 11 cases. Unilateral L5–S1 facet joint injury was detected in 13 cases; 14 cases had direct decompression (50 %) and 14 cases had indirect decompression (50 %). 19 patients (67.9 %) had excellent and good clinical outcome. Primary decompression had significantly increased the chances for neurological recovery (p = 0.024). Initial fracture kyphosis angles had a significant effect on neurological retrieval (p = 0.016). The mean of Gibbons score improved from 3.1 ± 0.83 preoperatively to 1.5 ± 0.84 at the end of follow-up with a highly significant impact (p = 0.001).
Conclusions
Surgical decompression and lumbopelvic segmental fixation can enhance neurological recovery and combat any structural instabilities associated with the displaced spinopelvic dissociation injuries with a hopeful clinical results.
Keywords: Spinopelvic dissociation, ‘U-shaped’ sacral fractures, Cauda equina syndrome, Lumbopelvic fixation, Surgical decompression
Introduction
Spinopelvic dissociations are the least common and the least studied sacral fractures. However, they are associated with higher rates of neurologic injury [1].
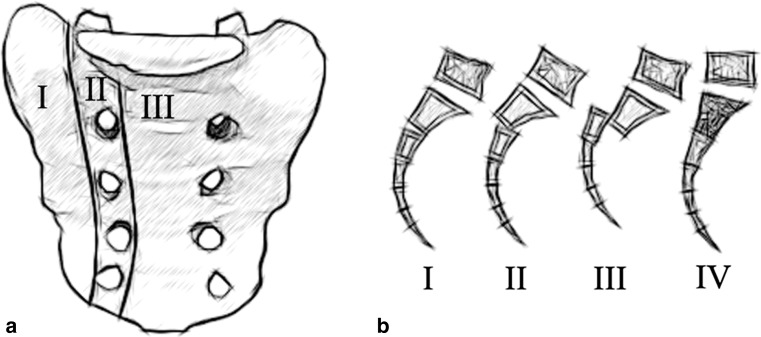
Currently, for sacral fractures, Denis’s classification (Fig. 1a) is the most commonly used [2]. It divides them into three zones: alar (zone 1), foraminal (zone 2) and central (zone 3). Zone 3 injuries are frequently transverse and cross into other zones. This system does not recognize the combination of the transverse and bilateral vertical fracture lines that induce spinopelvic dissociation. Roy-Camille et al. [3] described this injury but emphasized only the transverse sacral fracture (TSF), not the bilateral vertical elements. They identified three types, thereafter Strange-Vognsen and Lebech [4] (Fig. 1b) added a fourth type.
Fig. 1.
a Denis et al. [2] classification shows the three zones of longitudinal sacral fractures. b Roy-Camille et al. [3] classification shows the three types of combined longitudinal and transverse sacral fractures and the 4th type modified by Strange-Vognsen and Lebech [4]
The reported treatment methods for those concerned lesions have been associated with several shortcomings, including small number of cases, poor analysis of the prognostic factors, difficulty achieving and maintaining fracture stability, inconsistent neurological recovery and a high complication rate [1, 5–9].
The aim of this retrospective study is to evaluate the results of lumbopelvic fixation after direct or indirect decompression of these injuries with analysis of the different local factors which can affect the final outcome.
Patients and methods
Between January 2000 and January 2010, 28 polytrauma patients having displaced Roy-Camille type II and III injuries with cauda equina syndrome (CES) were managed surgically and followed up for an average of 26 months (range 20–37). Cases that had associated spinal injuries with neurological instability, mostly from severe fall from height injuries, were excluded to give a clear chance for only sacral CES recovery evaluation.
Preoperatively, Plain X-ray pelvic anteroposterior (AP), lateral, inlet and outlet views followed by the computed tomography (CT) scans in axial, coronal, and sagittal cuts were mandatory for all patients.
Clinical with full neurological examination was essential for CES diagnosis including testing of saddle area sensation, rectal sphincter tone, and the anal reflex. The presence of complete CES or bony fragments compromising the central canal and or the neural foramina with corresponding nerve roots injury indicated direct decompression.
Sorting of CES was based on Gibbons classification [10]; associated unilateral L5–S1 facet joint injury was classified according to Isler [11]; timing of posterior decompression with fixation was planned according to Tscherne et al. [12] management protocol for the polytrauma patients. According to that protocol primary surgical interference is created during the first 3 days; secondary interference is during 4–8 days; tertiary interference is afterward.
All cases had mechanical and chemical deep vein thrombosis (DVT) prophylaxis in addition to the perioperative parenteral antibiotic regime.
Technique of posterior decompression, reduction, and fixation
All patients were operated in a prone lined position, through a midline incision from L3 to S4 levels.
Under fluoroscopy control, pedicular screws were placed through L4 pedicles, the intact L5 pedicles and both iliac bones. For iliac screws, the entry point medial to the posterior superior iliac spine (PSIS) and more anteriorly were advanced into both iliac buttresses, under fluoroscopy (Fig. 2), aiming towards the anterior inferior iliac spine (AIIS).
Fig. 2.
An intraoperative C-arm control of iliac screw insertion. a The initial iliac oblique view confirms the screw direction from the posterior superior iliac spine (PSIS) to the anterior inferior iliac spine (AIIS). b The conjugated obturator-outlet view shows the contained screw within the teardrop formed by the inner and outer iliac tables (black arrows) and bony roof of the sciatic buttress (white arrow). c The conjugated obturator-inlet view confirms the intraosseous positioning of the iliac screw
Direct decompression (Fig. 3), when indicated, was achieved through laminectomy from L5 down to S4 with exploration of the dura and all insulted roots including partial anterior foraminotomy when needed. Indirect decompression, when indicated, was created through reduction.
Fig. 3.
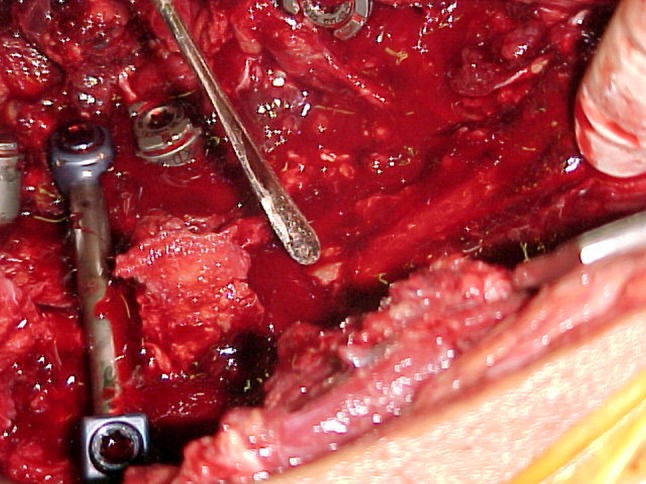
An intraoperative photo shows direct decompression of complete cauda equina syndrome through bilateral laminectomy from lumber five down to sacral four vertebrae
Reduction was achieved gently through longitudinal traction and manipulation through bone hooks and bilateral iliac Schanz screws joy sticking; it was maintained via large reduction and/or pelvic clamps.
Finally the rods were contoured to lie just posterior to the lumbosacral area and medial to the PSIS with just very short tip distal to the iliac screws. Thereafter, the rods were tightened to the screws heads and a transverse bar was compressing both rods. In case of direct decompression, posterolateral arthrodesis across the instrumented levels was accomplished.
The wound was closed tightly over a suction drain which was avoided in the cases associated with dural tears.
Postoperative care
Postoperatively, lumbosacral and pelvic radiographs were taken immediately and at regular intervals of 4–8 weeks during follow-up. Patients were rehabilitated immediately in bed and allowed to aided weight bear as tolerated on average of 4.3 weeks (range 3–7 weeks) according to the associated injuries. Full unaided weight bearing began 12.4 weeks on average (range 10–16 weeks) according to the evidence of good radiological healing.
Evaluation of the results
The results were evaluated according to the Hannover pelvic outcome scoring system because it takes both urologic deficiencies and bowel incontinence into account [13]. In this system the ratings of the radiologic result and the clinical result are assessed as one score on a 7-point scale, where the maximum of 7 points represent an excellent result, 6 points is a good result, 5 and 4 points is a fair result, and 3 and 2 points is a bad or poor result. Excellent and good scores were categorized as a satisfactory result; fair and poor scores as an unsatisfactory result.
Statistical analysis was done using SPSS version 11.0.1 for windows (SPSS Inc., Chicago, Illinois), one-way analysis of variance (ANOVA) test, and its non-parametric equivalent, the Kruskal–Wallis test, were used for variables that were small and not normally distributed. One-sample t test was applied for means comparison. A p value 0.05 or less was considered to be statistically significant.
Results
Perioperative data
The mean age was 33.7 years (range 21–54); the Hannover polytrauma score (PTS) averaged 26.2 points (range 21–34). Table 1 showed all demographic data of the patients.
Table 1.
Demographic data of the patients
| Factors | Numbers | % | |
|---|---|---|---|
| Groups | Subgroups | ||
| Gender | Males | 17 | 60.7 |
| Females | 11 | 39.3 | |
| Cauda equina lesion | Complete cauda | 11 | 39.3 |
| Incomplete cauda | 17 | 60.7 | |
| Initial neurological impairment | Gibbons type I | 0 | 0 |
| Gibbons type II | 8 | 28.6 | |
| Gibbons type III | 9 | 32.1 | |
| Gibbons type IV | 11 | 39.3 | |
| Zone III sacral fracture subtyping | Roy-Camille type II | 13 | 46.4 |
| Roy-Camille type III | 15 | 53.6 | |
| Age groups | 21–30 years | 7 | 25 |
| 31–40 years | 10 | 35.7 | |
| 41–50 years | 9 | 32.1 | |
| 51–60 years | 2 | 7.1 | |
| Mechanism of injury | Fall from height | 11 | 39.3 |
| Motorcycle accidents | 9 | 32.1 | |
| Pedestrian accidents | 8 | 28.6 | |
| Associated injuries | Head injuries | 9 | 32.1 |
| Chest injuries | 5 | 17.9 | |
| Abdominal injuries | 14 | 50 | |
| Urological injuries | 8 | 28.6 | |
| Extremities injuries | 9 | 32.1 | |
| Spine fractures | 5 | 17.9 | |
| Unilateral L5–S1 Facet joint injury | 13 | 46.4 | |
| Unilateral 5th Lumber pedicle fracture | 7 | 25 | |
| Unilateral 5th lumber transverse process fracture | 16 | 57.2 | |
| Morel-Lavallee lesions | 8 | 28.6 | |
| Transverse sacral fracture element | Through sacral one body | 10 | 35.7 |
| Through sacral two body | 9 | 32.1 | |
| Obliquely through sacral one and two bodies | 9 | 32.1 | |
| Initial fracture kyphosis angle | 0–19° | 5 | 17.9 |
| 20–39° | 8 | 28.6 | |
| 40–59° | 8 | 28.6 | |
| 60–79° | 7 | 25 | |
| Timing of fixation | Definitive primary anterior pelvic fixation | 18 | 64.3 |
| Secondary anterior pelvic fixation | 10 | 35.7 | |
| Primary posterior fixation | 14 | 50 | |
| Secondary posterior fixation | 7 | 25 | |
| Tertiary posterior fixation | 7 | 25 | |
| Decompression type | Indirect decompression | 14 | 50 |
| Direct decompression | 14 | 50 | |
| Residual neurological impairment | Gibbons type I | 19 | 67.8 |
| Gibbons type II | 5 | 17.9 | |
| Gibbons type III | 3 | 10.7 | |
| Gibbons type IV | 1 | 3.6 | |
Cauda equina syndrome was incomplete in 17 cases, while 11 cases had complete syndrome. Eight cases were Gibbons type II, nine cases were Gibbons type III, and 11 cases were Gibbons type IV.
Morel-Lavallee lesions (MLL) were encountered anterolaterally in five cases and posterolaterally in three cases, they were all managed during surgery by open drainage through a separate incision, debridement, and closed suction drainage.
Posterior fixation after decompression was created primarily for 12 cases including all cases with complete CES, secondarily for 9 cases, and during tertiary phase for 7 cases.
Direct decompression was indicated in 14 cases including partial anterior foraminotomy in five of them. All roots were found to be integral, a traumatic dural tear at the S1–S2 level in three patients was treated using 6-0 proline sutures.
It was noted, during reduction, that the lower the level of the transverse fracture element the difficult the reduction. Maintaining reduction was difficult in 20 cases, therefore, sacral one segment alar screws were added in absence of lateral comminution (16 cases) and very long iliac screws (120 mm) were used in the presence of alar comminution (4 cases).
The initial transverse sacral fracture kyphosis angle averaged 58.3° (range 15–79°); it averaged, postoperatively, 13.6° (range 0–22°) and no loss of reduction was confronted at the final follow up (Fig. 4). The difference showed highly significant angle improvement (p = 0.001).
Fig. 4.
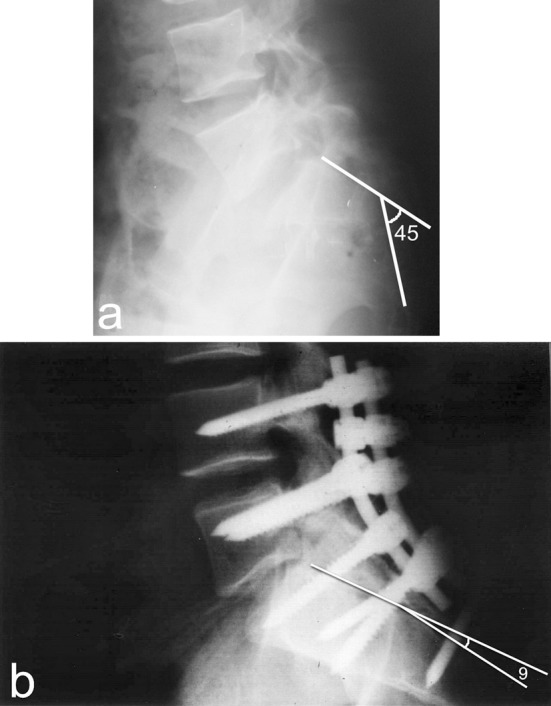
a A preoperative lateral radiograph shows 45° initial transverse sacral fracture kyphosis angle. b The same radiograph at final follow up with improvement of the angle down to 9°
Unilateral L5–S1 facet joint injury was detected in 13 cases. It was extraarticular (Isler type I) in two cases, intraarticular (Isler type II) in five cases, and complex injury (Isler type III) in six cases. During surgery type III injuries had arthrodesis surgery after direct decompression and reduction; type II injuries had only denervation of the joint capsule via electrocautery after indirect decompression (reduction); the two cases with extraarticular injuries had nothing after reduction.
Pelvic outcome scoring and factors affecting it
The pelvic outcome was excellent in 5 cases (Fig. 5 example), good in 14 cases (Fig. 6 example), fair in 7 patients, and poor in 2 patients. Satisfactory outcome was present in 19 patients (67.9 %). Unsatisfactory outcome was present in nine cases (32.1 %) due to the residual neurological deficits and posterior pelvic pain in all of them, difficulty in urination without incontinence or residual urine in three of them, partial impotence in two of them, and urinary incontinence in one of them.
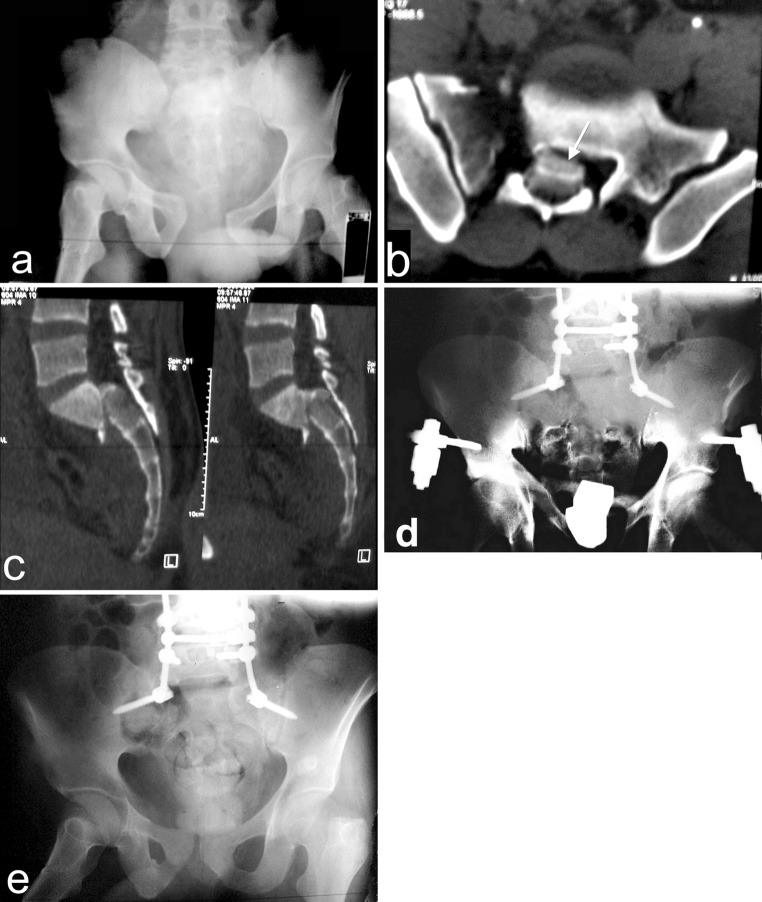
Fig. 5.
a An AP radiograph of a referred 32 years old male patient, He had incomplete CES (Gibbons type III). b The sagittal CT showing Roy-Camille type III injury. c The postoperative AP radiograph showing the added two lateral alar screws to augment fixation and maintain the difficult indirect decompression. d The AP radiograph 15 months after surgery, the patient had complete neurological recovery by the end of the 17th month postoperatively
Fig. 6.
a The AP radiograph of 25 years old male patient, he sustained a highly unstable pelvic injury with complete CES (Gibbons type IV). b An axial CT showing Denis type III sacral fracture with sacral canal blocking (white arrow). c The sagittal CT showing type II Roy-Camille spinopelvic dissociation. d The AP radiograph after 1 month from direct posterior decompression and lumbopelvic fixation. e The AP radiograph after 11 months from surgery showing good fusion. The CES regressed after 20 months to Gibbons type II
Pelvic outcome was better significantly among males, Roy-Camille type II fractures, road traffic injuries, first sacral body transverse fractures, when the initial transverse fracture kyphosis angle was <40°, and in the presence of distal alar and iliac screws (Table 2).
Table 2.
Factors affecting the final pelvic outcome and their significance
| Groups | Subgroups | Pelvic outcome | p value | ||||
|---|---|---|---|---|---|---|---|
| Excellent cases | Good cases | Fair cases | Poor cases | Total cases | |||
| Gender | Males | 3 | 10 | 3 | 1 | 17 | 0.038* |
| Females | 2 | 4 | 4 | 1 | 11 | ||
| Cauda equina lesion | Complete | 2 | 5 | 3 | 1 | 11 | 0.010* |
| Incomplete | 3 | 9 | 4 | 1 | 17 | ||
| Initial cauda equina typing | Gibbons type II | 2 | 4 | 1 | 1 | 8 | 0.033* |
| Gibbons type III | 1 | 5 | 3 | 0 | 9 | ||
| Gibbons type IV | 2 | 5 | 3 | 1 | 11 | ||
| Zone III sacral fracture subtyping | Roy-Camille type II | 3 | 7 | 3 | 0 | 13 | 0.038* |
| Roy-Camille type III | 2 | 7 | 4 | 2 | 15 | ||
| Age groups | 21–30 years | 3 | 3 | 1 | 0 | 7 | 0.282** |
| 31–40 years | 1 | 8 | 1 | 0 | 10 | ||
| 41–50 years | 1 | 3 | 4 | 1 | 9 | ||
| 51–60 years | 0 | 0 | 1 | 1 | 2 | ||
| Mechanism of injury | Pedestrian accidents | 2 | 5 | 1 | 0 | 8 | 0.004*** |
| Fall from height | 1 | 5 | 4 | 1 | 11 | ||
| Motorcycle accidents | 2 | 4 | 2 | 1 | 9 | ||
| Unilateral facet joint injury | Present | 3 | 5 | 4 | 1 | 13 | 0.010* |
| Absent | 2 | 9 | 3 | 1 | 15 | ||
| Unilateral lumber 5 pedicle fracture | Present | 1 | 2 | 2 | 2 | 7 | 0.600** |
| Absent | 4 | 12 | 5 | 0 | 21 | ||
| Morel-Lavallee lesion | Present | 2 | 4 | 1 | 1 | 8 | 0.324** |
| Absent | 3 | 10 | 6 | 1 | 20 | ||
| Transverse sacral fracture | Through sacral one body | 2 | 6 | 2 | 0 | 10 | 0.001*** |
| Through sacral two body | 1 | 4 | 3 | 1 | 9 | ||
| Obliquely through both | 2 | 4 | 2 | 1 | 9 | ||
| Initial fracture kyphosis angle | 0–19° | 2 | 2 | 1 | 0 | 5 | 0.016* |
| 20–39° | 2 | 4 | 2 | 0 | 8 | ||
| 40–59° | 1 | 4 | 2 | 1 | 8 | ||
| 60–79° | 0 | 4 | 2 | 1 | 7 | ||
| Timing of posterior fixation | Primary | 3 | 7 | 3 | 1 | 14 | 0.024*** |
| Secondary | 1 | 4 | 2 | 0 | 7 | ||
| Tertiary | 1 | 3 | 2 | 1 | 7 | ||
| Decompression | Direct | 3 | 6 | 4 | 1 | 14 | 0.010* |
| Indirect | 2 | 8 | 3 | 1 | 14 | ||
| Pelvic pedicular screws fixation | Alar + iliac | 3 | 9 | 3 | 1 | 16 | 0.010* |
| Iliac only | 2 | 5 | 4 | 1 | 12 | ||
* Statistically significant through Kruskal–Wallis test, **statistically insignificant through Kruskal–Wallis test, ***statistically significant through One Way Analysis of Variance test
Presence of unilateral 5th lumber vertebra pedicle fractures and Morel-Lavallee lesions had insignificant effect on the final outcome, whereas the associated facet joint injuries influenced it significantly, as five nonfused cases with initial type II Isler injury had persistent pain (Table 2).
Complete CES and accordingly Gibbons type IV injuries had a significant effect on the final results as four cases had unsatisfactory pelvic outcome after surgery. Prognostic factors that had affected significantly the pelvic outcome were summarized in Table 3.
Table 3.
The resulting significant prognostic factors
| Factors associated with significantly good prognosis | Factors associated with significantly bad prognosis |
|---|---|
| Male gender | Female gender |
| Incomplete cauda equine syndrome | Complete cauda equine syndrome |
| Gibbons types II and III injuries | Gibbons type IV injuries |
| Roy-Camille type II injuries | Roy-Camille type III injuries |
| Road traffic accidents | Fall from height injuries |
| Absence of associated L5–S1 facet joint injury | Presence of associated L5–S1 facet joint injury |
| Sacral one body transverse element | Sacral two body transverse element |
| Transverse fracture kyphosis angle <40° | Transverse fracture kyphosis angle 40° or more |
| Decompression within the first 8 days | Decompression after 8 days |
| Indirect decompression | Direct decompression |
| Iliac and alar screws fixation | Only Iliac screws fixation |
Neurological recovery
Early primary decompression had significantly increased the chances for CES recovery. Also indirect decompression significantly enhanced the neurological recovery of the incomplete CES as evidenced in Table 2.
Neurological recovery had been achieved in all Gibbons types of CES as 19 cases (67.9 %) had complete recovery, 8 cases (28.6 %) had partial recovery, and only one case (3.5 %) had no recovery. However, the initial incomplete CES injuries in 17 cases had significantly better neurological recovery in comparison to the initial complete CES in the other 11 cases (Table 4).
Table 4.
Cauda equina completeness and final neurological improvement
| Cauda equina syndrome | Final neurological outcome according to Gibbons | p value | ||||
|---|---|---|---|---|---|---|
| Type 1 | Type II | Type III | Type IV | Total (cases) | ||
| Incomplete | 13 | 3 | 1 | 0 | 17 | 0.038* |
| Complete | 6 | 2 | 2 | 1 | 11 | |
| Total | 19 | 5 | 3 | 1 | 28 | |
* Statistically significant through Kruskal–Wallis test
The mean of Gibbons scores improved from 3.1 ± 0.83 preoperatively to 1.5 ± 0.84 at the end of follow-up; the difference was highly significant (p = 0.001). Sensory impairment maximal recovery occurred in an average of 7.3 months (range 4–9 months); motor deficits maximal recovery happened in an average of 15.6 months (range 10–22 months); bowel incontinence had a maximal recovery in an average of 4.1 months (range 3–7 months); whereas urinary incontinence recovered maximally in an average of 8.2 months (range 5–11 months).
Complications
The faced complications with their management were registered in Table 5.
Table 5.
The encountered complications and their management
| Complications | No. | % | Management |
|---|---|---|---|
| Dural tears | 3 | 10.7 | Surgical repair and no suction drain application |
| Delayed posterior wound healing (range 18–28 days) | 8 | 28.6 | Repeated blood and plasma transfusion, early rehabilitation in bed, proper nutritional supply, and delayed suture removal (minimum 3 weeks) |
| Infection | 7 | 25 | Continuation of parenteral antibiotic and blood transfusion |
| Wound washout and debridement in three of them who had initial MLLa | |||
| Hardware prominence without skin breakage | 5 | 17.9 | Implants removal after 18 months |
| DVTb | 3 | 10.7 | Doubling the dose of chemical DVT prophylaxis |
| Fat embolism and ARDSc | 1 | 3.6 | ICU Medical control |
| L5–S1 facet arthropathy | 5 | 17.9 | Non-steroidal anti-inflammatory medications and local corticosteroid injections |
| Unilateral rod breakage (16–21 months after non-fusion surgery) | 3 | 10.7 | Nothing (no subjective complaints) |
aMorel-Lavallee lesion, b deep vein thrombosis, c adult respiratory distress syndrome
Discussion
The spinopelvic dissociation frequently causes neurological deficits ranging from incomplete monoradiculopathies to a complete cauda equina syndrome [14].
Although some authors [1, 8] recommend conservative treatment for these patients because intraoperative findings have shown torn, stretched, contused, or lacerated roots, surgical decompressions, in this study, lead to significant recovery in the associated Gibbons types of CES. Similarly Schildhauer et al. [5] had 83 % neurological improvement in spite of the presence of a high rate of sacral nerve root transection at the time of surgery. Hunt et al. [9] advised reduction of the fracture displacement as much as possible, and also creating decompression in the presence of neurological deficits and a grossly compromised sacral canal. Siebler et al. [1] documented that nonoperative treatment yields consistent healing and improvements in initial neurologic deficits but residual complaints were very common.
Neurologic improvement, in literature, could be expected in up to 83 % of patients with TSF regardless of operative or nonoperative management [1, 5, 8, 15]. However, in this series 96.5 % of the cases had partial or complete neurological recovery this may be due to surgical decompression which was created as soon as possible and absence of severe fall from height injuries with its related sacral roots transection as evidenced in many series [3, 15].
Although the timing of sacral root decompression has not been studied specifically in the literature, primary surgery had significantly increased chances for CES recovery in this study. This is contributed to early decompression of the insulted intact sacral roots. Similarly, many authors encourage early decompression of the neural elements, preferably within the first 24–72 h [5, 16, 17]. Yi and Hak [18] stated that decompression after fracture healing is more difficult because of epineural fibrosis and increased scarring of the central canal and foramina.
Indirect decompression cases had a significantly better pelvic outcome in this series. This may be related to absence of the compromised sacral canal with the loose bony fragments and the resulting dural tears, and absence of complete CES injuries among those cases.
The initial fracture kyphosis angle had a significant impact on this series neurological recovery. Angles more than 40° were associated with bad prognosis; this may be due to more stretching of the insulted roots. Similarly, Siebler et al. [1] found that 80 % of the cases with Gibbons types III and IV injuries were related to initial kyphosis angles more than 40° (43–60°).
In this study, 32.1 % of the cases had unsatisfactory outcome due to mainly the residual CES. Similarly, Denis et al. [2] declared that the residual neurological deficit is critical factor affecting the functional outcome. Lindahl and Hirvensalo [19] documented the permanent neurological injury as a strong prognostic factor.
Roy-Camille type III injuries had significantly worse outcome in this series. This may be related to the complete CES which was common among them, and to the presence of more anterior translation and more tethering of the roots.
First sacral body transverse fractures had significantly better outcome. This might be contributed to: the encountered easy complete reduction of them; the anatomical facts that sacral canal is wider at this level; and the more broad body than the second sacral one in the sagittal plane which gives less chance for complete displacement when the transverse fracture occurs.
The associated unilateral nonfused intraarticular facet joint injuries had a significant influence on our final outcome. To avoid this problem in the future, it is better to try primary L5–S1 posterolateral fusion with those injuries as the presence of intact contralateral facet joint and posterior arch cannot guard against the future instability and arthropathy.
The presence of unilateral 5th lumber pedicle fracture in 7 cases had no significant clinical effect in spite of lacking of the pedicle screw fixation at these pedicles. Moreover, no reduction loss was recorded regardless of the use of only one iliac screw on each side in 12 cases. This might be related to the good strength of the whole construct including the compressing transverse bar which provided good rotational stability and prevent splaying of the posterior pelvic ring.
Two distal alar and iliac pedicular screws had significantly better outcome than the only one distal iliac screw. This might be due to the alar screws bicortical purchasing advantage which limits micromotion, and enhances the chances for primary bone healing without excessive callus formation in the presence of nearby regenerating sacral roots. Biomechanical analysis has confirmed that segmental lumbopelvic instrumentation, is among the most stable methods of posterior pelvic fixation [20]. The superior clinical mechanical stability of such a construct has been proven also by many authors [5, 6].
MLL had no significant effect on this study although it participated in superficial infection of three cases and delayed wound healing in four cases. This finding confirmed the suggestion of Steiner et al. [21] who stated that surgical access for osteosynthesis and for debridement of MLL can be used without increased infection rate.
Hardware prominence was documented in 17.8 % of cases. The same complication confronted many other authors [5, 14, 18]. This can be especially problematic in multiply injured patients that exhibit substantial catabolic weight loss. Therefore, countersinking of the iliac screws heads as much as possible inside the more medial and anterior bony trough to the PSIS and shortening the distal rods tips are mandatory precautions.
After healing, asymptomatic unilateral distal rod breakage, due to motions of the sacroiliac joints, was documented in 10.7 % of cases. This finding augments suggestion of many authors for elective removal of the lumbopelvic instrumentation after solid union between 6 and 12 months from a nonfusion surgery [6, 20, 22].
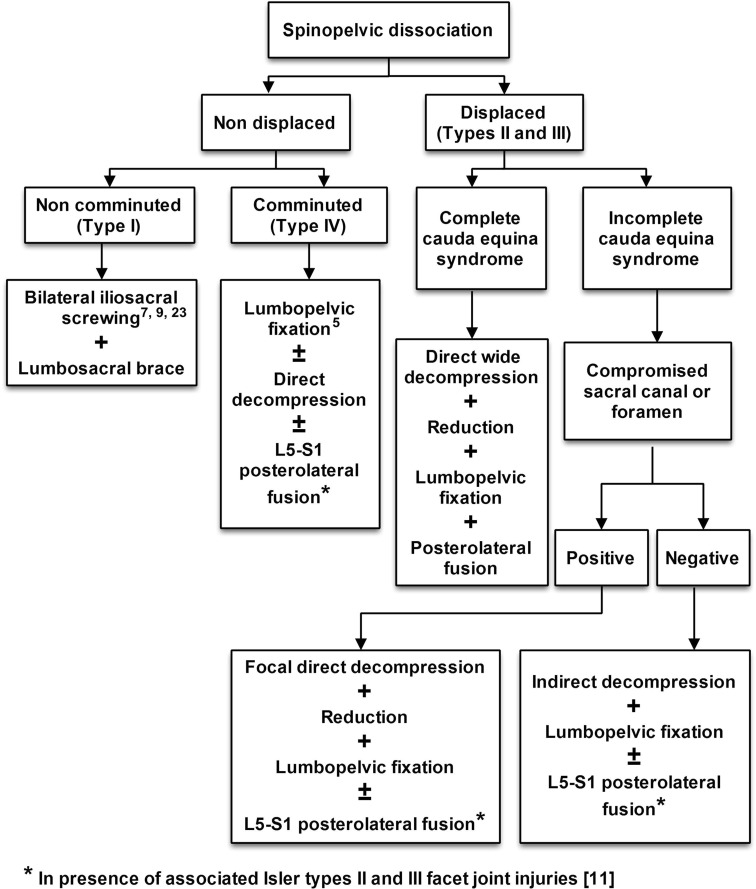
Displaced spinopelvic dissociation injuries have actually many interlocking instabilities and no general treatment paradigm has been formulated for them. According to the findings of this study and those literature [5, 7, 9, 23], an algorithm (Fig. 7) for the surgical management of these injuries is suggested. Lumbopelvic instrumentation had the upper hand in that algorithm as it can address all instabilities with more promising clinical, radiological and neurological results in addition to early mobilization of the polytrauma patients. Percutaneous bilateral iliosacral screwing and lumbosacral bracing can be used cautiously in undisplaced injuries. The value of this algorithm can be weighted in the future studies of those difficult and rare injuries.
Fig. 7.
A preliminary algorithm for surgical management of the spinopelvic dissociation injuries
Acknowledgments
I certify that there is no actual or potential conflict of interest in relation to this article.
Conflict of interest
None.
References
- 1.Siebler JC, Hasley BP, Mormino MA. Functional outcomes of Denis zone III sacral fractures treated nonoperatively. J Orthop Trauma. 2010;24(5):297–302. doi: 10.1097/BOT.0b013e3181ccb645. [DOI] [PubMed] [Google Scholar]
- 2.Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67–81. [PubMed] [Google Scholar]
- 3.Roy-Camille R, Saillant G, Gagna G, Mazel C. Transverse fracture of the upper sacrum: suicidal jumper’s fracture. Spine. 1985;10:838–845. doi: 10.1097/00007632-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Strange-Vognsen HH, Lebech A. An unusual type of fracture in the upper sacrum. J Orthop Trauma. 1991;5:200–203. doi: 10.1097/00005131-199105020-00014. [DOI] [PubMed] [Google Scholar]
- 5.Schildhauer TA, Bellabarba C, Nork SE, Barei DP, Routt ML, Jr, Chapman JR. Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma. 2006;20(7):447–457. doi: 10.1097/00005131-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bellabarba C, Schildhauer TA, Vaccaro AR, Chapman JR. Complications associated with surgical stabilization of high-grade sacral fracture dislocations with spino-pelvic instability. Spine. 2006;31(11 Suppl):S80–S88. doi: 10.1097/01.brs.0000217949.31762.be. [DOI] [PubMed] [Google Scholar]
- 7.Nork SE, Jones CB, Harding SP, Mirza SK, Routt MLC., Jr Percutaneous stabilization of U-shaped sacral fractures using iliosacral screws: technique and early results. J Orthop Trauma. 2001;15:238–246. doi: 10.1097/00005131-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Phelan ST, Jones DA, Bishay M. Conservative management of transverse fractures of the sacrum with neurological features. A report of four cases. J Bone Joint Surg [Br] 1991;73:969–971. doi: 10.1302/0301-620X.73B6.1955446. [DOI] [PubMed] [Google Scholar]
- 9.Hunt N, Jennings A, Smith M. Current management of U-shaped sacral fractures or spino-pelvic dissociation. Injury. 2002;33:123–126. doi: 10.1016/S0020-1383(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons KJ, Soloniuk DS, Razack N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72:889–893. doi: 10.3171/jns.1990.72.6.0889. [DOI] [PubMed] [Google Scholar]
- 11.Isler B. Lumbosacral lesions associated with pelvic ring injuries. J Orthop Trauma. 1990;4:1–6. doi: 10.1097/00005131-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Tscherne H, Regel G, Pape HC, Pohlemann T, Trettek C. Internal fixation of multiple fractures in patients with polytrauma. Clin Orthop Relat Res. 1998;347:62–78. doi: 10.1097/00003086-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Pohlemann T, Gänsslen A, Schellwald O, Culemann, Tscherne H. Outcome after pelvic ring injuries. Injury. 1996;27(Suppl 2):S-B31–S-B38. [PubMed] [Google Scholar]
- 14.Gribnau AJ, Hensbroek PB, Haverlag R, Ponsen KJ, Been HD, Goslings JC. U-shaped sacral fractures: surgical treatment and quality of life. Injury. 2009;40(10):1040–1048. doi: 10.1016/j.injury.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Robles LA. Transverse sacral fractures: a review. Spine J. 2009;9(1):60–69. doi: 10.1016/j.spinee.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Soultanis K, Karaliotas GI, Mastrokalos D, Sakellariou VI, Starantzis KA, Soucacos PN. Lumbopelvic fracture-dislocation combined with unstable pelvic ring injury: one stage stabilization with spinal instrumentation. Injury. 2010 doi: 10.1016/j.injury.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Avadhani A, Shetty AP, Rajasekaran S. Pediatric transverse sacral fracture with cauda equina syndrome. Spine J. 2010;10(2):e10–e13. doi: 10.1016/j.spinee.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Yi C, Hak DJ. Traumatic spinopelvic dissociation or U-shaped sacral fracture: a review of the literature. Injury. 2011 doi: 10.1016/j.injury.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Lindahl J, Hirvensalo E. Outcome of operatively treated type-C injuries of the pelvic ring. Acta Orthop. 2005;76(5):667–678. doi: 10.1080/17453670510041754. [DOI] [PubMed] [Google Scholar]
- 20.Schildhauer TA, Ledoux WR, Chapman JR, Henley MB, Tencer AF, Routt ML., Jr Triangular osteosynthesis and iliosacral screw fixation for unstable sacral fractures: a cadaveric and biomechanical evaluation under cyclic loads. J Orthop Trauma. 2003;17(1):22–31. doi: 10.1097/00005131-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Steiner CL, Trentz O, Labler L. Management of Morel-Lavallee lesion associated with pelvic and/or acetabular fractures. Eur J Trauma Emerg Surg. 2008;34:554–560. doi: 10.1007/s00068-007-7056-y. [DOI] [PubMed] [Google Scholar]
- 22.Keel MJ, Benneker LM, Siebenrock KA, Bastian JD. Less invasive lumbopelvic stabilization of posterior pelvic ring instability: technique and preliminary results. J Trauma. 2011;71(3):E62–E70. doi: 10.1097/TA.0b013e3182092e66. [DOI] [PubMed] [Google Scholar]
- 23.König MA, Jehan S, Boszczyk AA, Boszczyk BM. Surgical management of U-shaped sacral fractures: a systematic review of current treatment strategies. Eur Spine J. 2011 doi: 10.1007/s00586-011-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]