Abstract
Background
The life span of cancer patients has improved due to advancements in cancer management. With long survival periods, more patients show metastatic disease. Osteolytic tumours of spine are generated by metastatic deposits or primary tumours of the spine. A prospective study was performed to evaluate the efficacy and safety of percutaneous kyphoplasty in patients with osteolytic tumours of the thoracic and lumbar spine.
Materials and methods
Eleven patients (age range 52–77/average 65 years; 7 female, 4 male) with osteolytic tumours of the spine were treated with kyphoplasty. The main Tokuhashi score was registered preoperatively. Outcome was assessed prospectively by visual analogue scale (VAS) for pain, ECOG performance status, walking distance, standing and sitting time.
Results
Preoperative VAS (average 7.5; range 2.6–10) dropped to 3.0, 5 days postoperatively and remained below 5 for follow-up. Main Tokuhashi score was 6.3, ranging from 3 to 9. Survival time ranged from 2 to 293 (average 74.4) weeks. Average walking distance, standing and sitting time and ECOG performance score showed improvement. All patients returned home and no patient required re-operation or readmission due to local disease progression or recurrence.
Conclusion
Kyphoplasty is a suitable palliative treatment option for patients with advanced metastatic disease of the spine even with low Tokuhashi scores allowing rapid pain relief and mobilisation to increase the quality of life.
Keywords: Kyphoplasty, Lytic tumours, Spine, Palliative care, Long-term outcome
Introduction
Pain is very common in lytic lesions of the spine due to primary or secondary tumours [1, 2] and is more often the reason for seeking medical care than any neurological deficit in patients with spinal metastasis (95% of patients) [2, 3]. Pain in primary spinal tumours and cases of spinal metastasis may be caused by direct stimulation of sensory innervations by tumour, by peridural compression or from the instability of vertebral elements [4, 5] affecting and debilitating patients significantly [5–7]. As a result, mobility is decreased with simultaneously increased morbidity leading to impaired psychological and physical functioning for example depression and insomnia [6].
Different measures can be attempted to alleviate pain in patients with spinal tumours normally grouped into non-operative and operative measures. The non-operative options include bed rest, physiotherapy, analgesics, bracing and bisphosphanates. Although non-operative measures have relatively low risks, they cannot provide adequate stability to the spine and can affect patient’s independency frequently high doses of opioids for pain relief. The side effects of conservative medical treatment like constipation, sedation and gastrointestinal upset at high doses can be profound and sedation might reduce cognitive and physical functioning [8].
Operative measures can range from simple procedures like percutaneous kyphoplasty or vertebroplasty to open decompression and internal stabilization. However, the major procedures are reserved for patients with significant spinal instability and neurological deficit as they require patients to be in reasonably good general health due to the invasiveness of the surgery [1, 8]. Curative or excisional surgery should be considered only in those patients who have a Tokuhashi score of more than 9 [9] but before embarking on these major surgical interventions, the prognosis, patient’s general health and the risks and benefits of procedure should be taken into consideration [8, 10]. Only patients with a Tokuhashi score of more than 9 are considered to have a prognosis of survival of 12 months or more [9]. Cancer patients with generalised poor health and limited life expectancy are not the best candidates for major surgical procedures placing the focus to minimal invasive or palliative surgical treatment strategies [11]. It is one of the main aspects of palliative care to increase the quality of life through rapid and significant pain reduction and increased mobility to return home. This should be considered as a main benefit of surgical intervention [5, 12]. The aim of this investigation was the prospective evaluation of a consecutive cohort of patients presenting with osteolytic metastasis to the spine considered to be poor candidates for conventional open reconstructive surgery.
Materials and methods
The patients were enrolled from February 2001 to September 2002 and included in one of the first prospective series for long-term outcome in patients with low Tokuhashi scores. All procedures were performed after obtaining thorough informed consent. Eleven consecutive patients with lytic tumour lesions of the spine were treated with percutaneous kyphoplasty. Out of 11 patients, 7 were female and 4 were male with an age range 52–77 years and the average age was 65 years.
All procedures were performed under fluoroscopic guidance. Lumbar vertebrae were treated via a bipedicular approach and thoracic vertebrae via a unipedicular approach. One level was augmented as prophylactic vertebroplasty adjacent to two levels treated with kyphoplasty (patient 11) due to a perceived risk of impending fracture.
All patients had sustained pathological fractures due to osteolytic changes confirmed on imaging. Assessment of the degree of osteolysis was attempted, but abandoned as objective quantification was not possible beyond visual estimation on axial CT images. Multiple levels were treated where patients were judged to benefit from kyphoplasty of at least one pathological fracture and adjacent vertebrae had either shown signs of osteolytic collapse or were judged to be at risk thereof.
The patients were enrolled from February 2001 to September 2002 and included in one of the first prospective series for long-term outcome in patients with low Tokuhashi scores. All procedures were performed after obtaining thorough informed consent.
All the patients were followed up until death to assess the safety and efficacy of the procedure with outcome by visual analogue scale (VAS) for pain, walking distance, standing and sitting time as well as the Eastern Cooperative Oncology Group (ECOG) performance status. Since no objective and scientifically proven tool was available in the literature, a scoring system was also devised for walking distance, sitting and standing time to measure mobility outcome after the procedure (Table 1). The patients were assessed initially at admission, immediately after cement augmentation and discharge, at 6 weeks and at 3 months. From the third month onward, they were all assessed at 6 months intervals for 24 months and after this period patients with longer survival rates were followed up with telephone interviews at yearly intervals to evaluate if a re-admission due to spinal problems occurred.
Table 1.
Scores for standing time, sitting time and walking distance
| Score | Walking distance | Sitting time | Standing time |
|---|---|---|---|
| 0 | Walking not possible | Sitting not possible | Standing not possible |
| 1 | Walking distance <10 m | Sitting time <10 min | Standing time <10 min |
| 2 | Walking distance 10–50 m | Sitting time from 10 min to 1 h | Standing time from 10 min to 1 h |
| 3 | Walking distance 50–200 m | Sitting time >1 h, but with some difficulty | Standing time >1 h, but with some difficulty |
| 4 | Walking distance >200 m | Sitting time >1 h, but without any difficulty | Standing time >1 h, but without any difficulty |
Results
A total of 23 vertebrae, from T2 to L3, were treated with cement augmentation. The types of tumours included were non-Hodgkin lymphoma (2), myeloma (1), gastric carcinoma (1), cervix carcinoma (1), breast carcinoma (3), prostate carcinoma (1), small-cell lung carcinoma (1) and bladder carcinoma (1) with a pre-operative Tokuhashi score range from 3to 9.
The patients’ demographics are shown in Table 2. The average delay from time of admission to time of surgery was 4 days. The procedure was done under general anaesthesia in 10 patients and under local anaesthesia in 1 patient. Table 3 shows operative and follow-up data.
Table 2.
Patients’ demographics and tumour types
| Patient number | Age | Sex | Tumour type | Level of vertebra | Tokuhashi score | Days from admission to operation | Days in Hospital |
|---|---|---|---|---|---|---|---|
| 1 | 58 | F | Lymphoma | L4 | 8 | 4 | 11 |
| 2 | 52 | F | Gastric CA | T7 | 3 | 2 | 9 |
| 3 | 69 | M | Bladder CA | L5 | 5 | 8 | 18 |
| 4 | 63 | F | Breast CA | L1–L3 | 6 | 2 | 6 |
| 5 | 77 | M | Prostate CA | L3 | 7 | 7 | 10 |
| 6 | 52 | F | Cervix CA | T2 | 5 | 4 | 10 |
| 7 | 70 | M | Small cell lung CA | L1, L4, L5 | 6 | 2 | 6 |
| 8 | 62 | F | Breast CA | T9 | 6 | 2 | 5 |
| 9 | 74 | F | Lymphoma | L3–L5 | 6 | 3 | 7 |
| 10 | 55 | F | Breast CA | T5–T7 | 9 | 2 | 15 |
| 11 | 69 | M | Myeloma | L1–L5 | 6 | 9 | 29 |
Table 3.
Operative data, complications and follow-up times
| Patient number | Operation type | Anaesthesia | Operation time (min) | PMMA volume/vertebra (ml) | Blood loss (ml) | Complications | F/U time from procedure to death (weeks) |
|---|---|---|---|---|---|---|---|
| 1 | PK L4 | GA | 75 | 6 | 10 | None | 62 |
| 2 | PK T7 | GA | 95 | 5 | 40 | Epi PMMA leak | 13 |
| 3 | PK L5 | GA | 50 | 8 | 10 | None | 10 |
| 4 | PK L1–3 | GA | 120 | 4, 5, 6 | 15 | Epi PMMA leak | 15 |
| 5 | PK L3 | GA | 25 | 2.5 | 50 | None | 2 |
| 6 | PK T2 | GA | 45 | 3 | 10 | DVT | 6 |
| 7 | PK L1, L4, L5 | LA | 207 | 5.5, 7.5, 5.5 | 50 | None | 7 |
| 8 | PK T9 | GA | 26 | 2.5 | 10 | None | 10 |
| 9 | PK L3–5 | GA | 160 | 4, 4, 8 | 50 | None | 259 |
| 10 | PK T5, 6 PV T7 | GA | 80 | 2, 2, 1 | 10 | None | 141 |
| 11 | PK L1–L5 | GA | 90 | 6, 5, 5, 12, 8 | 80 | Adjacent fracture. PK 3 weeks later | 293 |
| Average | 88.5 min for PK | 10.7 ml | 30.5 ml/level for PK | 74.4 |
PK percutaneous kyphoplasty, PV percutaneous vertebroplasty, GA general anaesthesia, LA local anaesthesia, PMMA polymethyl methacrylate, Epi epidural, DVT deep vein thrombosis
The range of operative times was 25 (for single level kyphoplasty) to 207 min (for multi-level kyphoplasty). The average blood loss was 30.5 ml/level and there were no immediate symptomatic complications. The intra-operative biopsy confirmed the diagnosis in all cases (100% yield). Two patients had asymptomatic epidural cement leakage, which did not require any intervention. One patient had subsequent adjacent vertebral fracture 3 weeks after the procedure and required percutaneous kyphoplasty. One patient acquired deep vein thrombosis. The average hospital stay was 11.5 days (range 5–29).
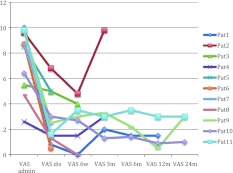
All of these patients were followed from the date of procedure until death and no patient was lost to follow-up. The average follow-up time from procedure until death was 74.4 weeks (range 2–293 weeks). The VAS improved from admission to discharge and remained constant for patients at 24 months follow-up time (Fig. 1). Even patients with short-term survival profited of the procedure in terms of pain relief.
Fig. 1.
VAS score indicating pain relief after kyphoplasty (VASadmin VAS score at admission, VASdis VAS score at discharge, VAS6w VAS score 6 weeks after kyphoplasty, VAS3m VAS score 3 months after kyphoplasty, VAS6m VAS score 6 months after kyphoplasty, VAS12m VAS score 12 months after kyphoplasty, VAS24m VAS score 24 months after kyphoplasty, Pat patient)
The sitting and standing time improved as well. The sitting and standing time score before the procedure and in the follow up period showed stable or improved results after kyphoplasty. Cement augmentation improved or maintained the sitting and standing times in all patients. The walking distance as measurement for independency and ability in daily life activities is improved as well over the follow-up period. The ECOG performance score showed improvement or a steady state compared to admission prior to the procedure. All results are summarised in Table 4.
Table 4.
Summary of the results after kyphoplasty
| Results | Pat Nr | Admission | Discharge | 6 weeks post-op | 3 months post-op | 6 months post-op | 12 months post-op | 24 months post-op |
|---|---|---|---|---|---|---|---|---|
| ECOG | 1 | 4 | 3 | 2 | 1 | 1 | 1 | |
| 2 | 2 | 2 | 2 | 3 | ||||
| 3 | 3 | 3 | ||||||
| 4 | 1 | 1 | 3 | 3 | ||||
| 5 | 2 | 2 | ||||||
| 6 | 4 | 4 | ||||||
| 7 | 3 | 3 | 2 | |||||
| 8 | 3 | 3 | 2 | |||||
| 9 | 4 | 2 | 1 | 1 | 1 | 3 | 2 | |
| 10 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |
| 11 | 4 | 3 | 3 | 3 | 2 | 2 | 2 | |
| Standing time | 1 | 0 | 2 | 2 | 3 | 3 | 2 | |
| 2 | 2 | 2 | 3 | 2 | ||||
| 3 | 1 | 2 | ||||||
| 4 | 2 | 3 | 3 | 2 | ||||
| 5 | 2 | 2 | ||||||
| 6 | 0 | 1 | ||||||
| 7 | 1 | 2 | 2 | |||||
| 8 | 2 | 3 | 2 | |||||
| 9 | 1 | 2 | 2 | 3 | 3 | 2 | 2 | |
| 10 | 2 | 1 | 3 | 3 | 3 | 2 | 3 | |
| 11 | 2 | 1 | 2 | 3 | 3 | 2 | 3 | |
| Sitting time | 1 | 0 | 2 | 3 | 3 | 3 | 3 | |
| 2 | 2 | 2 | 3 | 2 | ||||
| 3 | 1 | 1 | ||||||
| 4 | 4 | 4 | 4 | 3 | ||||
| 5 | 3 | 4 | ||||||
| 6 | 0 | 1 | ||||||
| 7 | 2 | 3 | 3 | |||||
| 8 | 2 | 2 | 3 | |||||
| 9 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 10 | 2 | 2 | 3 | 4 | 4 | 3 | 3 | |
| 11 | 3 | 3 | 4 | 3 | 3 | 2 | 3 | |
| Walking distance | 1 | 0 | 3 | 3 | 4 | 4 | 4 | |
| 2 | 3 | 4 | 3 | 2 | ||||
| 3 | 1 | 2 | ||||||
| 4 | 3 | 4 | 3 | 2 | ||||
| 5 | 3 | 4 | ||||||
| 6 | 0 | 1 | ||||||
| 7 | 2 | 2 | 3 | |||||
| 8 | 3 | 4 | 2 | |||||
| 9 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 10 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 11 | 3 | 4 | 4 | 4 | 3 | 4 | 3 |
Please see Table 1 for the value of each score
Discussion
The primary goal of kyphoplasty in osteolytic spinal tumours is pain alleviation and stabilization of the spine [11]. Since starting this series, these procedures have been reported by several authors as successful, minimally invasive procedures for managing lytic tumours of the spine [13]. The analgesic benefits of kyphoplasty can be achieved immediately after surgery or within hours of the procedure [11]. With better pain relief the psychological and physical functions improve, and these patients with metastatic cancer can achieve a better quality of life for the remaining part of their lives.
There has been no prospective study on the efficacy and safety of kyphoplasty where patients have been followed up on the time of procedure until their death and this is one of the first series of kyphoplasty in spinal lytic tumours in Europe, the first patient having been enrolled in February 2001. At that time kyphoplasty was still a very new procedure and most of the patients had general anaesthesia whilst at present, this procedure is mostly done under local anaesthesia. There is a wide range of available options for palliative pain management for patients with primary or secondary osteolytic spinal lesions. The analgesics and bisphosphonates can be helpful but they cannot provide stability to spine and the side effects can be cumbersome [8]. Radiation therapy can provide pain relief in more than half of the patients with radiosensitive tumours of the spine, but pain relief is not quick and radiotherapy cannot always prevent osteolytic vertebral collapse [10]. Kyphoplasty is not a contra-indication to radiotherapy and this procedure may be complementary in radiosensitive tumours as kyphoplasty provides immediate stabilisation and pain relief and then the tumour is treated by radiotherapy [11]. Kyphoplasty is also a safe procedure for patients on chemotherapy and might complement the treatment with local tumour and nerve tissue necrosis due to the local exothermic reaction and cytotoxicity of PMMA [1, 14, 15]. The cancer-patients with vertebral metastasis are usually in the advanced state of disease with other co-morbidities having a significant risk of preoperative and postoperative complications [11]. Therefore, major surgical procedures like resection of tumour mass and stabilisation are limited to patients who are otherwise healthy and have a good life expectancy with a Tokuhashi score greater than 9 [1, 10]. As mentioned before, most patients with spinal metastases have a score below 9 and are therefore not fit enough to undergo major surgery. Balloon kyphoplasty cuts down surgery time and allows early mobilization even for patients with Tokuhashi scores below or equal 9. The inpatient stay was not prolonged and all patients in this study with this score were able to return home without re-admission of recurrent local pain issues or further spinal interventions except one case of adjacent vertebral fracture which was treated successfully with percutaneous kyphoplasty. Avoiding major surgery and the associated risks, is paramount in patients with spinal metastases and limited life expectancy. The results for kyphoplasty so far have been very positive offering quick, reliable and significant pain relief in the majority of the patients [10]. Up to 90% of patients after kyphoplasty reported significant decrease or no pain at all after the procedure with stable results regarding analgesia and pain relief [15–17]. The complication rates of cement augmentation techniques are relatively high in cancer patients (5–10%) due to destruction of cortical bone by the tumour [15]. Complications include PMMA spillage into canal, higher in patients with malignancies, PMMA emboli and bone marrow emboli [11, 15, 18]. Careful patient selection is necessary for the success of these procedures. Absolute contra-indications are uncorrected coagulopathy, local infection at injection site and lack of surgical back up in the event of serious complication [10].
It is important in palliative care to record the changes in patients’ quality of life and proper outcome measures should be used for this purpose. The ECOG performance status is widely used to monitor patient’s response [19]. But even in the ECOG features like sitting and standing time as well as walking distance are poorly described and might camouflage a better treatment outcome than actually recorded in established outcome scores especially in palliative patients with metastatic disease. The pain-free sitting and standing time can be used as useful tools to record patients’ level of activity and the ease with which they can perform daily life activities. The walking distance also provides information about patients’ independence for routine tasks. These outcome measures are easy to record and can be used to compare a patient’s condition before and after the intervention, and then at subsequent follow-ups. Of course, this grading is not validated yet but it seems to focus specifically on the demands of palliative patients.
Limitations of this study include having no control group so that the procedure could be compared to other interventions especially for palliative treatment such as radiation and medical pain management. However, cement augmentation should be considered as a treatment option even for low Tokuhashi score patients to allow quick discharge for these patients hence an increased quality of life in the habitual environment. This study was done in one centre only and even though the sample size is not large, all of these patients were enrolled consecutively, prospectively and followed up on the time of procedure until death and no patient was lost to follow-up. The average follow-up period is also limited to the natural progression of the cancer disease but the longest survival was 259 weeks without re-admission for local pain recurrence. This underlines the use of cement augmentations even in palliative situations to offer a better quality of life and to avoid re-admission in final stages of cancer disease.
Conclusion
Kyphoplasty is a minimally invasive procedure with good efficacy for managing metastatic disease of the spine. The limited life expectancy and higher complication rates associated with surgery makes this procedure more favourable for cancer patients not fit enough for major surgeries like tumour debulking procedures, reflecting in a Tokuhashi score under or equal to 9. However, most patients, even with a low Tokuhashi score, need at least a preserved quality of life if not improvement at best. Pain and immobility seem to be the most limiting factors in these cases preventing patients from staying at home and reduce hospitalisation. Goals of palliative care include alleviation of pain (ideally, the patient is pain-free) and independence is regained or maintained. The patients can be mobilised after short bed rest, allowing the patient to go home or to be ready for further oncological treatment. Kyphoplasty can be combined with radiotherapy and chemotherapy. The quick pain relief as resulting from fracture stabilisation favours consideration of these procedures as effective and safe palliative tools in the management of patients with primary and secondary tumours of spine.
Conflict of interest
None.
References
- 1.Shindle MK, Shindle L, Gardner MJ, Lane JM. Supportive care aspects of vertebroplasty and kyphoplasty in patients with cancer. Support Cancer Ther. 2006;3:214–219. doi: 10.3816/SCT.2006.n.019. [DOI] [PubMed] [Google Scholar]
- 2.Sciubba DM, Nguyen T, Gokaslan ZL (2009) Solitary vertebral metastasis. Orthop Clin North Am 40:145–154 [DOI] [PubMed]
- 3.McLain R, Weinstein JN. Tumours of spine. In: Herkowitz H, Garfin S, Balderston R, editors. The spine. Philadelphia: WB Saunders Co; 1999. pp. 1171–1206. [Google Scholar]
- 4.Chen KY, Ma HI, Chiang YH. Percutaneous transpedicular vertebroplasty with polymethyl methacrylate for pathological fracture of the spine. J Clin Neurosci. 2009;16:1300–1304. doi: 10.1016/j.jocn.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Gofeld M, Bhatia A, Burton AW. Vertebroplasty in the management of painful bony metastases. Curr Pain Headache Rep. 2009;13:288–294. doi: 10.1007/s11916-009-0046-5. [DOI] [PubMed] [Google Scholar]
- 6.Hee HT. Percutaneous vertebroplasty: current concepts and local experience. Neurol India. 2005;53:475–482. doi: 10.4103/0028-3886.22617. [DOI] [PubMed] [Google Scholar]
- 7.Gertzbein SD. Metastatic spine tumours. In: Herkowitz HN, Dvorak J, Bell G, editors. The lumbar spine. 3. Philadelphia: Lippincott Williams & Wilikns; 2004. pp. 792–795. [Google Scholar]
- 8.Lemke DM, Hacein-Bey L. Metastatic compression fractures-vertebroplasty for pain control. J Neurosci Nurs. 2003;35:50–55. doi: 10.1097/01376517-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumour prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Wu AS, Fourney DR. Supportive care aspects of vertebroplasty in patients with cancer. Support Cancer Ther. 2005;2:98–104. doi: 10.3816/SCT.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 11.Wenger M. Vertebroplasty for metastasis. Med Oncol. 2003;20:203–209. doi: 10.1385/MO:20:3:203. [DOI] [PubMed] [Google Scholar]
- 12.Niv D, Gofeld M, Devor M. Causes of pain in degenerative bone and joint disease: a lesson from vertebroplasty. Pain. 2003;105:387–392. doi: 10.1016/S0304-3959(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 13.Trumm CG, Jakobs TF, Zech CJ, et al. CT fluoroscopy-guided percutaneous vertebroplasty for the treatment of osteolytic breast cancer metastases: results in 62 sessions with 86 vertebrae treated. J Vasc Interv Radiol. 2008;19:1596–1606. doi: 10.1016/j.jvir.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman I, Reinhardt MK (2003) Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res (415 Suppl):S176–S186 [DOI] [PubMed]
- 15.Ofluoglu O. Minimally invasive management of spinal metastases. Orthop Clin North Am. 2009;40:155–168. doi: 10.1016/j.ocl.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Fourney DR, Shomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(Suppl 1):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 17.Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20:2382–2387. doi: 10.1200/JCO.2002.09.097. [DOI] [PubMed] [Google Scholar]
- 18.Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18:311–323. doi: 10.1148/radiographics.18.2.9536480. [DOI] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of The Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]