Abstract
Background
Among index oropharyngeal cancer patients, second primary malignancies (SPMs) may be less common in human papillomavirus (HPV)-associated tumors than HPV-negative tumors. Further modification of these SPM risks by smoking has not been reported.
Methods
SPM outcomes of 356 incident oropharyngeal cancer patients were analyzed using Cox proportional hazards and Kaplan-Meier models. SPM risk and SPM-free survival were compared in HPV-seronegative patients, HPV-seropositive never smokers, and HPV-seropositive ever smokers.
Results
HPV-seropositive patients had a lower 5-year SPM rate than HPV-seronegative patients (5.6% vs. 14.6%, p=0.051). Compared to HPV-seronegative patients, HPV-seropositive never smokers had a 73% reduced SPM risk, and HPV-seropositive ever smokers had a 27% reduced SPM risk (trend p=0.028). While HPV-seronegative patients had SPMs in traditional locations, 70% of SPMs among HPV-seropositive patients were outside typical tobacco-related sites.
Conclusions
HPV serologic status and smoking may stratify patients with index oropharyngeal cancers in terms of risk and location of SPMs.
Keywords: Head and neck neoplasms, second primary, smoking, oropharyngeal cancer, human papillomavirus
INTRODUCTION
Squamous cell carcinoma of the head and neck (SCCHN) is one of the more common malignancies worldwide.1 Advances in multidisciplinary care have improved survival rates of patients with index SCCHNs, yet the development of a second primary malignancy (SPM) remains a major cause of morbidity and mortality in this group. A recent large population-based study showed that patients with SCCHN have a high risk of developing a SPM, predominantly in the head and neck, lung, or esophagus.2 SPMs account for most of the long-term morbidity in patients with SCCHN who live more than 3 years after diagnosis,3 and survival after diagnosis of SPMs is notably poorer than survival after diagnosis of similar index cancers.4–6 For this reason, optimal screening for SPMs is essential to the care of patients with SCCHN and represents a major survivorship issue.
SCCHN is strongly associated with alcohol and tobacco use, but an epidemic of human papillomavirus (HPV)-associated squamous cell carcinoma of the oropharynx (SCCOP) has emerged over the past two decades.7–14 HPV-associated SCCOP commonly occurs in nonsmokers and younger patients and is associated with certain sexual practices.14-16 Patients with HPV-positive SCCOP have better survival than those with HPV-negative tumors,17 and HPV-positive SCCOP has clinical behavior different from that of the traditional smoking- or alcohol-associated malignancies.18 Recent data suggest that the survival of patients with HPV-positive SCCOP can be refined further by smoking exposure.17 Others17,18 have previously reported significantly lower rates of SPMs among patients with HPV-positive than HPV-negative SCCOP, but to our knowledge, the influence of smoking on SPM risk among HPV-positive patients has not been reported.
It is crucial to elucidate how HPV-associated SCCOP compares with HPV-negative SCCOP so that physicians can effectively tailor treatment, screening, and follow-up strategies on the basis of patients’ HPV status. We sought to determine the influence of HPV positivity and smoking status on the risk and locations of SPMs among patients with SCCOP. We conducted our study using a large database of SCCHN patients treated at a tertiary cancer center in the United States.
MATERIALS AND METHODS
Study Subjects
This research was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. All patients were participants in a molecular epidemiologic protocol of incident SCCHN between 1995 and 2003. Patients were included if they had previously untreated, pathologically confirmed SCCOP (tonsil, base of tongue, soft palate/uvula, or posterior/lateral oropharyngeal wall) (N=406). Patients who received a consultation alone without treatment at our center (N=36) were excluded from the analysis, as were patients who received only palliative care (N=14). After these exclusion criteria were applied, 356 patients remained for analysis.
Throughout their treatment and posttreatment courses, patients had regularly scheduled clinical and radiographic examinations with surgeons, radiation oncologists, and medical oncologists specializing in head and neck cancer. On the basis of modified criteria of Warren and Gates19, second lesions were considered SPMs if they were of different histopathologic type than the index tumor, occurred more than 5 years after treatment for the index tumor, or were clearly separated from the index tumor by normal epithelium based on clinical and radiographic assessment. Pulmonary lesions were considered to be SPMs if they had a nonsquamous histology, or if they were isolated squamous lesions appearing greater than 5 years after the initial SCCHN and were believed to be SPMs by the thoracic medical oncologist and the thoracic surgeon. If there was a difference of opinion or question regarding the origin of any second lesion (i.e., recurrence vs. SPM), the second lesion was classified as a recurrence rather than a SPM. SPMs were then categorized as being in one of three classes: SCCHN, other tobacco-related cancer (lung and bronchus, esophagus, or urinary bladder), or other. Nonmelanoma skin cancers were not recorded or considered SPMs.
HPV status was determined via serologic methods as part of a research study of a sample of oropharyngeal cancer patients between 1995 and 2003. Serologic methods have been described in detail previously and were based on an established assay for antibody response to HPV16 virus–like particles.20 Prior studies have shown significant correlation between HPV seropositivity and the presence of HPV16 DNA within tumor tissue.21 All patients completed a prospective standard epidemiologic questionnaire at time of enrollment, which included smoking and alcohol consumption data. Ever smokers were defined as those who had smoked more than 100 cigarettes in their lifetime, and ever drinkers were defined as those who had drunk alcoholic beverages at least once per week for over a year at any period in their lifetime. A subject’s comorbidities were determined on the basis of his or her medical history at initial encounter and were scored using the Adult Comorbidity Evaluation 27, an index extensively used in studies of head and neck cancer.22
Statistical Analysis
Analysis was done using Stata statistical software (Release 12; StataCorp, College Station, TX, USA). The threshold for statistical significance was set at p<0.05, and all tests were two-sided. Chi-square tests were used to assess differences in demographic and clinical variables between HPV-seronegative and HPV-seropositive patients and differences in HPV status among subjects who developed SPM and those who did not. Cox proportional hazards models were generated to calculate hazard ratios to detect any differences in SPM-free survival between groups. Multivariable models were created by minimizing Akaike’s information criterion. Kaplan-Meier curves were generated and log-rank tests were performed to detect statistically significant differences in SPM-free survival. Time to event was calculated from the date of diagnosis of the index oropharyngeal cancer to the date of SPM occurrence. Patients without a known SPM at the date of last contact, those who were lost to follow-up, and those who expired after their initial date of diagnosis were censored. Multiple imputation was used to account for potential bias in estimates due to missing HPV16 serologic status. We created 20 datasets using a Markov chain Monte Carlo algorithm, and Rubin’s formula was used to combine the analyses (Stata Release 12 with Stata Multiple-Imputation Reference Manual: Release 12 online documentation, StataCorp, College Station, TX).23–25
RESULTS
Of the 356 patients with incident oropharyngeal cancer who were treated at our institution between 1995 and 2003, 182 (51%) had HPV serologic test results available, and results were positive in 80 patients (44%). Among patients with HPV serologic test results available, the median follow-up time was 104 months for those still alive at last follow-up.
Risk of SPMs by HPV Status
Overall, HPV-seropositive patients had a lower 5-year SPM rate than HPV-seronegative patients (5.6% vs. 14.6%, p=0.051, Table 1). The 5-year SPM rate was lower for HPV-seropositive patients than for HPV-seronegative patients in 11 of 12 demographic subgroups (Table 1) and 11 of 14 clinical subgroups (Table 2). However, these differences were significant only for never smokers, patients having limited comorbidities, patients with moderate or poorly differentiated cancers, and patients treated with radiation alone (Tables 1 and 2).
Table 1.
Five-year second primary malignancy rates after index oropharyngeal cancer for HPV-seronegative and HPV-seropositive patients segregated by demographic variables.
| Variable | Patients, | HPV negative
|
HPV positive
|
Log-rank |
|---|---|---|---|---|
| (N=102) | (N=80) | |||
| 5-yr SPM rate, % | 5-yr SPM rate, % | |||
| No. | (95% CI) | (95% CI) | p value | |
| Total | 182 | 14.6 (8.6–24.4) | 5.6 (2.1–14.3) | 0.051 |
| Age | ||||
| ≤55 years | 99 | 8.1 (2.7–23.2) | 2.0 (0.3–13.4) | 0.059 |
| > 55 years | 83 | 20.6 (11.2–35.9) | 13.7 (4.5–37.6) | 0.517 |
| Sex | ||||
| Male | 150 | 10.6 (5.2–21.1) | 4.8 (1.6–14.4) | 0.057 |
| Female | 32 | 29.6 (13.4–57.4) | 11.1 (1.6–56.7) | 0.722 |
| Race | ||||
| Non-Hispanic white | 163 | 13.9 (7.7–24.3) | 5.8 (2.2–14.7) | 0.052 |
| Other | 19 | 19.2 (5.0–59.0) | 0.0 | 0.488 |
| Smoking | ||||
| Never | 65 | 17.6 (7.7–37.4) | 0.0 | 0.018 |
| Ever | 117 | 13.2 (6.5–25.9) | 10.5 (4.0–26.1) | 0.657 |
| Alcohol use | ||||
| Never | 35 | 26.6 (10.8–56.5) | 5.9 (0.1–35.0) | 0.487 |
| Ever | 147 | 12.0 (6.2–22.7) | 5.4 (1.7–16.0) | 0.056 |
| Comorbidities | ||||
| None or mild | 173 | 13.7 (7.8–23.4) | 4.5 (1.5–13.4) | 0.043 |
| Moderate or severe | 9 | 0.0 | 25.0 (4.0–87.2) | 0.937 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; SPM, second primary malignancy.
Table 2.
Five-year second primary malignancy rates after index oropharyngeal cancer for HPV-seronegative and HPV-seropositive patients segregated by clinical variables.
| Variable | Patients, | HPV Negative
|
HPV Positive
|
Log-rank |
|---|---|---|---|---|
| (N=102) | (N=80) | |||
| 5-yr SPM rate, % | 5-yr SPM rate, % | |||
| No. | (95% CI) | (95% CI) | p value | |
| Total | 182 | 14.6 (8.6–24.4) | 5.6 (2.1–14.3) | 0.051 |
| Subsite | ||||
| Tonsil/base of tongue | 169 | 14.1 (8.1–24.1) | 5.9 (2.2–15.1) | 0.088 |
| Other | 13 | 20.0 (3.1–79.6) | 0.0 | 0.246 |
| Histologic grade* | ||||
| Well | 8 | 0.0 | 0.0 | -- |
| Moderate – Poor | 147 | 15.1 (8.4–26.3) | 4.8 (1.6–14.2) | 0.029 |
| Not recorded | 27 | 16.1 (4.3–50.6) | 11.1 (1.6–56.7) | 0.990 |
| T classification | ||||
| 1 or 2 | 103 | 11.4 (5.3–23.6) | 2.5 (0.4–16.5) | 0.107 |
| 3 or 4 | 79 | 20.3 (9.5–40.4) | 10.2 (3.4–28.8) | 0.264 |
| N classification | ||||
| 0 | 27 | 13.5 (3.5–44.2) | 0.0 | 0.331 |
| 1–3 | 155 | 14.9 (8.3–26.0) | 6.2 (2.4–15.7) | 0.084 |
| TNM stage | ||||
| I or II | 12 | 16.7 (2.5–72.7) | 0.0 | 0.607 |
| III or IV | 170 | 14.5 (8.3–24.7) | 5.9 (2.2–14.9) | 0.075 |
| Treatment | ||||
| Radiation | 79 | 21.1 (10.6–39.3) | 2.0 (0.3–13.4) | 0.005 |
| Chemoradiation | 84 | 12.2 (5.3–27.0) | 13.7 (4.5–37.6) | 0.606 |
| Other | 19 | 0.0 | 0.0 | 0.183 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; SPM, second primary malignancy.
Well category includes grades of well differentiated and moderately well differentiated tumors. Moderate – Poor category includes grades of moderately, moderately poorly, and poorly differentiated tumors.
In a Kaplan-Meier analysis, HPV-seropositive patients had better SPM-free survival than HPV-seronegative patients (p= 0.051, Figure 1A). HPV-seropositive patients had half the SPM risk of HPV-seronegative patients (HR 0.48, 95% CI 0.23–1.02, p= 0.056, Table 3). After multivariable adjustment, this lower risk was still apparent (30% reduced risk), though not significant (HR 0.70, 95% CI 0.31–1.57, p= 0.382).
Figure 1.
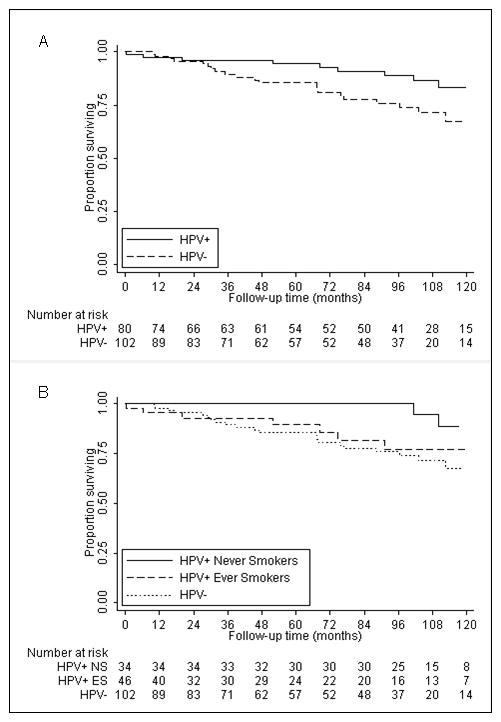
Kaplan-Meier analysis of second primary malignancy–free survival after index oropharyngeal cancer segregated by (A) human papillomavirus (HPV) serologic status and (B) HPV serologic status and smoking status.
Table 3.
Five-year second primary malignancy rates and risks associated with HPV serologic status and smoking status after index oropharyngeal cancer.
| Patient type | Complete data (N=182)
|
Imputed data (N=356)
|
|||||
|---|---|---|---|---|---|---|---|
| Patients, | 5-yr SPM rate, % | Log-rank | Crude HR | Adjusted HR | Crude HR | Adjusted HR | |
| No. | (95% CI) | p value | (95% CI) | (95% CI)* | (95% CI) | (95% CI)* | |
| HPV− | 102 | 14.6 (8.6–24.4) | Ref. | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| HPV+ | 80 | 5.6 (2.1–14.3) | 0.051 | 0.48 (0.23–1.02) | 0.70 (0.31–1.57)† | 0.45 (0.22–0.90) | 0.59 (0.28–1.24)† |
| HPV+ ever smokers | 46 | 10.5 (4.0–26.1) | 0.480 | 0.73 (0.31–1.71) | 1.05 (0.43–2.58) | 0.69 (0.31–1.52) | 0.81 (0.35–1.86) |
| HPV+ never smokers | 34 | 0.0 | 0.021 | 0.27 (0.08–0.90) | 0.31 (0.09–1.11) | 0.23 (0.07–0.74) | 0.29 (0.09–0.98) |
| 0.023‡ | 0.028‡ | 0.099‡ | 0.010‡ | 0.048‡ | |||
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HPV+, HPV positive; HPV−, HPV negative; Ref., reference group; SPM, second primary malignancy.
Adjusted for sex, grade, N classification, and comorbidity.
Adjusted for sex, grade, N classification, smoking, and comorbidity.
P(trend) for HPV+ never smokers vs. HPV+ ever smokers vs. HPV−.
Risk of SPMs by HPV and Smoking Status
In a Kaplan-Meier analysis, HPV-seropositive ever smokers had an SPM-free survival more similar to that of HPV-seronegative patients than to that of HPV-seropositive never smokers (Figure 1B). The HPV-seropositive never smokers, but not the HPV-seropositive ever smokers, had a significantly lower SPM rate and risk than HPV-seronegative patients (Table 3). Furthermore, there was a significant trend of lower SPM rate and risk with the combination of HPV seropositivity and lack of smoking (Table 3). Compared to HPV-seronegative patients, HPV-seropositive ever smokers had a 27% reduced SPM risk (HR 0.73, 95% CI 0.31–1.71, p= 0.468), while HPV-seropositive never smokers had a 73% reduced SPM risk (HR 0.27, 95% CI 0.08–0.90, p= 0.033). These findings were no longer significant after multivariable adjustment, although HPV-seropositive never smokers had only one-third the SPM risk of HPV-seronegative patients (HR 0.31, 95% CI 0.09–1.11, p= 0.072, Table 3).
In order to explore whether these findings applied to all 356 patients enrolled and treated during the time period of study, multiple imputation analysis was used to impute missing HPV serologic status in the remaining 174 patients. Patients with complete data were compared to those with missing data across all variables, and no significant differences in the distribution of these variables were noted between groups (data not shown). Also, there were no statistically significant differences in the 5-year SPM rates between those with and without HPV data, or within any of their subgroups (data not shown). After multiple imputation to account for missing HPV serologic data, HPV seropositivity was associated with a similar (although significant) magnitude reduction in SPM risk as was seen in patients with complete HPV data (Table 3). A significant trend for decreasing SPM risk with HPV seropositivity and lack of smoking was found in both crude and adjusted analyses (Table 3). After adjustment for sex, grade, N classification, smoking, and comorbidity, HPV-seropositive patients had a 41% reduced SPM risk compared to HPV-seronegative patients (HR 0.59, 95% CI 0.28–1.24, p= 0.163). After segregation for smoking status, HPV-seropositive ever smokers and never smokers had 19% (HR 0.81, 95% CI 0.35–1.86, p= 0.613) and 71% (HR 0.29, 95% CI 0.09–0.98, p= 0.047) reduced SPM risks, respectively, in the adjusted analysis.
Locations of SPMs by HPV and Smoking Status
Finally, SPM sites segregated by HPV serologic status and smoking are presented in Table 4. HPV-seronegative patients tended to develop SPMs in the more traditional sites, such as head and neck, lung and bronchus, esophagus, and urinary bladder. However, 7 of the 10 SPMs among HPV-seropositive patients were located outside these traditional locations (Table 4).
Table 4.
Sites of second primary malignancies after index oropharyngeal cancer segregated by HPV serologic status and smoking status.
| Site | HPV−
|
HPV+ ever smokers
|
HPV+ never smokers
|
|||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | |
| Total second primary malignancies | 24 | (100) | 7 | (100) | 3 | (100) |
| Head and neck | 5 | (20.8) | 1 | (14.3) | 0 | (0.0) |
|
|
|
|
|
|||
| Oral cavity | 1 | 1 | 0 | |||
| Oropharynx | 1 | 0 | 0 | |||
| Larynx | 2 | 0 | 0 | |||
| Hypopharynx | 1 | 0 | 0 | |||
| Tobacco related | 10 | (41.7) | 1 | (14.3) | 1 | (33.3) |
|
|
|
|
|
|||
| Lung and bronchus | 8 | 1 | 1 | |||
| Esophagus | 1 | 0 | 0 | |||
| Urinary bladder | 1 | 0 | 0 | |||
| Others | 9 | (37.5) | 5 | (71.4) | 2 | (66.7) |
|
|
|
|
|
|||
| Prostate | 3 | 1 | 1 | |||
| Kidney | 1 | 1 | 0 | |||
| Breast | 1 | 0 | 0 | |||
| Colon | 0 | 1 | 0 | |||
| Thyroid | 0 | 0 | 1 | |||
| Liver | 1 | 0 | 0 | |||
| Endometrium | 0 | 1 | 0 | |||
| Appendix | 1 | 0 | 0 | |||
| AML | 1 | 0 | 0 | |||
| Soft tissue | 1 | 0 | 0 | |||
| Unknown | 0 | 1 | 0 | |||
Abbreviations: HPV, human papillomavirus; HPV+, HPV positive; HPV−, HPV negative; AML, acute myeloid leukemia
DISCUSSION
This study sheds light on the combined impact of HPV serologic status and smoking history on the risk of SPM among patients with index oropharyngeal cancer. Overall, HPV-seropositive patients had a lower risk of SPM than HPV-seronegative patients. However, HPV-seropositive patients with a history of smoking had SPM-free survival and risk profiles more similar to those of HPV-seronegative patients than to those of HPV-seropositive never smokers.
Our findings regarding HPV serologic status are consistent with the concept that HPV-positive and HPV-negative oropharyngeal carcinomas are separate clinical entities, each with their own unique risk factors, clinical presentation, outcomes, and follow-up requirements. Our findings are in agreement with those of Ang et al.17 and Huang et al.,18 who reported lower SPM rates among patients with HPV-positive than among those with HPV-negative oropharyngeal cancers. HPV-positive tumors often arise in an environment with limited tobacco exposure and have fewer mutations and chromosomal abnormalities than HPV-negative tumors, which are typically smoking related.26,27 It would seem logical that the risks and locations of SPMs would differ between HPV-positive and HPV-negative index tumors as well. In fact, national data have demonstrated that while in the past, patients with index oropharyngeal cancer had very high SPM rates, similar to those of other SCCHN populations, more recently patients with oropharyngeal cancer have the lowest SPM rates among SCCHN populations, apparently as a result of the emerging epidemic of HPV-associated oropharyngeal cancer.28
Tobacco use is well known as the primary risk factor for the development of SCCHN and subsequent SPM. The concept of field cancerization associated with the use of tobacco products is well described and contributes to the increased risk of SPM among patients with SCCHN2,3 and also patients with HPV-negative oropharyngeal cancer, who, similar to traditional SCCHN patients, tend to be older and more likely to have a smoking history than patients with HPV-positive oropharyngeal cancer.29,30 As expected, most of the SPMs in HPV-seronegative patients in our study developed in smoking-associated locations such as the head and neck, the lung and bronchus, the esophagus, and the urinary bladder. In contrast, HPV-seropositive patients had overall fewer SPMs and proportionally fewer SPMs in the traditional smoking-associated locations. Our findings regarding SPM locations in HPV-seronegative patients are similar to those of Ang et al.17 and Huang et al.18: in all three studies, 63–75% of SPMs in patients with HPV-negative disease were found in the traditional smoking-associated locations (head and neck, lung and bronchus, esophagus, and bladder).17,18 When these 3 studies are taken together, only 46% (32 of 70) of SPMs among patients with HPV-associated disease occurred in smoking-associated locations. While previous studies suggest that oropharyngeal cancers are also associated with SPMs at sites traditionally associated with HPV, such as the cervix, penis, and anus,2,31 we did not observe any SPMs at anogenital sites, and only two anogenital cancers out of 60 SPMs were reported among HPV-associated oropharyngeal cancer patients in the Ang et al.17 and Huang et al.18 reports.
While others17,18 have previously reported significantly lower rates of SPMs among patients with HPV-associated SCCOP than among patients with HPV-negative SCCOP, a difference in SPM risks or locations between HPV-associated SCCOP patients with or without smoking exposures has not to our knowledge ever before been reported. In this cohort, HPV-seropositive never smokers had a 5-year SPM-free survival rate of 100%. These findings have important implications in terms of the follow-up care for oropharyngeal cancer patients. Although our study did not evaluate overall survival as an endpoint, it is certainly possible that the lower SPM rates observed in patients with HPV-associated oropharyngeal cancer contribute, in part, to the well known excellent survival of these patients.17 Other authors have previously shown that smokers with HPV-positive oropharyngeal cancer have lower overall and disease-specific survival rates than never smokers with HPV-positive oropharyngeal cancer,32,33 and this study confirms a similar effect in terms of SPM-free survival.
This study does have limitations that are important to recognize. Many of the results did not reach statistical significance despite trends in the data, likely because of our limited sample size. This limitation is counter-weighted by strong follow-up in the study sample: every patient had at least 8 years of potential follow-up time, and the observed median follow-up time was 104 months. Another major limitation of this study is the use of HPV serologic methods to segregate SPM risk groups. While HPV serologic status does reflect past exposures, not all exposed patients will seroconvert or maintain an antibody response. It would be preferable to determine HPV status directly from tumor specimens by HPV in situ hybridization and measurement of p16 expression. Directly obtaining HPV status from tumor specimens is a more accurate way of classifying cancer patients. Although we did not have tumor tissues from most subjects from this period, one of our priorities for the future is to present our subsequent experience with SPM risk in a larger sample of patients with oropharyngeal cancer with both rigorous and lengthy follow-up and HPV status determined by testing of tumor tissues.
We conclude that both HPV exposures and smoking influence the risks and locations of SPM development among patients with oropharyngeal cancer. Furthermore, these results suggest that while SPM risk may be particularly low among never smokers with HPV-associated oropharyngeal cancer, ever smokers with HPV-positive cancers may require more heightened awareness of SPM risk. While we did not observe any SPMs at other sites associated with HPV among our patients, we believe that the existing literature suggests that clinicians following patients with HPV-associated oropharyngeal cancer should also be aware of the risk of anogenital SPMs.
Acknowledgments
The authors wish to thank Ms Stephanie P. Deming for manuscript editing.
Sources of Support: Funded in part by start-up funds from The University of Texas MD Anderson Cancer Center (to E.M.S.); NIH Head and Neck SPORE grant P50CA097007 Career Development Award (to E.M.S.); The University of Texas MD Anderson Cancer Center Institutional Research Grant (to E.M.S.); NIH grant K-12 88084 (to E.M.S., faculty trainee; to R.C. Bast, P.I.); NIH grant R03CA128110-01A1 (to E.M.S.); NIH grant R01ES011740 (to Q. Wei); NIH grant R01CA131274 (to Q. Wei); and NIH Cancer Center Support grant P30CA016672 (to John Mendelsohn).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(5):671–679. doi: 10.1007/s10552-011-9739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgis EM, Miller RH. Second primary malignancies in the head and neck cancer patient. Ann Otol Rhinol Laryngol. 1995;104(12):946–954. doi: 10.1177/000348949510401206. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N, Nayak VK. Survival outcomes for second primary head and neck cancer: a matched analysis. Otolaryngology Head Neck Surg. 2005;132(1):63–68. doi: 10.1016/j.otohns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto E, Shibuya H, Yoshimura R-ichi, Miura M. Site specific dependency of second primary cancer in early stage head and neck squamous cell carcinoma. Cancer. 2002;94(7):2007–2014. doi: 10.1002/cncr.10444. [DOI] [PubMed] [Google Scholar]
- 6.Di Martino E, Sellhaus B, Hausmann R, et al. Survival in second primary malignancies of patients with head and neck cancer. J Laryngol Otol. 2002;116(10):831–838. doi: 10.1258/00222150260293664. [DOI] [PubMed] [Google Scholar]
- 7.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9(6):665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 8.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 9.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363(9420):1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 12.Hansson BG, Rosenquist K, Antonsson A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125(12):1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 13.Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 14.Smith EM, Ritchie JM, Summersgill KF, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108(5):766–772. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 15.Dahlstrom KR, Li G, Tortolero-Luna G, Wei Q, Sturgis EM. Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and patients with squamous cell carcinoma at other head and neck sites. Head Neck. 2011;33(6):847–855. doi: 10.1002/hed.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and - unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SH, Perez-Ordonez B, Liu F-F, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Warren S, Gates O. Multiple primary tumors. American Journal of Cancer. 1932;16:1358–1414. [Google Scholar]
- 20.Dahlstrom KR, Adler-Storthz K, Etzel CJ, et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res. 2003;9(7):2620–2626. [PubMed] [Google Scholar]
- 21.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 22.Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol. 2010;46(10):712–719. doi: 10.1016/j.oraloncology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Ali AMG, Dawson S-J, Blows FM, et al. Comparison of methods for handling missing data on immunohistochemical markers in survival analysis of breast cancer. Br J Cancer. 2011;104(4):693–699. doi: 10.1038/sj.bjc.6606078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai M, Kubo J, Esserman D, Terry MB. The handling of missing data in molecular epidemiology studies. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1571–1579. doi: 10.1158/1055-9965.EPI-10-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heitjan DF. Incomplete data: what you don’t know might hurt you. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1567–1570. doi: 10.1158/1055-9965.EPI-11-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15(5):1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 27.Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA. Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous. Eur J Cancer. 2007;43(2):415–432. doi: 10.1016/j.ejca.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka D, Yamashita S, Tomioka T, et al. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history: a target for risk diagnosis and prevention of esophageal cancers. Cancer. 2009;115(15):3412–3426. doi: 10.1002/cncr.24394. [DOI] [PubMed] [Google Scholar]
- 30.López-Blanc SA, Collet AM, Gandolfo MS, et al. Nucleolar organizer regions (AgNOR) and subepithelial vascularization as field cancerization markers in oral mucosa biopsies of alcoholic and smoking patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):747–753. doi: 10.1016/j.tripleo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Sikora AG, Morris LG, Sturgis EM. Bidirectional association of anogenital and oral cavity/pharyngeal carcinomas in men. Arch Otolaryngol Head Neck Surg. 2009;135(4):402–405. doi: 10.1001/archoto.2009.19. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122(12):2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]


