Abstract
Purpose
To determine if differences in safety or efficacy exist between balloon kyphoplasty (BKP), vertebroplasty (VP) and non-surgical management (NSM) for the treatment of osteoporotic vertebral compression fractures (VCFs).
Methods
As of February 1, 2011, a PubMed search (key words: kyphoplasty, vertebroplasty) resulted in 1,587 articles out of which 27 met basic selection criteria (prospective multiple-arm studies with cohorts of ≥20 patients). This systematic review adheres to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Results
Pain reduction in both BKP (−5.07/10 points, P < 0.01) and VP (−4.55/10, P < 0.01) was superior to that for NSM (−2.17/10), while no difference was found between BKP/VP (P = 0.35). Subsequent fractures occurred more frequently in the NSM group (22 %) compared with VP (11 %, P = 0.04) and BKP (11 %, P = 0.01). BKP resulted in greater kyphosis reduction than VP (4.8º vs. 1.7°, P < 0.01). Quality of life (QOL) improvement showed superiority of BKP over VP (P = 0.04), along with a trend for disability improvement (P = 0.08). Cement extravasation was less frequent in the BKP (P = 0.01). Surgical intervention within the first 7 weeks yielded greater pain reduction than VCFs treated later.
Conclusions
BKP/VP provided greater pain relief and fewer subsequent fractures than NSM in osteoporotic VCFs. BKP is marginally favored over VP in disability improvement, and significantly favored in QOL improvement. BKP had a lower risk of cement extravasation and resulted in greater kyphosis correction. Despite this analysis being restricted to Level I and II studies, significant heterogeneity suggests that the current literature is delivering inconsistent messages and further trials are needed to delineate confounding variables.
Keywords: Vertebral compression fractures, Osteoporosis, Balloon kyphoplasty, Vertebroplasty, Systematic review
Introduction
Vertebral compression fractures (VCF) constitute a major health problem affecting more than 1.4 million people each year worldwide [1], leading to pain, significant morbidity [2, 3], and healthcare expenses [4]. Non-surgical management (NSM) may not relieve pain, frequently leads to prolonged immobilization, and may lead to pulmonary deterioration, persistent pain, progressive kyphotic deformity, weight loss, depression, and overall compromise in life quality [2, 5, 6]. In addition, patients with VCF are prone to new adjacent fractures (a fivefold increase in risk) [7]. In one prospective study, elderly women with at least 1 VCF had an age-adjusted increased risk of mortality of 32 %; survival impact was more profound with greater numbers of vertebral fractures [3].
Minimally invasive techniques such as vertebroplasty (VP) and balloon kyphoplasty (BKP) have been employed to treat painful VCFs. There is class I evidence to support the superiority of vertebral augmentation procedures (VAPs) over NSM [8–10], as well as non-randomized prospective studies [11–13], systematic reviews [14–19], and numerous retrospective series supporting safety and effectiveness of these procedures. However, recently published randomized controlled trials (RCTs) that showed no superiority of VP over NSM [20] or over a simulated procedure (sham) [21, 22] have raised questions regarding the value of VP. These trials have been criticized for potential methodological flaws confounding the outcomes [23]. Meta-analysis may help resolve controversies by combining data and increasing the power of the analysis. Therefore, we performed a new systematic review evaluating the latest published literature related to the treatment of VCFs.
The null hypothesis in the current review is that there is no difference in safety or efficacy between BKP, VP, and NSM. The objective of this study was to determine if differences existed between BKP and VP, BKP and NSM, and VP and NSM in the treatment of symptomatic osteoporotic VCFs. To this end, we reviewed only the published prospective studies to date (class I and II data). In addition, we used meta-regression and subgroup analyses to identify potential predictors of outcomes.
Materials and methods
Literature search and selection
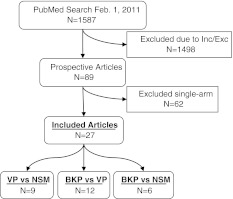
As of February 1, 2011, a PubMed search using “kyphoplasty” and “vertebroplasty” as keywords resulted in 1,587 articles, out of which 27 studies satisfied the inclusion/exclusion criteria. Inclusion criteria were prospective comparative studies of VAPs (Fig. 1), studies enrolling ≥20 patients, and studies performed for mid/lower thoracic and lumbar vertebral fractures due to osteoporosis. Exclusion criteria were single-arm studies, BKP studies not using inflatable balloons, studies not available in English, systematic reviews and meta-analyses, studies including traumatic non-osteoporotic or cancer-related fractures, and studies not reporting clinical outcomes. Of the 27 identified studies, 8 described randomized studies. Nine articles compared VP to NSM (6 articles reported on 5 randomized studies, and 3 articles reported on 2 non-randomized studies). Six articles compared BKP to NSM (1 article reported on 1 randomized study, and 5 articles reported on 2 non-randomized studies). Finally, 12 articles compared BKP to VP (1 article reported on 1 randomized study, and 11 articles reported on 11 non-randomized studies). Some of the studies reported effects for the same group of patients and were combined into one analyzable “study” (Kasperk/Grafe et al., 4 total [11, 12, 24, 25] and Rousing et al., 2 total [20, 26]; see Table 1). This systematic review was reported in accordance with the PRISMA statement [27]. Study bias was assessed with the 6-category risk of bias assessment suggested in the Cochrane Handbook [28] and advocated in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [29] (Table 2).
Fig. 1.
Literature search and selection
Table 1.
Summary of study characteristics
| Author/year of publication | Baseline characteristics | Pain relief | Disability | Quality of life | Kyphotic angle (grades) | Vertebral height | Cement leakage | New VF | AE | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Grafe [11]/ [24]; Kasperk 2005 [12]/2010 [25] | 1. BKP (40) vs. NSM (20) 2. Chronic # 3. Postop, 3 months, 6 months, 1 year, 3 years |
Pain score (0–100) BKP superior to NSM (P: 0.008 at 1 year, P: 0.02 at 3 years) |
EVOS NS difference |
NR | NR | BKP superior to NSM (P < 0.0001) | 9 %, none symptomatic | BKP superior to NSM (P: 0.03 at 3 years) | None | BKP reduces pain, new VCFs and doctor’s visits in chronic osteoporotic VCFs for at least 1 year. Both PMMA and CaP equally effective in reducing pain and improving mobility. Recommend CaP is better for young patients |
| 2. Komp [13] | 1. BKP (21) vs. NSM (19) 2. Acute # 3. 6 weeks, 6 months |
VAS score BKP superior to NSM (P: NR) |
ODI BKP superior to NSM (P: NR) |
NR | NR | Poorly reported | Poorly reported | BKP: 36 % NSM: 64 % P: NR |
None | BKP is superior to NSM |
| 3. Wardlaw [8] (Free) |
1. BKP (149) vs. NSM (151) 2. Acute # 3. 1 month, 3 months, 6 months, 12 months RCT |
VAS score BKP superior to NSM (P < 0.0001) |
RM BKP superior to NSM (P < 0.0001 at 1 month and P = 0.0012 at 1 year) |
SF-36 BKP superior to NSM (P < 0.0001 in 1 month, P = 0.0064 at 6 months) except at 1 year (P = 0.2) EQ-5D BKP superior to NSM (P = 0.0003 at 1 month, P = 0.02 at 1 year) |
NR | NR | 27 %, none symptomatic | BKP: 33 % NSM: 25 % P: NR |
Similar to both groups: BKP: 9 deaths, no procedure related, 3PE. NSM: 7 deaths, no PE |
BKP is superior to NSM in acute VCFs in terms of pain and QOL/disability. Differences between groups diminished by 1 year |
| 4. Dong [63] | 1. BKP (20) vs. VP (18) 2. NR 3. Postop, 3 months |
VAS score Both showed significant improvement (P < 0.001) BKP equivalent to VP |
NR Pulmonary function: BKP improved VC more than VP (P < 0.01) |
NR | BKP superior to VP (P < 0.01) | BKP superior to VP (P < 0.01) | NR | NR | NR | Both procedures have significant pain relief and improve lung function; BKP improves vital capacity more than VP |
| 5. Grohs [64] | 1. BKP (28) vs. VP (23) 2. Subacute # 3. Postop, 4 months, 1 year, 2 years |
VAS score Both showed significant improvement (P < 0.05) BKP superior to VP (P < 0.05) |
ODI BKP: uperior to NSM (P < 0.05) except from 2 years (NS) |
NR | BKP: 6 % decrease (P < 0.0001) VP: No decrease |
BKP: 5.8 % increase (P < 0.0001) VP: No increase |
BKP: 22.8 %, asymptomatic VP: 27.5 %, 6.9 % into canal (NR if needed surgery) |
BKP: 17 % VP: 3.5 %, |
NR | In subacute # BKP is superior in reducing the kyphotic wedge and pain for 2 years. Disability improvement was superior up to 1 year |
| 6. Liu [65] | 1. BKP (50) vs. VP (50) 2. Acute # 3. Postop, 6 months RCT |
VAS score BKP equivalent to VP |
NR | NR | BKP superior to VP (P < 0.001) | BKP superior to VP (P < 0.001) | NR | BKP: 2 VP: 0 |
NR | Similar clinical results between BKP and VP. Better height restoration with BKP |
| 7. Lovi [62] | 1 BKP (36) vs. VP (118) 2. Acute # 3. 1 month, 3 months, 6 months, 2 years 4. BKP > 30 % collapse VP < 30 % |
VAS score BKP equivalent to VP |
ODI BKP equivalent to VP |
NR | BKP had more restoration than VP (P: NR) |
BKP less extravasation than VP (P < 0.05) | BKP: none VP: 3.3 %, (P: NR) |
BKP: None VP: 1 canal leak- non operative. 1 pt died (not procedure related) |
Similar pain relief and function score BKP less cement leakage |
|
| 8. De Negri [59] | 1. BKP (15) vs. VP (18) 2. Acute and subacute 3. Postop, 2 days, 1 month, 3 months, 6 months |
VAS score BKP equivalent to VP |
ODI BKP equivalent to VP |
NR | NR | NR | BKP: None VP: 33 % none symptomatic |
NR | None | There no difference between two groups in pain relief and disability improvement Cement leakage occur in VP |
| 9. Pflugmacher [65] | 1. BKP (22) vs VP (20) 2. Acute # 3. Postop, 3 months, 6 months, 1 year |
VAS score BKP equivalent to VP |
ODI BKP equivalent to VP |
NR | BKP superior to VP (P < 0.05) |
BKP superior to VP (P < 0.05) |
NR | NR | None | BKP restore significant body height in acute # |
| 10. Rollinghof [39] | 1. BKP (49) vs. VP (41) 2. Acute # 3. Preop, postop, 1 year |
VAS score BKP equivalent to VP |
ODI BKP equivalent to VP |
NR | BKP equivalent to VP | BKP superior to VP (P < 0.05) |
BKP:22.6 % no complications VP: 25.5– 5.9 % in canal-needed surgery |
BKP: 13.2 % VP: 7.8 % (P: NR) |
BKP: None VP: 2 paresis from cement leakage into canal |
Mean vertebral body height restoration was significantly higher in BKP |
| 11. Santiago [67] | 1. BKP (22) vs VP (20) 2. Acute # 3.1 month, 6 months, 1 year |
VAS score BKP equivalent to VP |
ODI BKP equivalent to VP |
NR | NR | BKP equivalent to VP | BKP equivalent to VP | BKP superior to VP (P:0.01) |
None | No difference between operations |
| 12. Schofer [68] | 1. BKP (30) vs. VP (30) 2. Acute # 3. 1 day, last follow-up |
VAS score BKP equivalent to VP |
NR |
SF36 BKP equivalent to VP |
BKP superior to VP (P < 0.001) |
NR | BKP less extravasation than VP (P < 0.02) | BKP: none VP: 3.3 % |
None | Similar pain relief and QOL BKP less cement leakage and better reduction |
| 13. Alvarez [58] | 1. VP (101) vs NSM (27) 2. Fractures >6 weeks and <1 year 3. Postop, 3 months, 6 months, 1 year |
VAS score (0–10) VP superior to NSM up to 6 months (P < 0.001 postop. and at 3 months, P = 0.03 at 6 months, NS at 1 year) |
ODI VP superior to NSM up to 3 months (P < 0.01) NSM superior to VP AT 6 months and 1 year (P < 0.01) |
SF36 VP superior to NSM at 3 months VP equivalent to NSM at 6 months and 1 year |
NR | NR | VP: 59.6 %, 1 case (0.66 %) into canal | VP inferior to NSM (P < 0.01) | VP: 1 transient paraparesis-reoperated 5 %transient radiculopathy (nonop) | VP is more effective than NSM in pain relief and function in the early postop period. No difference was observed after 6 months |
| 14. Diamond [76] | 1. VP (88) vs. NSM (38) 2. Acute # 3. Postop, 6 weeks, 6 months, 1 year, 2 years |
VAS score (0–25) VP superior to NSM only in 6 weeks (P = 0.004) |
Barthel Index VP superior to NSM only in 6 weeks (P = 0.02) |
NR | NR | NR | NR | VP equivalent to NSM | – | VP is more effective than NSM in pain relief and function in the early postop period. No difference was observed after 6 months |
| 15. Rousing [20], [26] | 1. VP (26) vs. NSM (24) 2. Acute # 3. 3 months, 12 months RCT |
VAS score VP equivalent to NSM up to 1 year (P = 0.3) |
NR | SF36 VP equivalent to NSM up to 1 year |
NR | NR | NR | VP/NSM: RR: 1.3 |
None | No difference between VP and NSM in 3 months and 12 months |
| 16. Voormolen [9] (VERTOS I) | 1. VP (18) vs. NSM (16) 2. Acute # 3. Postop, 2 weeks RCT |
VAS score VP superior to NSM 14/16 pts crossovered from NSM to VP: |
RM VP superior to NSM |
QUALEFFO Mixed results in the subscores between groups |
NR | NR | NR | NR | NR | VP is more effective than NSM in pain relief and function in the immediate postop period (2 weeks) |
| 17. Buchbinder [22] | 1. VP (38) vs Sham (40) 2. Acute # (also Subacute) 3. 1 week, 1 month, 3 months, 6 months RCT-Sham group |
VAS score VP equivalent to SHAM |
RM VP equivalent to SHAM |
AQoL, QUALEFFO, EQ-5D VP equivalent to SHAM |
NR | NR | 37 %, none symptomatic | VP: 3 SHAM: 4 |
1 osteomyel in VP, reoperated | No difference between VP and SHAM up to 6 months |
| 18. Kallmes [21] (INVEST) | 1. VP (68) vs. Sham (63) 2. Subacute # (also Acute and Chronic) 3. Postop, 2 weeks, 1 month, 3 months RCT-Sham group |
VAS score VP equivalent to SHAM At 1 month trend favoring VP (P = 0.06). Higher crossover rate in SHAM (P < 0.001) |
RM VP equivalent to SHAM (P > 0.3) |
EQ-5D, SF-36 VP equivalent to SHAM |
NR | NR | NR | NR | One pt in VP has injury to thecal sac, managed conserv. | No difference between VP and SHAM up to 1 month |
| 19. Klazen [41] (VERTOS II) | 1. VP (101) vs. NSM (101) 2. Acute # 3. Postop, 1 week, 1 month, 3 months, 6 months, 1 year RCT |
VAS score VP superior to NSM (P < 0.0001) |
RM VP superior to NSM (P < 0.0001) |
QUALEFFO, EQ-5D VP superior to NSM (P < 0.0001) |
NR | NR | 72 % had asymptomatic cement leakage | VP equivalent to NSM (P = 0.44) | 25 % in VP had clinical silent PE | VP is more effective than NSM in acute fractures for at least 1 year |
| 20. Bae [69] | 1. BKP (20) vs. VP (20) 2. Subacute and Chronic # 3. 1 month, 3 months, 1 year, 2 years 4. Cortoss instead of PMMA |
VAS score Significant improvement in both groups BKP equivalent to VP (P < 0.05) |
ODI Significant improvement in both groups BKP equivalent to VP (P < 0.05) |
SF-12 BKP equivalent to VP (P < 0.05) |
NR | NR | BKP equivalent to VP | BKP equivalent to VP | None | Both BKP and VP were equally effective in improving pain and disability/QOL |
| 21. Movrin [77] | 1. BKP (46) vs. VP (27) 2. Acute and Subacute # 3. Postop, 1 year |
VAS score Significant improvement in both groups BKP equivalent to VP (P = 0.2 postop, P = 0.09 at 1 year) |
NR | NR | BKP superior to VP (P < 0.001) |
NR | BKP equivalent to VP (Trend favoring BKP, P = 0.08) |
BKP equivalent to VP | NR | Both BKP and VP were equally effective in improving pain, and have low incidence of new VCFs. BKP is superior in kyphosis correction |
| 22. Kumar [78] | 1. BKP (24) vs. VP (28) 2. Subacute # 3. 1 week, 3 months, 10 months |
VAS score Significant improvement in both groups BKP superior to VP (P = 0.05 at 1 week, NS at 3 months, P = 0.0005 at 10 months) |
ODI Significant improvement in both groups BKP superior to VP (P = 0.04) |
SF-36/EQ-5D Significant improvement in both groups BKP superior to VP At 1 week and 10 months but not at 3 months |
BKP superior to VP (No reduction observed in VP) |
NR | BKP equivalent to VP | BKP equivalent to VP | None | Both BKP and VP were equally effective in improving pain, disability and QOL; BKP yielded superior results maintained until last follow-up |
Table 2.
Bias assessment by study summary
| Study name | Sequence generation | Allocation concealment | Blinding | Incomplete outcomes | Selective outcomes | Other sources |
|---|---|---|---|---|---|---|
| Alvarez | No | No | No | No | NA | NA |
| Bae | No | No | No | No | Yes | NA |
| Buchbinder | Yes | Yes | Yes | No | Yes | NA |
| De Negri | No | NA | NA | NA | Yes | NA |
| Diamond | No | No | No | Yes | No | NA |
| Dong | No | No | No | No | NA | NA |
| Grohs | No | NA | NA | NA | Yes | NA |
| Kallmes | Yes | Yes | Yes | No | Yes | NA |
| KasperkGrafe | No | No | No | Yes | Yes | NA |
| Klazen | Yes | No | No | No | Yes | NA |
| Komp | No | No | No | Yes | Yes | No |
| Kumar | No | No | No | Yes | Yes | NA |
| Liu | Yes | Yes | NA | Yes | No | NA |
| Lovi | No | NA | NA | Yes | Yes | No |
| Movrin | No | No | No | No | Yes | No |
| Pflugmacher | No | NA | NA | Yes | Yes | NA |
| Rollinghoff | No | NA | NA | Yes | Yes | NA |
| Rousing | Yes | Yes | No | No | Yes | NA |
| Santiago | No | NA | NA | No | No | No |
| Schofer | No | No | No | No | Yes | NA |
| Vormoolen | Yes | Yes | No | No | Yes | NA |
| Wardlaw | Yes | Yes | No | Yes | Yes | NA |
Statistical methods
The primary analytic approach was to pool treatment subgroups to calculate a mean effect, and then to compare subgroups in a pair-wise manner using the Z test [30]. This method allowed for the assessment of the maximum number of effects in a body of evidence that comprised 27 publications on 3 treatments. Sham arms were considered to be part of the NSM group. Considering sham as an independent treatment was desired but not possible due to only two studies reporting on sham treatment. We applied a mixed-effect model when performing pair-wise comparisons. In general, we sought a minimum of four studies contributing to each subgroup in order to generate an estimated within-group effect.
We also used the method of indirect treatment comparisons (ITC) [31] and direct treatment comparisons. While these comparisons were limited by available studies, the ITC and direct methods preserve randomization [32] and thus is a worthwhile method to assess stability of our conclusions using the primary statistical approach.
Mean, SD, and N, if not directly reported, were imputed from other summary statistics [33]. For effects measured repeatedly over time, such as pain scores, mean differences from baseline were used in a meta-regression of days from baseline to assess for time-dependent effects. When the meta-regression yielded a non-significant slope, we combined multiple time point measures to yield a more precise per-study effect size. If the original scale of measure for an effect could not be preserved, we calculated standardized mean differences [34].
All summary effect sizes were assessed for heterogeneity using the I2 statistic. We identified a priori baseline fracture age as a potential covariate. A meta-regression was performed to assess for the significance of the slope and to search for any trends [35]. We set our Type-I error at α = 0.05.
Unless otherwise noted, data are reported as mean effect sizes with the 95 % confidence interval in parentheses. The review was conducted in Comprehensive Meta-Analysis Version 2 [36]. Indirect treatment analysis was conducted using the ITC Software Application [37]. Analysis was performed by one of the co-authors (G.C) and results were confirmed by an independent statistician (B.S).
Results
Analysis of pooled treatment arms
Results are summarized in Tables 3 (includes mean values, SE, and I2) and 4 (pair-wise treatment comparisons).
Table 3.
Summary of endpoints
| Effect | Statistic | Balloon kyphoplasty (BKP) | Vertebroplasty (VP) | Non-surgical management (NSM) | BKP-VP1 | BKP-NSM1 | VP-NSM1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | Mean | SE | I2 | k | Mean | SE | I2 | k | Mean | SE | I2 | |||||
| Age | Raw mean | 9 | 71.17 | 0.84 | 84 | 15 | 73.89 | 0.70 | 81 | 8 | 75.35 | 1.03 | 83 | 0.01 | <0.01 | 0.24 |
| Pain reduction (0−10) | Raw mean | 11 | −5.07 | 0.45 | 99 | 14 | −4.55 | 0.34 | 99 | 9 | −2.17 | 0.38 | 99 | 0.35 | <0.01 | <0.01 |
| Subsequent adjacent fracture | Event rate | 9 | 0.10 | – | 26 | 9 | 0.08 | – | 39 | – | – | – | – | 0.51 | – | – |
| Subsequent fracture | Event rate | 11 | 0.11 | – | 78 | 13 | 0.11 | – | 78 | 8 | 0.22 | – | 20 | 0.96 | 0.04 | 0.01 |
| Cement extravasation | Event rate | 9 | 0.17 | – | 38 | 11 | 0.33 | – | 89 | – | – | – | – | 0.01 | – | – |
| Spinal canal extravasation | Event rate | 6 | 0.01 | – | 0 | 8 | 0.02 | – | 10 | – | – | – | – | 0.30 | – | – |
| VB height restoration2 | Mean difference2 | 6 | 1.87 | 0.62 | 60 | – | – | – | – | – | – | – | – | <0.01 | – | – |
| Disability reduction | Standardized mean3 | 8 | −3.93 | 0.91 | 91 | 10 | −1.95 | 0.70 | 83 | 6 | −0.77 | 0.19 | 0 | 0.08 | <0.01 | 0.10 |
| Quality of life improvement | Mean difference4 | 4 | 7.13 | 1.20 | 91 | 5 | 2.70 | 1.82 | 94 | – | – | – | – | 0.04 | – | – |
| Kyphotic angle reduction | Raw mean | 8 | 4.85 | 1.00 | 96 | 6 | 1.74 | 0.63 | 96 | – | – | – | – | <0.01 | – | – |
1Columns contain P values unless otherwise noted
2Difference in mean values approximates change in millimeters. Positive values favor BKP. Data in BKP column
3Standardized mean difference incorporates both RMDQ and ODI. A more negative number indicate greater reduction in disability
4Mean difference expressed using a combined SF-36/SF-12 PCS scale
Table 4.
Pair-wise treatment comparison results
| Effect | BKP vs. VP | BKP vs. NSM | VP vs. NSM |
|---|---|---|---|
| Pain reduction (0–10) | NS (P = 0.356) | BKP (P < 0.001) | VP (P < 0.001) |
| Subsequent adjacent fracture | NS (P = 0.510) | ||
| Subsequent fracture | NS (P = 0.967) | BKP (P = 0.045) | VP (P = 0.015) |
| Cement extravasation | BKP (P = 0.015) | ||
| Spinal canal extravasation | NS (P = 0.304) | ||
| VB height restoration | BKP (P = 0.003) | ||
| Disability reduction | NS (P = 0.088) | BKP (P = 0.001) | NS (P = 0.109) |
| Quality of life improvement | BKP (P = 0.043) | ||
| Kyphotic angle reduction | BKP (P = 0.009) |
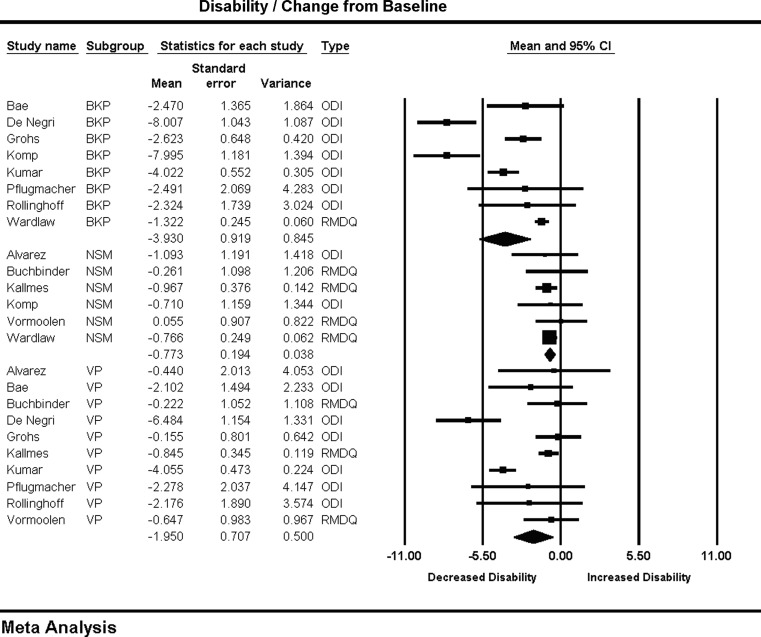
Disability improvement is reported as a standardized mean difference utilizing two scales: the Oswestry Disability Index (ODI) and Roland Morris Disability Questionnaire (RMD). A meta-regression showed no significant time-dependent effect on disability scores for any treatment arm. In terms of disability reduction, BKP, −3.93 (−5.73, −2.12), showed a trend toward greater improvement than VP, −1.95 (−3.33, −0.56) (P = 0.08), and significantly better than NSM, −0.77 (−1.15, −0.39) (P = 0.008). The difference between VP and NSM in terms of disability reduction was not significant (P = 0.23) (Fig. 2). There was substantial heterogeneity in the BKP (I2 = 91) and VP (I2 = 83) arms.
Fig. 2.
Change in disability from baseline and forest plot
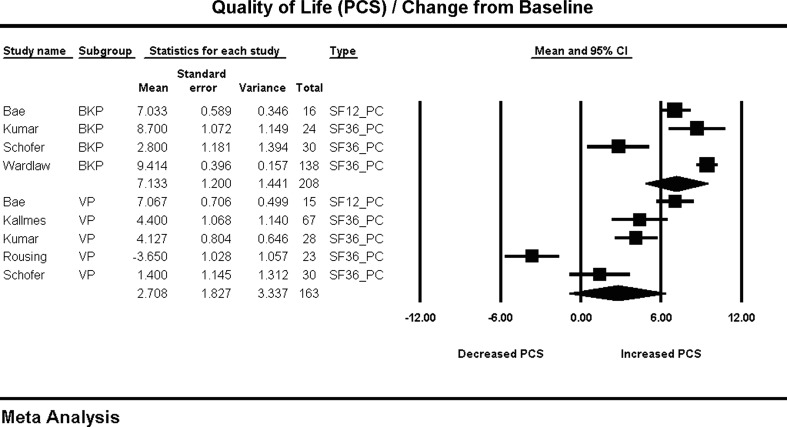
QOL improvement is reported as Physical Component Summary (PCS) units from the combined SF-36 and SF-12 surveys. BKP, 7.13 (4.78, 9.48), showed significantly more PCS improvement than VP, 2.70 (−0.87, 6.28) (P = 0.043) (Fig. 3). It should be cautioned that these results are based on only four studies for BKP with an I2 = 92, and five studies for VP with an I2 = 6.
Fig. 3.
Change in quality of life from baseline and forest plot
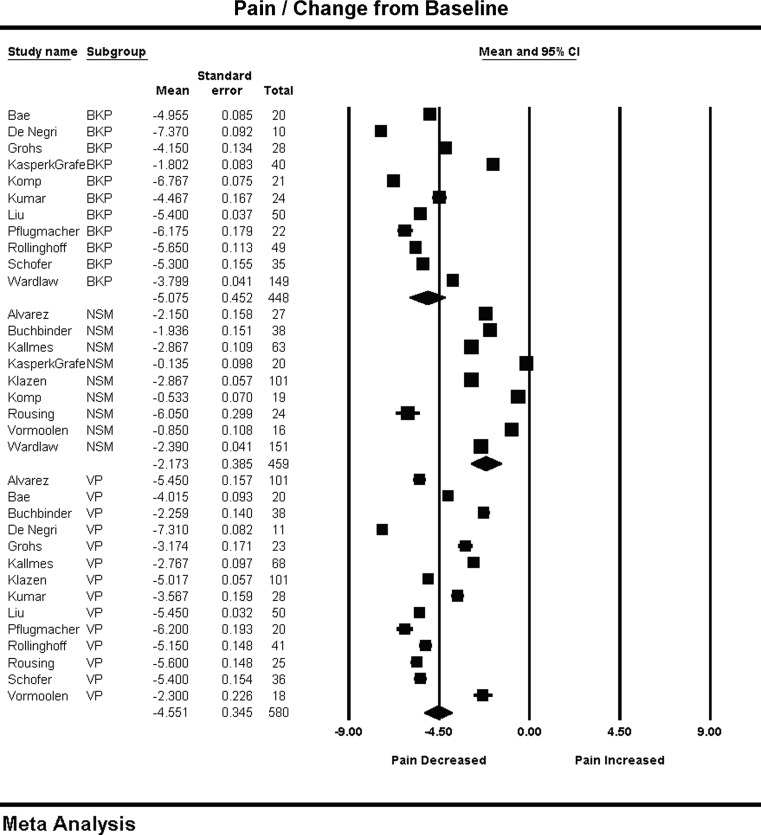
Pain ratings were rescaled to a 0 to 10 scale, with 0 being no pain and 10 being the worst pain imaginable. The meta-regression of days from procedure versus pain rating for each of the treatment arms demonstrated no significant correlation. The range of pain relief (0 = no pain relief, −10 = maximum possible pain relief) across all studies was −5.07 (−5.96, −4.18) for BKP, −4.55 (−5.22, −3.87) for VP, and −2.17 (−2.92, −1.41) for NSM. A wide scatter in ranges of pain relief for BKP (I2 = 99), VP (I2 = 99), and NSM (I2 = 99) was evident as shown in Fig. 4. Both BKP (P < 0.01) and VP (P < 0.01) performed significantly better than NSM, while no significant difference was observed between the two interventional procedures (P = 0.35).
Fig. 4.
Change in pain from baseline and forest plot
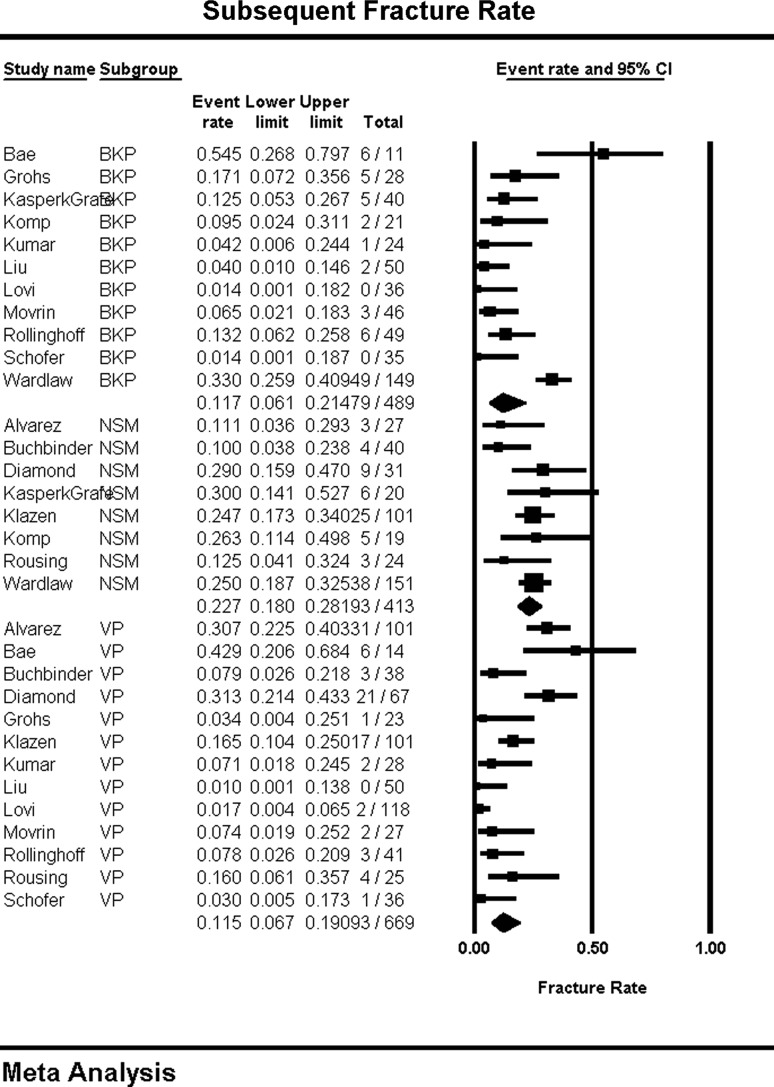
Subsequent adjacent fractures and overall subsequent fractures are reported as event rates. For overall subsequent fractures (95 % CI in parentheses), both BKP, 11.7 (6.1, 21.4) % (P = 0.04), and VP, 11.5 (6.7, 19.0) % (P = 0.01), showed significantly lower rates of fracture than NSM, 22.7 (18.0, 28.1) % (Fig. 5), while there was no significant difference between BKP and VP (P = 0.96). Significant heterogeneity was observed in BKP (I2 = 78) and VP (I2 = 78) while NSM showed more consistent results (I2 = 20). For subsequent adjacent fracture, there was not a detectable difference between BKP, 10.4 (6.7, 16.0) %, and VP, 8.4 (5.3, 13.2) % (P = 0.51).
Fig. 5.
Subsequent fracture rate and forest plot
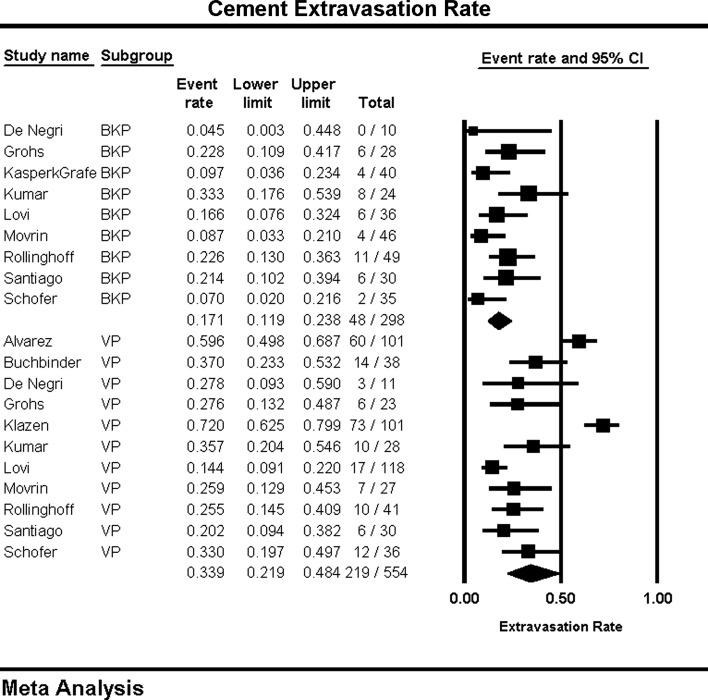
Cement extravasation, reported as an event rate, was significantly less frequent for BKP, 18.1 (13.9, 23.2) % than for VP, 41.1 (36.6, 45.8) % (P = 0.01) (Fig. 6). The VP group (I2 = 89) showed substantially more heterogeneity than the BKP group (I2 = 38). Spinal canal extravasations occurred too infrequently in both groups to provide a meaningful analysis. There were no reported spinal canal extravasations for BKP and 7 reported for VP.
Fig. 6.
Cement extravasation rate and forest plot
For vertebral height restoration, we converted the relative values to quasi-absolute measures by assuming normal vertebral body height to be 30 mm as reported for a large population of osteoporotic women in a previous study [38]. The calculation of Hedges’s g showed a significant difference favoring BKP over VP for height restoration, 1.87 (0.64, 3.11) (P = 0.003). However, heterogeneity was moderate (I2 = 60).
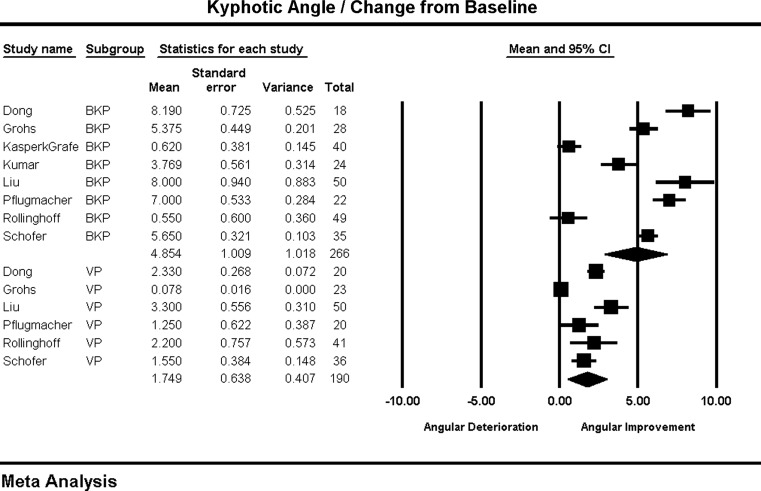
Kyphotic angle was reported as a degree difference in index VB angulation, defined as |A|−|B| where A is pre-op angle and B is post-op angle. BKP, 4.85° (2.87°, 6.83°), was superior to VP, 1.74° (0.49°, 3.00°) (P = 0.009) (Fig. 7). Both the BKP group (I2 = 96) and the VP group (I2 = 96) have substantial heterogeneity. Most BKP studies reporting 3.7º–8º reduction, with 2 outliers that showed minimal change [12, 39].
Fig. 7.
Kyphotic angle and forest plot
Analysis of randomized trials
Results are summarized in Table 5, which compares mean values (where applicable) between RCTs, non-RCTs and pooled studies. Here, we report the comparison between RCTs-only. Seven randomized trials were recognized: one between BKP/VP (Liu [40]), one comparing BKP to NSM (Wardlaw [8]) and five comparing VP either to NSM (Rousing [20], Voormolen [9], Klazen [41]) or to SHAM procedure (Buchbinder [22], Kallmes [21]).
Table 5.
Comparison results stratified by study type
| Outcome | Subgroup | P, BKP–VP | P, BKP–NSM | P, VP–NSM |
|---|---|---|---|---|
| Disability reduction | All | 0.08 | <0.01 | 0.10 |
| RCTs only | 0.164 | 0.078 | 0.989 | |
| Non-RCTs | 0.189 | 0.004 | 0.161 | |
| QOL improvement | All | 0.043 | – | – |
| RCTs only | 0.025 | – | – | |
| Non-RCTs | 0.361 | – | – | |
| Pain reduction | All | 0.35 | <0.01 | <0.01 |
| RCTs only | 0.467 | 0.034 | 0.062 | |
| Non-RCTs | 0.868 | <0.001 | <0.001 | |
| Subsequent fracture | All | 0.96 | 0.04 | 0.01 |
| RCTs only | 0.892 | 0.684 | 0.119 | |
| Non-RCTs | 0.959 | 0.031 | 0.050 | |
| Kyphotic angle reduction | All | 0.008 | – | – |
| RCTs only | 0.001 | – | – | |
| Non-RCTs | 0.016 | – | – |
P values from the original (all) analyses, as well as sub-divided by RCT or non-RCT studies
In terms of disability reduction, there was no difference between BKP/VP (P = 0.16) or VP/NSM (P = 0.1), with BKP showing a trend toward greater improvement than conservative management (P = 0.078). One study was available for BKP, three for VP and four for NSM. QOL improvement was superior for BKP versus VP (P = 0.02) based on only one randomized trial for BKP and two studies for VP. Pain ratings were similar between procedures (P = 0.46), while BKP performed better than NSM (P = 0.03) and VP showed a trend toward more pain relief than NSM (P = 0.06) with six studies were available for VP/NSM and two for BKP. For overall subsequent fractures no differences were encountered: BKP versus VP (P = 0.8), BKP versus NSM (P = 0.6) and VP versus NSM (P = 0.11) with four studies available for VP/NSM and two studies for BKP. As far as kyphosis correction BKP was superior to VP (P = 0.001) with only one RCT available for VP/BKP. Finally no randomized trials reported cement extravasation for BKP so comparisons were not feasible.
Additional analyses
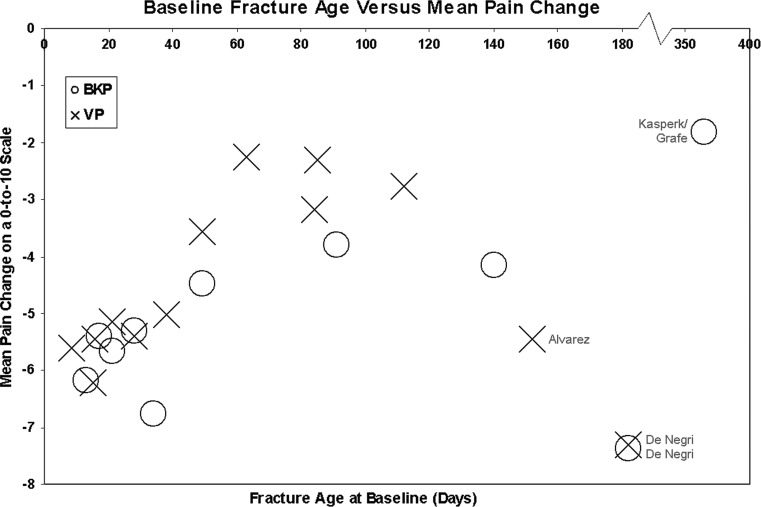
The sensitivity analyses on average baseline index fracture age against subsequent fractures, cement extravasation, and disability did not yield significant results. The meta-regression of pain reduction against baseline fracture age exhibited a clear pattern, with clinically significant pain reduction before 7 weeks (~−5.0 to −7.0 points) and substantially less pain reduction between 7 weeks and 4 months, especially for VP (~−2.3 to −3.5 points for VP and (~−3.8 to −4.5 points for BKP) (Fig. 8).
Fig. 8.
Meta-regression of fracture age versus pain reduction in vertebroplasty and kyphoplasty
We attempted confirmatory analysis of pain change, subsequent fractures, and disability using the techniques of direct and indirect treatment comparison, though in many cases low study count caused a difficulty in interpretation. For pain change, indirect treatment comparison trended toward BKP over VP (effect size −1.99 (95 % CI −5.28, 1.29). The wide confidence band is likely due to only three studies contributing to the BKP–NSM path. Direct treatment comparison significantly favored BKP over VP (effect size −0.39 [−0.74, −0.04], P = 0.02). For subsequent fractures, inconsistent results and low study count resulted in wide confidence bands. Thus, the results neither refuted nor confirmed the results of the grouped treatment analysis. For disability, indirect treatment comparison trended toward BKP over VP (effect size −3.75 [−10.39, 2.88]), while direct treatment comparison also trended toward BKP over VP (effect size −0.80 [−1.81, 0.19]). Low study count likely accounted for the wide confidence intervals.
Discussion
Traditionally, VP has been accepted as a successful procedure for treating VCFs; but three recently published RCTs comparing VP with a sham procedure [21, 22] or NSM [20] have created contention about the efficacy of VP. Potential flaws confounding the outcomes have been previously outlined: these include low accrual rates at busy centers, inclusion of patients with subacute/chronic fractures, sham design, and no reported clinical examination to determine the source of pain [23]. These studies do not report on what happened to the majority of patients that fulfilled the inclusion criteria but opted not to participate in the study. Non-uniform evaluation of fractures with MRI [20, 21], higher crossover rates in the NSM arm [21] and other pain generators unrelated to the fracture (e.g., discogenic/facetogenic pain) are additional problems. Most of the limitations of those RCTs were presented by Bono et al. [23] on behalf of the North American Spine Society, and were responded to by study authors [42]. Nevertheless, these trials demonstrate the likelihood that a subset of patients will not benefit from VP. Further randomized studies are needed to address this issue, although a subsequently published RCT (VERTOS II) found clear superiority of VP versus NSM [41]. The current study represents an updated systematic review of prospective studies of VAP and NSM for the treatment of osteoporotic VCFs. We supplement the most recent meta-analysis, published by Han et al. [43] which pertains only to comparative trials between VP/BKP, by also including analysis of randomized and non-randomized controlled trials comparing VAPs with NSM.
Disability/QOL
Disability instruments such as the ODI and RMD and QOL scales such as SF-12/36 are standard questionnaires designed to minimize subjective variability and allow for reproducible and comparable measures [44, 45]. BKP was shown to be superior to NSM in terms of reduced disability and non-significantly better than VP, whereas VP was not significantly different from NSM. Direct treatment comparisons and ITC provide secondary evidence of the disability benefit of BKP over VP. QOL improvement (PCS component) was also superior in BKP over VP. Similar observations were made in randomized trials although this effect was milder (no difference between VP/BKP and trend favoring BKP vs. NSM). This potential advantage of BKP in disability and QOL was not observed (or could not be validated due to insufficient data) in previous analyses [15, 18, 19, 43]. In addition to a possible procedural effect, this may reflect different patient selection criteria and clinical acumen and expertise of practitioners of BKP compared to VP. Caution should be used due to the pooled nature of this analysis, the heterogeneity of the results and the low number of studies.
Pain relief
Pain relief as measured by the VAS was similar between BKP and VP, while both treatments were significantly better than NSM. When considering only randomized trials, the effect was diminished only for VP (non-significant trend favoring VP vs. NSM). This 4- to 5-point difference between VAPs and NSM should be considered not only statistical but also clinical important (more than 30 % improvement from baseline pain) [46, 47]. However, this should be interpreted with caution because of significant heterogeneity (I2 = 99) in pain relief for all three treatment groups. Surprisingly, even between RCTs, great variance exists, for instance in VERTOS II [41] there is almost a double size effect in pain reduction comparing with the INVEST [21] or the Buchbinder trial [22]. This is partially attributable to the non-uniform scales across studies but also points to the unreliability of this method; patient rated VAS pain scores may be referring to maximum pain, average or current pain, or pain with or without medications, positional pain, etc. predisposing to heterogeneity in responses. The wide scatter in ranges of pre-operative pain and pain relief suggests that pain using a single VAS measure as a sole measure of treatment efficacy is inconsistent and unreliable.
Subsequent/adjacent fractures
The results of the current study are consistent with prior meta-analyses and prospective and comparative studies reporting higher rates of subsequent vertebral fractures after non-surgical treatment of osteoporotic VCFs when compared to VP and BKP [11–13, 15, 16]. The mechanism whereby vertebral augmentation may reduce the risk of subsequent fracture might be that anterior column support along with reduction of kyphosis lessens the flexion moment on the surrounding vertebrae, thus reducing the likelihood of further fractures [48]. Of note, the subsequent fracture rate in the NSM group is in accordance with the literature (around 22 % at 1 year after the initial fracture [7]). Even if we base our conclusions on randomized-only trials, VAPs are at least equivalent with NSM and are not associated with an increased risk of subsequent fractures.
Cement extravasation
There was significantly less cement extravasation in the BKP arm than in the VP arm, with high heterogeneity in the VP group. In contrast, the BKP arm yielded more consistent results. The lower rate of cement extravasation after BKP is consistent with previous studies [15, 18, 49–52]. A number of factors may have contributed to the heterogeneity in the VP group: procedural technique, variation in considering extravasation as a complication [51], different postoperative radiological follow-up (plain films vs. computed tomography [51, 53]), cement viscosity (inverse relationship [52]), cement pressure [49], fracture level (higher extravasation rates above T7), and cement volume (dose dependent [54]). The optimum cement amount per level has not been established, and cement volume does not seem to correlate well with either clinical success [55] or restoration of vertebral stiffness or strength [56, 57]. The lower rate of cement extravasation seen after BKP may be attributed to the cavity created by the inflatable balloon that allows for low-pressure and higher-viscosity controlled cement filling. In addition with balloon expansion, cancellous bone is compacted, thus creating a dam effect during cement filling. A recent meta-analysis of only comparative trials found no difference between BKP/VP [43]; this may be due to the study design which did not allow for the inclusion of significant RCTs or prospective studies comparing VP to NSM. Several of these trials noted a significantly increased rate of cement leakage with VP (Klazen-72 % of patients [41], Buchbinder-37 % [22], Alvarez-60 % [58], De Negri-33 % [59]).
Height restoration/kyphotic angle reduction
The random effect model showed a significant difference in VB height restoration in favor of BKP, which also showed significantly greater kyphotic angle reduction. The BKP arm was more heterogeneous, but this was due to the presence of two studies that paradoxically report significant VB height restoration with no change in kyphosis [12, 39]. This finding likely reflects the correction of biconcave fractures that are not associated with the development of kyphotic angulation.
Kyphosis reduction may be attributed to postural reduction in the prone position for the procedure and/or balloon expansion. The beneficial effects of restoration of spinal sagittal balance are well documented [60]. Theoretically, improvement in spinal alignment will reduce the flexion moments around the affected vertebrae and relax the paraspinal muscles, leading to more upright posture, reduced pain, and fewer subsequent fractures. Studies give conflicting results, with some authors favoring either no correlation of deformity correction with clinical outcome [12, 39, 61, 62], positive correlation [63, 64], or did not report or investigate this outcome [40, 65–68]. Insufficient study data made it difficult to perform a statistical analysis to test for a relationship, so the question still remains.
Serious adverse events
Most studies did not either report [9, 40, 63, 64] or encounter [11–13, 20, 59, 65, 67, 68] any serious adverse events (SAEs). In the remaining studies, most SAEs were related to spinal canal/foramen cement leakage. In VP studies, three patients had postoperative paraparesis related to cement extravasation and required reoperation that reversed their symptoms [39, 58]. Two patients had postoperative radiculopathy and were successfully managed non-operatively [58, 62]. Additionally in the VP group the authors reported one psoas hematoma, one injury to the thecal sac (managed conservatively [21]), and one osteomyelitis requiring further surgery [22]. No cases of symptomatic cement extravasation were reported in the BKP arm, while one case of osteomyelitis was recorded [69].
The only study describing SAEs in detail was the FREE trial [8] (BKP vs. NSM randomized) where the profile was similar in both groups: 58 events in 149 cases in the BKP arm and 54/151 in the NSM arm. In the same study, three cases of pulmonary embolism were reported more than 6 weeks after the procedure. In VERTOS II (VP vs. NSM), the authors performed postoperative computed tomography in two-thirds of the patients and found clinically silent cement embolus in peripheral pulmonary vessels in one-fourth of them [70]. Overall, the literature suggests that both procedures had safe SAE profiles with occasional case reports of symptomatic cement extravasation in the VP arm.
Optimal intervention time
There is controversy regarding the optimal time of intervention, with some authorities recommending early intervention [61, 71] and others suggesting that late augmentation does not compromise outcome [25, 72]. The majority of VP studies that yielded significant pain relief (greater than a 4 point drop) had a mean fracture age less than 7 weeks (see Fig. 8). The most important observation was that in both arms there appears to be a period of substantially greater pain relief (approximately less than 7 weeks), after which results were suboptimal or inconsistent.
Limitations
Unlike previous systematic reviews and meta-analyses [14–19, 50, 73, 74], the current study included only prospective comparative studies. This restriction limited the total number of studies, and therefore the power of the analysis. However, by including only class I and II evidences, our analysis was less prone to bias than retrospective or single-arm studies. Significant heterogeneity in effect sizes and data reporting methods (e.g., RM/ODI, VB height restoration, clinical/subclinical fractures) limited interpretation of treatment differences in many cases and likely represents the developmental nature of the level I and II studies.
The six-point assessment of bias revealed that 50 % of studies had incomplete outcomes, while 68 % had a risk of bias due to lack of randomization. The review used mixed-effect analysis of study treatment effects subgrouped by bias ratings (Table 2). For NSM, pain reduction was affected by randomization (P = 0.001) and incomplete outcome reporting (P = 0.053), while subsequent fracture rate was marginally affected by incomplete outcome reporting (P = 0.073). For VP, disability improvement was marginally affected by randomization (P = 0.059), and significantly affected by incomplete outcome reporting (P < 0.001). The BKP group did not show any detectable effect of bias on outcomes.
These sensitivity analyses have the potential to be highly variable due to the relatively low number of studies contributing to the analyses. Lower quality trials, defined by a higher risk for bias, have been shown to significantly amplify beneficial results [75].
Conclusions
Our analysis indicated that vertebral augmentation was superior to NSM in the treatment of osteoporotic VCFs in terms of reducing pain and subsequent fractures. Balloon kyphoplasty was superior to VP and NSM in terms of QOL. As expected, kyphosis reduction was variable but was superior for BKP than for VP, along with a lower cement extravasation rate for BKP. Surgical interventions on VCFs within the first 7 weeks show evidence of greater pain reduction. The significant heterogeneity of effects, even among randomized trials, indicates that the current class I and II evidences are delivering inconsistent messages.
Acknowledgments
We thank Mohammed Eleraky, MD (Moffitt Cancer Center, Tampa, FL) for validation and verification of the extracted data.
Conflict of interest
Frank M. Phillips, Jan Van Meirhaeghe, James R. Berenson and Frank D. Vrionis are consultants for Medtronic and have received grants and honoraria. Brent J. Small and Gary Chung were paid from Medtronic to conduct an independent statistical analysis.
Glossary
- VCF(s)
Vertebral compression fracture(s)
- BKP
Balloon kyphoplasty
- VP
Vertebroplasty
- VAPs
Vertebral augmentation procedures
- NSM
Non-surgical management
- RCT(s)
Randomized controlled trial(s)
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- ITC
Indirect treatment comparisons
- RMD
Rolland morris disability
- ODI
Oswestry disability index
- QOL
Quality of life
- PCS
Physical component summary
- VAS
Visual analog scale
- SAE(s)
Serious adverse event(s)
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Lyles KW, Gold DT, Shipp KM, Pieper CF, Martinez S, Mulhausen PL. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med. 1993;94(6):595–601. doi: 10.1016/0002-9343(93)90210-G. [DOI] [PubMed] [Google Scholar]
- 3.Kado DM, Duong T, Stone KL, Ensrud KE, Nevitt MC, Greendale GA, Cummings SR. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003;14(7):589–594. doi: 10.1007/s00198-003-1412-5. [DOI] [PubMed] [Google Scholar]
- 4.Borgstrom F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjo K, Thorngren KG, Sernbo I, Rehnberg C, Jonsson B. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17(5):637–650. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 5.Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27–31. doi: 10.1016/8756-3282(92)90193-Z. [DOI] [PubMed] [Google Scholar]
- 6.Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, Ziegler R, Leidig-Bruckner G. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8(3):261–267. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw D, Cummings SR, Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, Eastell R, Shabe P, Talmadge K, Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 9.Voormolen MH, Mali WP, Lohle PN, Fransen H, Lampmann LE, Graaf Y, Juttmann JR, Jansssens X, Verhaar HJ. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28(3):555–560. [PMC free article] [PubMed] [Google Scholar]
- 10.Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, Bastian L, Ashraf T, Vrionis F. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 11.Grafe IA, Fonseca K, Hillmeier J, Meeder PJ, Libicher M, Noldge G, Bardenheuer H, Pyerin W, Basler L, Weiss C, Taylor RS, Nawroth P, Kasperk C. Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int. 2005;16(12):2005–2012. doi: 10.1007/s00198-005-1982-5. [DOI] [PubMed] [Google Scholar]
- 12.Kasperk C, Hillmeier J, Noldge G, Grafe IA, Dafonseca K, Raupp D, Bardenheuer H, Libicher M, Liegibel UM, Sommer U, Hilscher U, Pyerin W, Vetter M, Meinzer HP, Meeder PJ, Taylor RS, Nawroth P. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. 2005;20(4):604–612. doi: 10.1359/JBMR.041203. [DOI] [PubMed] [Google Scholar]
- 13.Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs. 2004;11(Suppl 1):13–15. [Google Scholar]
- 14.Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(12):1749–1758. doi: 10.1007/s00586-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RS, Taylor RJ, Fritzell P (2006) Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (Phila Pa 1976) 31 (23):2747–2755. doi:10.1097/01.brs.0000244639.71656.7d [DOI] [PubMed]
- 16.Bouza C, Lopez T, Magro A, Navalpotro L, Amate JM. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(7):1050–1067. doi: 10.1007/s00586-005-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulme PA, Krebs J, Ferguson SJ, Berlemann U (2006) Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976) 31 (17):1983–2001. doi:10.1097/01.brs.0000229254.89952.6b [DOI] [PubMed]
- 19.Hadjipavlou AG, Tzermiadianos MN, Katonis PG, Szpalski M. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br. 2005;87(12):1595–1604. doi: 10.1302/0301-620X.87B12.16074. [DOI] [PubMed] [Google Scholar]
- 20.Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J (2009) Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila Pa 1976) 34 (13):1349–1354. doi:10.1097/BRS.0b013e3181a4e628 [DOI] [PubMed]
- 21.Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, Edwards R, Gray LA, Stout L, Owen S, Hollingworth W, Ghdoke B, Annesley-Williams DJ, Ralston SH, Jarvik JG. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 23.Bono CM, Heggeness M, Mick C, Resnick D, Watters WC (2010) 3rd North American Spine Society: newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J 10(3):238–240. doi:10.1016/j.spinee.2009.09.007 [DOI] [PubMed]
- 24.Grafe IA, Baier M, Noldge G, Weiss C, Da Fonseca K, Hillmeier J, Libicher M, Rudofsky G, Metzner C, Nawroth P, Meeder PJ, Kasperk C (2008) Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine (Phila Pa 1976) 33(11):1284–1290. doi:10.1097/BRS.0b013e3181714a84 [DOI] [PubMed]
- 25.Kasperk C, Grafe IA, Schmitt S, Noldge G, Weiss C, Da Fonseca K, Hillmeier J, Libicher M, Sommer U, Rudofsky G, Meeder PJ, Nawroth P (2010) Three-year outcomes after kyphoplasty in patients with osteoporosis with painful vertebral fractures. J Vasc Interv Radiol 21(5):701–709. doi:10.1016/j.jvir.2010.01.003 [DOI] [PubMed]
- 26.Rousing R, Hansen KL, Andersen MO, Jespersen SM, Thomsen K, Lauritsen JM (2010) Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine (Phila Pa 1976) 35 (5):478–482. doi:10.1097/BRS.0b013e3181b71bd1 [DOI] [PubMed]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT GS (2009) Cochrane Handbook for systematic reviews of interventions Version 5.0.2. www.cochrane-handbook.org. Accessed May 2010
- 29.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein M. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 31.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 32.Wells G, Sultan S, Chen L, Khan M, Coyle D. Indirect evidence: indirect treatment comparisons in meta-analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 33.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 35.Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. 2. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 36.Borenstein M, Hedges L, Higgins J, Rothstein H (2005) Comprehensive Meta-analysis Version 2
- 37.Wells G, Sultan S, Chen L, Khan M, Coyle D (2009) Indirect treatment comparison [computer program]. Version 1.0. Canadian Agency for Drugs and Technologies in Health, Ottawa
- 38.Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, Johnell O. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12(7):519–528. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 39.Rollinghoff M, Siewe J, Zarghooni K, Sobottke R, Alparslan Y, Eysel P, Delank KS. Effectiveness, security and height restoration on fresh compression fractures–a comparative prospective study of vertebroplasty and kyphoplasty. Minim Invasive Neurosurg. 2009;52(5–6):233–237. doi: 10.1055/s-0029-1243631. [DOI] [PubMed] [Google Scholar]
- 40.Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, Lin TB. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21(2):359–364. doi: 10.1007/s00198-009-0952-8. [DOI] [PubMed] [Google Scholar]
- 41.Klazen CA, Lohle PN, Vries J, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, Rooij WJ, Schoemaker MC, Juttmann JR, Lo TH, Verhaar HJ, Graaf Y, Everdingen KJ, Muller AF, Elgersma OE, Halkema DR, Fransen H, Janssens X, Buskens E, Mali WP. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376:1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 42.Buchbinder R, Kallmes DF. Vertebroplasty: when randomized placebo-controlled trial results clash with common belief. Spine J. 2010;10(3):241–243. doi: 10.1016/j.spinee.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Han S, Wan S, Ning L, Tong Y, Zhang J, Fan S. Percutaneous vertebroplasty versus balloon kyphoplasty for treatment of osteoporotic vertebral compression fracture: a meta-analysis of randomised and non-randomised controlled trials. Int Orthop. 2011 doi: 10.1007/s00264-011-1283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holm I, Friis A, Storheim K, Brox JI (2003) Measuring self-reported functional status and pain in patients with chronic low back pain by postal questionnaires: a reliability study. Spine (Phila Pa 1976) 28(8):828–833 (pii:00007632-200304150-00017) [PubMed]
- 45.Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res. 2005;14(7):1651–1668. doi: 10.1007/s11136-005-1743-0. [DOI] [PubMed] [Google Scholar]
- 46.Carragee EJ, Cheng I. Minimum acceptable outcomes after lumbar spinal fusion. Spine J. 2010;10(4):313–320. doi: 10.1016/j.spinee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Gatchel RJ, Mayer TG. Testing minimal clinically important difference: additional comments and scientific reality testing. Spine J. 2010;10(4):330–332. doi: 10.1016/j.spinee.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan HA, Brown CW, Phillips FM. Osteoporotic spinal deformity: a biomechanical rationale for the clinical consequences and treatment of vertebral body compression fractures. J Spinal Disord Tech. 2004;17(3):236–242. doi: 10.1097/00024720-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Phillips FM, Todd Wetzel F, Lieberman I, Campbell-Hupp M (2002) An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine (Phila Pa 1976) 27 (19):2173–2178. doi:10.1097/01.BRS.0000025689.39941.26 (discussion 2178–2179) [DOI] [PubMed]
- 50.Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K (2009) Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine (Phila Pa 1976) 34 (11):1228–1232. doi:10.1097/BRS.0b013e3181a3c742 [DOI] [PubMed]
- 51.Schmidt R, Cakir B, Mattes T, Wegener M, Puhl W, Richter M. Cement leakage during vertebroplasty: an underestimated problem? Eur Spine J. 2005;14(5):466–473. doi: 10.1007/s00586-004-0839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bohner M, Gasser B, Baroud G, Heini P. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials. 2003;24(16):2721–2730. doi: 10.1016/S0142-9612(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 53.Stoffel M, Wolf I, Ringel F, Stuer C, Urbach H, Meyer B. Treatment of painful osteoporotic compression and burst fractures using kyphoplasty: a prospective observational design. J Neurosurg Spine. 2007;6(4):313–319. doi: 10.3171/spi.2007.6.4.5. [DOI] [PubMed] [Google Scholar]
- 54.Ryu KS, Park CK, Kim MC, Kang JK. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg. 2002;96(1 Suppl):56–61. doi: 10.3171/spi.2002.96.1.0056. [DOI] [PubMed] [Google Scholar]
- 55.Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, Chastanet P, Clarisse J. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200(2):525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 56.Molloy S, Mathis JM, Belkoff SM (2003) The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine (Phila Pa 1976) 28 (14):1549–1554 (pii: 00007632-200307150-00014) [PubMed]
- 57.Liebschner MA, Rosenberg WS, Keaveny TM (2001) Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine (Phila Pa 1976) 26(14):1547–1554 [DOI] [PubMed]
- 58.Alvarez L, Alcaraz M, Perez-Higueras A, Granizo JJ, de Miguel I, Rossi RE, Quinones D (2006) Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine (Phila Pa 1976) 31(10):1113–1118. doi:10.1097/01.brs.0000216487.97965.38 [DOI] [PubMed]
- 59.Negri P, Tirri T, Paternoster G, Modano P. Treatment of painful osteoporotic or traumatic vertebral compression fractures by percutaneous vertebral augmentation procedures: a nonrandomized comparison between vertebroplasty and kyphoplasty. Clin J Pain. 2007;23(5):425–430. doi: 10.1097/AJP.0b013e31805593be. [DOI] [PubMed] [Google Scholar]
- 60.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F (2005) The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 30(18):2024–2029 (pii: 00007632-200509150-00005) [DOI] [PubMed]
- 61.Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomized study. Eur Spine J. 2004;13(6):496–501. doi: 10.1007/s00586-004-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovi A, Teli M, Ortolina A, Costa F, Fornari M, Brayda-Bruno M (2009) Vertebroplasty and kyphoplasty: complementary techniques for the treatment of painful osteoporotic vertebral compression fractures. A prospective non-randomised study on 154 patients. Eur Spine J 18 Suppl 1:95–101. doi:10.1007/s00586-009-0986-9 [DOI] [PMC free article] [PubMed]
- 63.Dong R, Chen L, Gu Y, Han G, Yang H, Tang T, Xiaoqing C. Improvement in respiratory function after vertebroplasty and kyphoplasty. Int Orthop. 2009;33(6):1689–1694. doi: 10.1007/s00264-008-0680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech. 2005;18(3):238–242. [PubMed] [Google Scholar]
- 65.Pflugmacher R, Kandziora F, Schroder R, Schleicher P, Scholz M, Schnake K, Haas N, Khodadadyan-Klostermann C. Vertebroplasty and kyphoplasty in osteoporotic fractures of vertebral bodies—a prospective 1-year follow-up analysis. Rofo. 2005;177(12):1670–1676. doi: 10.1055/s-2005-858631. [DOI] [PubMed] [Google Scholar]
- 66.Pflugmacher R, Taylor R, Agarwal A, Melcher I, Disch A, Haas NP, Klostermann C. Balloon kyphoplasty in the treatment of metastatic disease of the spine: a 2-year prospective evaluation. Eur Spine J. 2008;17(8):1042–1048. doi: 10.1007/s00586-008-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santiago FR, Abela AP, Alvarez LG, Osuna RM, Garcia MD. Pain and functional outcome after vertebroplasty and kyphoplasty. A comparative study. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Schofer MD, Efe T, Timmesfeld N, Kortmann HR, Quante M. Comparison of kyphoplasty and vertebroplasty in the treatment of fresh vertebral compression fractures. Arch Orthop Trauma Surg. 2009;129(10):1391–1399. doi: 10.1007/s00402-009-0901-1. [DOI] [PubMed] [Google Scholar]
- 69.Bae H, Shen M, Maurer P, Peppelman W, Beutler W, Linovitz R, Westerlund E, Peppers T, Lieberman I, Kim C, Girardi F (2010) Clinical experience using Cortoss for treating vertebral compression fractures with vertebroplasty and kyphoplasty: twenty four-month follow-up. Spine (Phila Pa 1976) 35(20):E1030–1036. doi:10.1097/BRS.0b013e3181dcda75 [DOI] [PubMed]
- 70.Venmans A, Klazen CA, Lohle PN, van Rooij WJ, Verhaar HJ, de Vries J, Mali WP (2010) Percutaneous vertebroplasty and pulmonary cement embolism: results from VERTOS II. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A2127 [DOI] [PMC free article] [PubMed]
- 71.Phillips FM, Ho E, Campbell-Hupp M, McNally T, Todd Wetzel F, Gupta P (2003) Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 28(19):2260–2265. doi:10.1097/01.BRS.0000085092.84097.7B (discussion 2265–2267) [DOI] [PubMed]
- 72.Crandall D, Slaughter D, Hankins PJ, Moore C, Jerman J. Acute versus chronic vertebral compression fractures treated with kyphoplasty: early results. Spine J. 2004;4(4):418–424. doi: 10.1016/j.spinee.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488–497. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Gill JB, Kuper M, Chin PC, Zhang Y, Schutt R., Jr Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007;10(4):583–590. [PubMed] [Google Scholar]
- 75.Moher D, Pham B, Jones A, Cook D, Jadad A, Moher M, Tugwell P, Klassen T. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? The Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 76.Diamond TH, Bryant C, Browne L, Clark WA. Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust. 2006;184(3):113–117. doi: 10.5694/j.1326-5377.2006.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 77.Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg. 2010;130(9):1157–1166. doi: 10.1007/s00402-010-1106-3. [DOI] [PubMed] [Google Scholar]
- 78.Kumar K, Nguyen R, Bishop S (2010) A comparative analysis of the results of vertebroplasty and kyphoplasty in osteoporotic vertebral compression fractures. Neurosurgery 67(3 Suppl Operative):on171–188 doi:10.1227/01.NEU.0000380936.00143.11 (discussion ons188) [DOI] [PubMed]