Abstract
Introduction
Vertebral deformities often occur in patients who recall no trauma, and display no evident fracture on radiographs. We hypothesise that vertebral deformity can occur by a gradual creep mechanism which is accelerated following minor damage. “Creep” is continuous deformation under constant load.
Materials and methods
Forty-five thoracolumbar spine motion segments were tested from cadavers aged 42–92 years. Vertebral body areal BMD was measured using DXA. Specimens were compressed at 1 kN for 30 min, while creep in each vertebral body was measured using an optical MacReflex system. After 30 min recovery, each specimen was subjected to a controlled overload event which caused minor damage to one of its vertebrae. The creep test was then repeated.
Results
Vertebral body creep was measurable in specimens with BMD <0.5 g/cm2. Creep was greater anteriorly than posteriorly (p < 0.001), so that vertebrae gradually developed a wedge deformity. Compressive overload reduced specimen height by 2.24 mm (STD 0.77 mm), and increased vertebral body creep by 800 % (anteriorly), 1,000 % (centrally) and 600 % (posteriorly). In 34 vertebrae with complete before-and-after data, anterior wedging occurring during the 1st creep test averaged 0.07° (STD 0.17°), and in the 2nd test (after minor damage) it averaged 0.79° (STD 1.03°). The increase was highly significant (P < 0.001). Vertebral body wedging during the 2nd creep test was proportional to the severity of damage, as quantified by specimen height loss during the overload event (r2 = 0.51, p < 0.001, n = 34).
Conclusions
Minor damage to an old vertebral body, even if it is barely discernible on radiographs, can accelerate creep to such an extent that it makes a substantial contribution to vertebral deformity.
Keywords: Vertebral deformity, Fracture, Creep, BMD, Cadaveric, Damage
Introduction
Vertebral fracture is an important cause of pain and disability in elderly people, [1, 2] and its impact is increasing in our ageing society. Visual assessment of vertebral fracture on plain radiographs is notoriously subjective [3] so semi-quantitative and quantitative schemes have been developed to measure vertebral body deformity as a more reproducible indicator of fracture [1, 2, 4, 5].
Several types of vertebral deformity can be distinguished, [6] but the most common involves disproportionate height loss from the anterior vertebral body [5]. Vertebral bodies become wedged anteriorly, thereby increasing thoracic kyphosis [7] and decreasing lumbar lordosis. The resulting loss of self esteem arising from an exaggerated kyphosis can rival more medically related aspects of the problem, and many elderly people seek expensive ‘cement augmentation’ therapies to halt their progressive deformity [8].
Vertebral deformity can often be found in individuals who do not recollect any incident that could have resulted in a vertebral fracture, so that the deformity does not come to medical attention [4, 9]. This suggests that vertebrae may possibly deform as a result of gradual time-dependent processes as well as from injuries [10].
Bone has long been known to display time-dependent ‘visco-elastic’ materials properties when subjected to sustained or repetitive loading [11, 12]. Mechanical experiments on small bone samples have reported residual deformation after such loading [10, 13] even though the bone samples were macroscopically undamaged. Following on from this work, we used a sensitive optical technique to measure deformations of whole cadaveric vertebrae that were subjected to physiological loading for periods of up to 2 h. Results showed that many vertebral bodies did indeed deform in a slow continuous manner under a constant compressive load, and that these “creep" deformations were incompletely reversed when loading was removed [14, 15]. The phenomenon was extremely variable, but the anterior vertebral body consistently lost more height than the posterior, so that vertebral bodies developed an anterior wedge angulation which averaged 0.08°. Although these experiments were performed on dead tissues at 21°C, they established the principle that vertebral deformity can be created by chronic loading as well as by injury. The mechanisms of bone creep are obscure, but may possibly involve fluid flow, or slipping between structural elements [14].
The purpose of the present experiment is to explain why some vertebrae “creep” so much more than others. Our previous work showed that creep was greater in old specimens with low bone mineral density (BMD), and was particularly high in four vertebrae with radiographic evidence that suggested minor pre-existing bone damage [14]. We now hypothesise that minor damage to a vertebral body, which may be insufficient to be recognized as a “fracture” on radiographs, may nevertheless accelerate bone creep so that it leads to substantial vertebral deformity.
Methods
Cadaveric material
Human thoraco-lumbar spines were removed within 72 h of death from 26 cadavers (15 males and 11 females). Death was unrelated to any condition known to affect bone metabolism, and none of the subjects had experienced prolonged bed rest prior to death. Spines were dissected into 45 specimens consisting of two vertebrae (19 specimens) or three vertebrae (26 specimens), including the intervertebral disc(s) and ligaments. (In two-vertebra specimens, each vertebra is loaded naturally on one side by an intervertebral disc, whereas both sides of the central vertebra are loaded naturally in a three-vertebra specimen, and it was of interest to gauge the effect of these influences on vertebral creep.) After dissection, all specimens were stored at −20 °C. Average specimen age was 75 years (range 42–92 years). All spinal levels from T7 to L4 were included. Grade of disc degeneration was assessed from macroscopic features evident at dissection after testing, using the first four points of a scale of 1–5 which emphasizes tissue integrity [16, 17]. Before mechanical testing, the areal BMD of each vertebral body was measured in the sagittal plane using dual-energy X-ray absorptiometry (DXA).
Mechanical testing apparatus
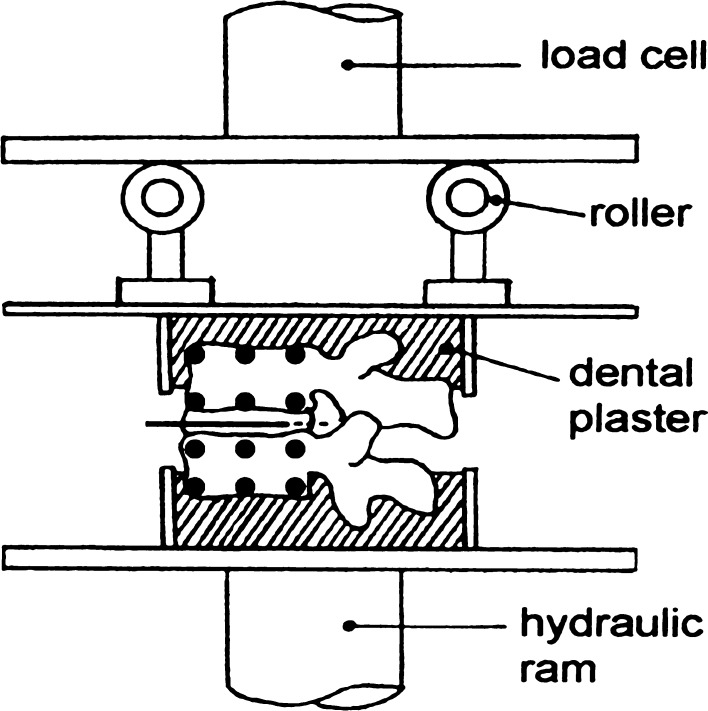
Each motion segment was secured in two cups of dental plaster (Fig. 1) in such a manner that compressive loading could be applied evenly to its outer surfaces by a hydraulic materials testing machine [14]. Two low-friction rollers of variable height allowed compression to be applied to a specimen maintained at some constant angle of flexion or extension. An initial period of compressive creep loading (300 N for 15 min) was applied in order to reduce post-mortem disc hydration to typical physiological levels [18]. All experiments were performed at room temperature, with polyethylene film wrapped around the specimen to keep its surface wet.
Fig. 1.
A two-vertebra specimen subjected to compressive loading by a hydraulic materials testing machine (anterior on left). The height of each roller could be adjusted to allow the specimen to be loaded in pure compression (as shown) or in flexion (height of the anterior roller increased). Black circles indicate the six reflective markers attached to each vertebral body. (A photograph of such a specimen was published previously [14].)
Creep loading
Each specimen was positioned in 0° of flexion, and subjected to a constant compressive load of 1 kN for 30 min. This force is appropriate to simulate moderate activity in upright postures [19]. (Applied force was not normalised for endplate area because area is not the most important determinant of vertebral strength [20].) Four specimens with particularly low BMD were tested at 0.5 kN to avoid premature damage. In all cases, the applied load increased linearly over 5 s. ‘Elastic deformation’ was defined as all deformation recorded during load application (5 s) and in the first 2 s after load application. ‘Creep deformation’ was measured as the continuing deformation during the following 30 min of static loading. Specimens were allowed to recover for a further 30 min under a nominal load of 3 N. Some limited height recovery after creep was noted in our previous experiment [14], but recovery was not measured in the present experiment because there is evidence that full recovery would take many hours [10].
Measuring bone deformations
Vertebral body deformations in the sagittal plane were monitored using an optical 2D MacReflex system (Qualisys Ltd., Goteborg, Sweden), which located the centres of six reflective markers glued to map pins inserted into the lateral cortex of each vertebral body (Fig. 1). Resolution was better than 10 μm [21, 22]. (These pins may possibly have caused some minor damage to trabeculae, but any such damage would have been the same before-and-after the overload event and so would not influence the ‘before vs. after’ results.) Two markers each defined the anterior, middle and posterior heights of each of the 116 vertebral bodies tested, and deformations were expressed as a proportion of initial vertebral height at zero load. During creep, deformations were measured at 1 Hz. Measuring vertebral deformation in a single (sagittal) plane increases the precision of this system, as discussed previously [14]. Digital smoothing (30-point moving-average) was used to reduce random noise. Bone strain was measured as the % change in the vertical distance between each pair of reflective markers [14].
Preliminary tests on a porcine vertebra compared MacReflex measurements of vertebral body height loss with height loss measured by the linear variable displacement transducer (LVDT) of the materials testing machine. LVDTs are highly sensitive and accurate, and so can be used to calibrate the optical MacReflex measurements.
Inducing vertebral damage
By increasing the height of the front roller (Fig. 1), each specimen was positioned in moderate (6–8°) flexion to simulate a typical stooped posture, and then compressed at 3 mm/s to simulate back muscle forces during manual labour [23]. A force–deformation graph was plotted in “real time”, with deformation being measured by the LVDT mounted on the actuator of the materials testing machine, and force being measured by the machine’s load cell (Fig. 1). Loading was removed as soon as the ‘yield point’ was reached, as indicated by a distinct reduction in stiffness (gradient of the force–deformation graph). Previous work has shown that minor compressive damage induced in this manner affects only one vertebra [4], and this was confirmed in the present experiment by dissection, and by close comparison of before-and-after radiographs taken in the sagittal and frontal planes. Damage severity was quantified as the change in specimen height after damage, measured while the specimen was subjected to a reference compressive force of 1 kN.
Creep loading after damage
The creep loading test was repeated on all specimens, using the same load as in the first creep test.
Statistical analysis
A repeated measures mixed ANOVA was used to detect differences in pre-damage creep strains between the three different regions of the vertebral body (within-subject factor), and between gender and degree of disc degeneration (between-subject factors). A repeated measures ANOVA was used to detect differences in strains before and after damage. Linear regression was used to examine the influence of age and BMD.
Results
Accuracy of the MacReflex
Vertebral height loss in the anterior cortex of the pig vertebra, as measured by the optical MacReflex system, was linearly related to height loss measured by the Dartec LVDT (r2 = 0.94). The gradient of the relationship (0.984) was close to 1, indicating that MacReflex measurements were accurate as well as linear.
Creep in undamaged vertebrae
Loss of signal from one or more reflective markers meant that complete creep data could not be obtained from 10 vertebral bodies before damage, and 11 vertebral bodies after damage. Usually this was caused by blood staining, or physical disturbance of a reflective marker during testing.
Typical creep curves (Fig. 2) were analysed in order to measure the initial elastic deformation, and the total creep deformation occurring over 30 min. Group t tests showed no significant differences (P > 0.05) between results from vertebrae in two- or three-vertebra specimens, so results were pooled for all vertebrae. Baseline data from specimens before damage (Fig. 3a) showed that creep deformations were comparable in size to the initial elastic deformations, and both were greater anteriorly than posteriorly (p < 0.01). Greater anterior creep caused the wedging of vertebral bodies to increase during the first creep test by 0.06° (STD 0.18°, n = 106, p < 0.001). Small angles of vertebral deformation can easily be accommodated under load (Fig. 1) by complementary deformations of the adjacent discs, which are much more compliant.
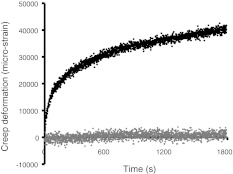
Fig. 2.
Typical ‘creep’ curves for the anterior region of an L3 vertebral body (male, 89 years) during a 30-min test. Initial elastic deformations have been omitted to enhance the contrast between the very slight creep of the undamaged vertebra (lower graph) and the much greater creep that follows vertebral microdamage (upper graph). Note that creep appears to be a continuous process, even in the damaged vertebra, and that the rate of creep (gradient of the graphs) falls steadily with time. Data are shown before smoothing
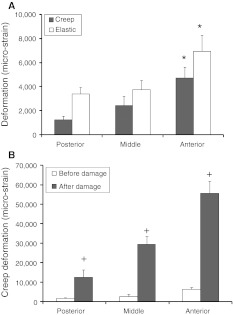
Fig. 3.
a Before damage, creep deformations were comparable in size to initial elastic deformations, and both types of deformation increased from posterior to anterior. *anterior > posterior (p < 0.01). b Creep deformations increased greatly after minor vertebral damage, especially in the anterior vertebral body. Plus symbol indicates after damage > before damage (p < 0.001). Error bars indicate the SEM. 10,000 microstrain = 1 % height loss
Creep in undamaged vertebrae was not influenced significantly by age, gender, or grade of disc degeneration (possibly because most specimens were old and degenerated), but it did depend on BMD. Areal BMD of all 116 vertebral bodies, measured in the sagittal plane, averaged 0.58 (STD 0.28) g/cm2. BMD had little influence on creep deformation in male specimens (p > 0.05), but in female specimens, decreasing BMD was associated with increasing creep of the anterior cortex, and with increasing anterior wedging, during the creep test (Fig. 4). These influences were significant in female specimens with BMD <0.5 g/cm2. The ratio of creep/elastic deformation (all specimens) was significantly larger in the anterior vertebral body compared to the posterior (p = 0.032) suggesting that the anterior region is not only more deformable but also particularly susceptible to creep.
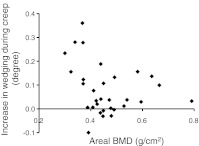
Fig. 4.
Anterior wedging of undamaged female vertebral bodies during the first 30 min creep test increased as BMD decreased, in the range BMD <0.5 g/cm2. (r2 = 0.33, p < 0.01, n = 24). This relationship was insignificant for male specimens
Vertebral damage
The mean compressive force at the yield point was 2.73 kN (range: 1.20–5.50 kN). Usually a single cranial endplate was depressed, either centrally or anteriorly, with disruption to adjacent trabeculae. Specimen height (vertebrae plus discs) was reduced by an average 2.24 mm (range 1.09–4.72 mm) after damage. Radiographic appearances of damage resulting from this testing protocol are shown in Fig. 5. Such images have previously been considered by an experienced radiologist [4] who reported that 40 % of (different) specimens tested according to the same protocol showed no discernible fracture on the after-damage radiographs. In the present experiment, specimens revealed slight signs of damage only if the before-and-after radiographs were compared carefully (Fig. 5). Hence, we use the term ‘minor’ damage to characterise the effect of the controlled mechanical overload.
Fig. 5.
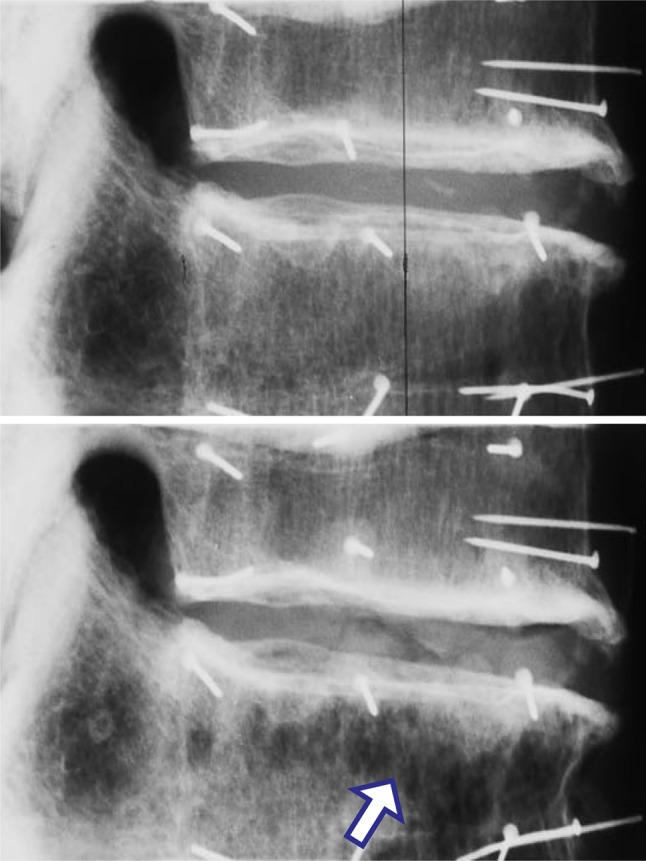
Lateral radiographs of a two-vertebra specimen, before (upper) and after (lower) minor compressive damage (male 66 years, T10-11, anterior on the right). In the lower radiograph, note the disturbed anterior trabeculae (arrow). Damage reduced the height of this specimen by 2.29 mm. Pins were inserted into the vertebrae for the attachment of reflective markers
Effect of minor damage on vertebral body creep
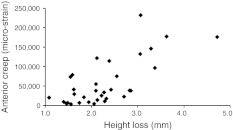
Creep deformation increased greatly after damage, especially in the anterior and middle regions of the vertebral body (Fig. 3b). On average, creep deformations of the anterior, middle and posterior vertebral body increased by 800, 1,000, and 600 % respectively (all p < 0.001). Rounded numbers are quoted because the small before-damage measurements of strain were influenced by random errors, which were then magnified when % increases were calculated. In those 34 specimens (of both genders) for which there was complete before-and-after creep data from all 6 reflective markers, anterior wedging during the 30 min creep test increased by approximately 1,000 %, from 0.07° (STD 0.17°) before damage, to 0.79° (STD 1.03°) after damage (p < 0.001). Creep deformation of the anterior cortex increased in proportion to the severity of damage (Fig. 6), as measured by specimen height loss immediately after the overload event (r2 = 0.51, p < 0.001, n = 34). Regional creep strains could exceed 200,000 micro-strains (i.e. 20 % deformation) in the most damaged specimens (Fig. 6).
Fig. 6.
Creep deformation of the anterior vertebral body increased in proportion to the severity of damage, as measured by specimen height loss during the overloading event (r2 = 0.51, p < 0.001, n = 34). 10,000 microstrain = 1 % height loss
Discussion
Summary of findings
Minor vertebral body damage that could barely be detected on radiographs was sufficient to increase continuous “creep” deformations of old human vertebrae by 600–1,000 %. Creep was greatest in the anterior vertebral body (where trabecular density is least [24, 25]) and where most damage occurred during compressive overload. After damage, 30 min of moderate sustained loading increased vertebral body wedging by an average 0.79°, which is similar to the average 0.95° wedging caused by the injury itself, and reported previously for this in vitro compressive injury model [26]. Note that creep is a slow continuous process, even in the damaged vertebra (Fig. 2), and should not be confused with repeated injuries. These results therefore support our hypothesis: they indicate that minor damage to a vertebral body accelerates bone creep to the extent that it can make a substantial contribution to vertebral deformity.
Strengths and weaknesses of the study
A strength is that elderly human vertebrae were used, and were loaded in a physiological manner by means of their adjacent intervertebral discs. Similar creep results were obtained from two- and three-vertebra specimens, suggesting that vertebral body deformations in two-vertebra specimens are not greatly influenced by the adjacent dental plaster. Comparing creep before and after damage minimized the influence of inter-specimen factors such as age, genetic inheritance, and BMD. Another strength is the sensitivity and accuracy of the optical strain-measuring system, which enabled strains to be compared in different regions of the vertebral body.
The main weakness of the study is the use of dead tissue, tested at laboratory temperature. As discussed previously [14], death and frozen storage would have little influence on the results, although creep would be expected to increase at body temperature [27, 28]. Creep in small samples of bone recovers substantially if the tissue is unloaded for a long period [10], and approximately 40 % of the creep in whole human vertebrae during a 30-min test was reversed after 2 h [14]. Another confounding factor is that the rate of creep decreases with time (Fig. 2) to such an extent that creep measured during 2 h is not much greater than that measured during 30 min [14]. Therefore, the present study cannot specify how rapidly vertebral body wedging would accumulate in a living spine: that would depend on the relative duration of loading and unloading, as well as on temperature and BMD. The present results merely show that creep processes in old human vertebrae increase substantially following minor damage, so that the anterior wedging deformity during creep is comparable to that which is caused by injury.
Relationship to other studies
The present results are compatible with previous measurements of creep in human [14] and animal [29] vertebrae, although the latter study appeared not to distinguish between creep in the bone and cartilage endplates. Present results are also compatible with measurements of creep and residual deformation in trabecular bone samples [10, 30]. Our previous work showed that old human vertebrae creep as much as their adjacent intervertebral discs, and deform elastically by a similar amount [15]. Evidently, viewing the elderly human spine as a segmented column of rigid vertebrae separated by deformable discs is an oversimplification.
Explanation of results
The origins of bone creep are obscure. Creep in soft tissues such as articular cartilage and intervertebral discs is mostly due to fluid expulsion [18], and can be reversed after loading by the osmotic pressure of proteoglycans sucking the water back in again [31]. Fluid flow within canaliculi [32] may play a similar role in bone creep, with the slowness of the process being determined by the small diameter/length ratio of canaliculi. However, the fact that some bone creep is either permanent, or recovered very slowly [14], suggests additional mechanisms unrelated to fluid flow. Possibilities include the propagation of microcracks [33–35], slipping between osteons [36], viscoelastic deformation of collagen [27], and slipping between [28, 37] or within [28] hydroxyapatite crystals. Plastic deformation must be involved, because total strains (creep plus elastic) measured in the present experiment greatly exceed those at the elastic limit of trabecular bone [30, 38].
The results of the present experiment give some additional insights into the creep mechanism, particularly when creep is compared in the anterior and posterior regions of the vertebral body. The ratio of creep to elastic deformation increases anteriorly (Fig. 3a), indicating that extra creep anteriorly is not simply due to the applied loading being concentrated in this region by a degenerated intervertebral disc [25, 39]. The anterior bone must be intrinsically more susceptible to creep. Trabecular spacing is greatest anteriorly [25] and may have been further increased by the overload event which created most damage anteriorly (Fig. 5). This suggests that creep is accelerated by the bending of relatively isolated and unsupported trabeculae. Modelling studies predict that bone strain rises to particularly high levels in those regions of individual trabeculae that are deformed most in bending [37]. In these regions, local strains may exceed the elastic limit so that plastic deformation occurs, by one or more of the mechanisms suggested above. According to this view, vertebral bodies creep because the least supported trabeculae within them undergo progressive plastic deformations, reducing support for the cortical shell, which then slowly (and perhaps irreversibly) deforms. Abnormal load-sharing in degenerated spines probably explains an initial loss of trabeculae from the anterior vertebral body [25] which is then vulnerable to microdamage and to accelerated creep deformations, leading to anterior wedging and spinal deformity. Once initiated, spinal kyphosis is likely to progress because of the increased back muscle forces required to balance the forward bending moment arising from anterior displacement of the upper body [40].
Clinical relevance
Vertebral deformity in patients with osteoporosis can be caused by fracture, or by a continuous creep process, or by a combination of both whereby one or more minor injuries serve to accelerate the creep process. We suggest that a combined aetiology is most likely, because minor vertebral injuries are naturally more likely to occur than major ones. We speculate that each minor injury would be followed by accelerated creep for a period of days or weeks, before cell-mediated healing processes would (partially) restore the bone’s mechanical properties [41]. Vulnerable patients should perhaps be advised to minimize the time they spend in a stooped posture, because spinal flexion concentrates loading on to the anterior vertebral body, especially when the intervertebral discs are degenerated [42].
Unanswered questions and future research
We are currently investigating how temperature increases vertebral creep (and creep recovery) in vitro, and how cement augmentation techniques [26] can diminish it. Longitudinal studies on osteoporotic patients are required to establish the contribution of creep to the deformity of living bones.
Acknowledgments
This study was funded in the UK by Action Medical Research and the Annett Charitable Trust.
Conflict of interest
None of the authors has any potential conflict of interest.
Ethics approval
This study was approved by Frenchay NHS Research Ethics Committee, Bristol, UK
Contributor Information
Jin Luo, Email: Jin.Luo@roehampton.ac.uk.
Phillip Pollintine, Email: Phill.Pollintine@bris.ac.uk.
Edward Gomm, Email: edwardgomm@mac.com.
Patricia Dolan, Email: Trish.Dolan@bris.ac.uk.
Michael A. Adams, Phone: +44-117-9288363, FAX: +44-117-9254794, Email: M.A.Adams@bris.ac.uk
References
- 1.Burger H, Daele PL, Grashuis K, Hofman A, Grobbee DE, et al. Vertebral deformities and functional impairment in men and women. J Bone Miner Res. 1997;12:152–157. doi: 10.1359/jbmr.1997.12.1.152. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger B, Black DM, Nevitt MC, Rundle AC, Cauley JA, et al. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7:449–456. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 3.Grados F, Fechtenbaum J, Flipon E, Kolta S, Roux C, et al. Radiographic methods for evaluating osteoporotic vertebral fractures. Jt Bone Spine. 2009;76:241–247. doi: 10.1016/j.jbspin.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Jiang G, Luo J, Pollintine P, Dolan P, Adams MA, et al. Vertebral fractures in the elderly may not always be “osteoporotic”. Bone. 2010;47:111–116. doi: 10.1016/j.bone.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, European Vertebral Osteoporosis Study Group et al. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. Osteoporos Int. 1999;9:206–213. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 6.Rao RD, Singrakhia MD. Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty in its management. J Bone Jt Surg Am. 2003;85-A:2010–2022. [PubMed] [Google Scholar]
- 7.Cortet B, Roches E, Logier R, Houvenagel E, Gaydier-Souquieres G, et al. Evaluation of spinal curvatures after a recent osteoporotic vertebral fracture. Jt Bone Spine. 2002;69:201–208. doi: 10.1016/S1297-319X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein JN. Balancing science and informed choice in decisions about vertebroplasty. N Engl J Med. 2009;361:619–621. doi: 10.1056/NEJMe0905889. [DOI] [PubMed] [Google Scholar]
- 9.Jager PL, Jonkman S, Koolhaas W, Stiekema A, Wolffenbuttel BH et al (2011) Combined vertebral fracture assessment and bone mineral density measurement: a new standard in the diagnosis of osteoporosis in academic populations. Osteoporos Int 22(4):1059–1068 [DOI] [PMC free article] [PubMed]
- 10.Yamamoto E, Paul Crawford R, Chan DD, Keaveny TM. Development of residual strains in human vertebral trabecular bone after prolonged static and cyclic loading at low load levels. J Biomech. 2006;39:1812–1818. doi: 10.1016/j.jbiomech.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Currey JD. Anelasticity in bone and echinoderm skeletons. J Exp Biol. 1965;43:279–292. [Google Scholar]
- 12.Zilch H, Rohlmann A, Bergmann G, Kolbel R. Material properties of femoral cancellous bone in axial loading. Part II: Time dependent properties. Arch Orthop Trauma Surg. 1980;97:257–262. doi: 10.1007/BF00380706. [DOI] [PubMed] [Google Scholar]
- 13.Burgers TA, Lakes RS, Garcia-Rodriguez S, Piller GR, Ploeg HL. Post-yield relaxation behavior of bovine cancellous bone. J Biomech. 2009;42:2728–2733. doi: 10.1016/j.jbiomech.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Pollintine P, Luo J, Offa-Jones B, Dolan P, Adams MA. Bone creep can cause progressive vertebral deformity. Bone. 2009;45:466–472. doi: 10.1016/j.bone.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Pollintine P, Tunen MS, Luo J, Brown MD, Dolan P, et al. Time-dependent compressive deformation of the ageing spine: relevance to spinal stenosis. Spine (Phila Pa 1976) 2010;35:386–394. doi: 10.1097/BRS.0b013e3181b0ef26. [DOI] [PubMed] [Google Scholar]
- 16.Adams MA, Dolan P, Hutton WC. The stages of disc degeneration as revealed by discograms. J Bone Jt Surg [Br] 1986;68:36–41. doi: 10.1302/0301-620X.68B1.3941139. [DOI] [PubMed] [Google Scholar]
- 17.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 18.McMillan DW, Garbutt G, Adams MA. Effect of sustained loading on the water content of intervertebral discs: implications for disc metabolism. Ann Rheum Dis. 1996;55:880–887. doi: 10.1136/ard.55.12.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;24:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Brinckmann P, Biggemann M, Hilweg D. Prediction of the compressive strength of human lumbar vertebrae. Clin Biomech. 1989;4(Suppl 2):S1–S27. doi: 10.1016/0268-0033(89)90071-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhao F, Pollintine P, Hole BD, Dolan P, Adams MA. Discogenic origins of spinal instability. Spine. 2005;30:2621–2630. doi: 10.1097/01.brs.0000188203.71182.c0. [DOI] [PubMed] [Google Scholar]
- 22.Green TP, Allvey JC, Adams MA. Spondylolysis. Bending of the inferior articular processes of lumbar vertebrae during simulated spinal movements. Spine. 1994;19:2683–2691. [PubMed] [Google Scholar]
- 23.Dolan P, Earley M, Adams MA. Bending and compressive stresses acting on the lumbar spine during lifting activities. J Biomech. 1994;27:1237–1248. doi: 10.1016/0021-9290(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhao FD, Pollintine P, Hole BD, Adams MA, Dolan P. Vertebral fractures usually affect the cranial endplate because it is thinner and supported by less-dense trabecular bone. Bone. 2009;44:372–379. doi: 10.1016/j.bone.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 25.Adams MA, Pollintine P, Tobias JH, Wakley GK, Dolan P. Intervertebral disc degeneration can predispose to anterior vertebral fractures in the thoracolumbar spine. J Bone Miner Res. 2006;21:1409–1416. doi: 10.1359/jbmr.060609. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Bertram W, Sangar D, Adams MA, Annesley-Williams DJ, et al. Is kyphoplasty better than vertebroplasty in restoring normal mechanical function to an injured spine? Bone. 2010;46:1050–1057. doi: 10.1016/j.bone.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Bowman SM, Gibson LJ, Hayes WC, McMahon TA. Results from demineralized bone creep tests suggest that collagen is responsible for the creep behavior of bone. J Biomech Eng. 1999;121:253–258. doi: 10.1115/1.2835112. [DOI] [PubMed] [Google Scholar]
- 28.Rimnac CM, Petko AA, Santner TJ, Wright TM. The effect of temperature, stress and microstructure on the creep of compact bovine bone. J Biomech. 1993;26:219–228. doi: 10.1016/0021-9290(93)90360-Q. [DOI] [PubMed] [Google Scholar]
- 29.Veen AJ, Mullender MG, Kingma I, Van Deen JH, Smit TH. Contribution of vertebral bodies, endplates, and intervertebral discs to the compression creep of spinal motion segments. J Biomech. 2008;41:1260–1268. doi: 10.1016/j.jbiomech.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Bowman SM, Keaveny TM, Gibson LJ, Hayes WC, McMahon TA. Compressive creep behavior of bovine trabecular bone. J Biomech. 1994;27:301–310. doi: 10.1016/0021-9290(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 31.Veen AJ, Dieen JH, Nadort A, Stam B, Smit TH. Intervertebral disc recovery after dynamic or static loading in vitro: Is there a role for the endplate? J Biomech. 2007;40:2230–2235. doi: 10.1016/j.jbiomech.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39:1735–1743. doi: 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zioupos P, Hansen U, Currey JD. Microcracking damage and the fracture process in relation to strain rate in human cortical bone tensile failure. J Biomech. 2008;41:2932–2939. doi: 10.1016/j.jbiomech.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Norman TL, Little TM, Yeni YN. Age-related changes in porosity and mineralization and in-service damage accumulation. J Biomech. 2008;41:2868–2873. doi: 10.1016/j.jbiomech.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Fleck C, Eifler D. Deformation behaviour and damage accumulation of cortical bone specimens from the equine tibia under cyclic loading. J Biomech. 2003;36:179–189. doi: 10.1016/S0021-9290(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 36.Caler WE, Carter DR. Bone creep-fatigue damage accumulation. J Biomech. 1989;22:625–635. doi: 10.1016/0021-9290(89)90013-4. [DOI] [PubMed] [Google Scholar]
- 37.Mercer C, He MY, Wang R, Evans AG. Mechanisms governing the inelastic deformation of cortical bone and application to trabecular bone. Acta Biomater. 2006;2:59–68. doi: 10.1016/j.actbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Morgan EF, Bayraktar HH, Yeh OC, Majumdar S, Burghardt A, et al. Contribution of inter-site variations in architecture to trabecular bone apparent yield strains. J Biomech. 2004;37:1413–1420. doi: 10.1016/j.jbiomech.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Jt Surg Br. 1996;78:965–972. doi: 10.1302/0301-620X78B6.1287. [DOI] [PubMed] [Google Scholar]
- 40.Briggs AM, Wrigley TV, Dieen JH, Phillips B, Lo SK, et al. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006;15:1785–1795. doi: 10.1007/s00586-006-0158-0. [DOI] [PubMed] [Google Scholar]
- 41.Lynch JA, Silva MJ. In vivo static creep loading of the rat forelimb reduces ulnar structural properties at time-zero and induces damage-dependent woven bone formation. Bone. 2008;42:942–949. doi: 10.1016/j.bone.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams MA, McNally DS, Chinn H, Dolan P. Posture and the compressive strength of the lumbar spine. Clin Biomech. 1994;9:5–14. doi: 10.1016/0268-0033(94)90052-3. [DOI] [PubMed] [Google Scholar]