Abstract
The developmental relations among different behaviors can take many forms. At one extreme, two behaviors emerge independently of one another and, at the other extreme, the emergence of one behavior depends on the prior emergence of the other. Whether the two behaviors in the latter case should be designated as developmentally homologous is explored in this essay by reviewing differing approaches to conceptualizing the development of sleep. It is argued that whereas the concept of developmental homology may offer little new to the understanding of sleep development, the conventional notion of evolutionary homology remains to be fully exploited. Identifying homologous sleep processes will benefit from the adoption of a developmental comparative approach that emphasizes real-time sleep dynamics and individual sleep components. Because evolution occurs through the modification of developmental processes, a new commitment to a developmental comparative approach to sleep is a necessary next step toward a better understanding of its evolution.
Keywords: REM sleep, brainstem, cortex, delta activity, atonia, myoclonic twitching, development, evolution, rat
Homology has been and continues to be a bedrock concept for evolutionary biologists (Hall, 2003). Despite some recent challenges and important modifications (e.g., deep homology; see Shubin et al., 1997), homology remains a critical concept for evaluating evidence of common descent. But can we and should we export this evolutionary notion of homology into the realm of individual development? In considering this question in the months before and after our workshop, I considered and adopted several positions in succession. One of those positions went something like this:
We should be wary of potentially superficial similarities between evolutionary and developmental notions of homology. As conventionally used by evolutionary biologists, two homologous structures (or behaviors) allow us to infer descent from a common ancestor. But to say that two structures (or behaviors) observed at two ages are developmentally homologous is to make a claim about one developing from the other. In the former case, our claim of homology extends beyond the two observable structures (or behaviors) to say something about an evolutionary relationship with an unobserved ancestor (i.e., “A and B commonly descended from C”). But in the latter case, our claim of homology seems to say little more than “B developed from A,” which packs little or no theoretical punch. Rather, it simply restates what we already knew: that A is a developmental precursor of B or that A and B are developmentally continuous.
I am aware that one can argue that a common ancestor has a similar relationship to its descendants as a developmental precursor has to its adult form; this is an interesting argument and perhaps even a valid one. Nevertheless, I left the workshop wondering whether the notion of developmental homology does any useful work for us at all.
But I soon realized I had a problem: I had invoked homology in my own writings on the development of sleep. This realization, or rather recollection, impelled me to reconsider my use of this term. Why did I use that term? What specific meaning did I ascribe to it?
Before describing that work, I would first like to briefly revisit a classic research story from our field that dealt explicitly with the issues just described. Specifically, I turn first to the work of Hall and colleagues on the relationship between suckling in infant rats and other forms of feeding in adults. The question addressed by that research was whether the obvious similarities between suckling and feeding as ingestive behaviors reflect deep mechanistic commonalities that permit insight into the early origins of adult feeding. In their review of this work, Hall and Williams (1983) begin by discussing the concept of developmental continuity:
A strategy in developmental analysis is to observe and manipulate a behavioral system at different stages during its maturation in order to trace how that system is assembled and organized. A critical assumption of this particular developmental approach is one of “continuity,” that the system being examined at one stage in development is the same system as that examined at a later stage. Often, in the context of this approach, the system examined in early development is viewed as the infantile behavioral form that is gradually elaborated into the adult pattern. In recent years, this developmental strategy has been applied to the study of feeding behavior in rats. (p. 220)
What makes this opening paragraph so startling is that Hall and Williams devote the remainder of their review showing how suckling and feedling violate the expected conformity with developmental continuity. In fact, rather than provide insight into the development of feeding, suckling appears to be a distinct ingestive system with its own internal and external physiological controls and, perhaps, neural substrates. Not only can infant rats feed independently of suckling, but pups raised without the opportunity to suckle have no trouble learning to feed. Thus, rather than think of suckling as a developmental precursor of feeding, Hall and Williams ask us to consider suckling as an entity unto itself. Suckling is, they suggest, an ontogenetic adaptation—a transient developmental feature that fits the ecological niche of the infant mammal.
It is worth noting that Hall and Williams do not invoke homology in their discussion of developmental continuity, but they could have. For example, they could have begun their paper by asking whether suckling and feeding are homologous behaviors and could have concluded that they are instead analogous behaviors, serving the same function (i.e., providing nutrition) in different ways. But it is also possible that there is a deeper commonality between suckling and feeding that has yet to be discovered. In making this suggestion, I am invoking the notion of deep homology, as described by Shubin et al. (1997):
Determination of whether two structures are homologous depends on the hierarchical level at which they are compared. For example, bird wings and bat wings are analogous as wings, having evolved independently for flight in each lineage. However, at a deeper hierarchical level that includes all tetrapods, they are homologous as forelimbs, being derived from a corresponding appendage of a common ancestor. Similarly, we suggest that whereas vertebrate and insect wings are analogous as appendages, the genetic mechanisms that pattern them may be homologous at a level including most protostomes and deuterostomes. (p. 647)
Can we export deep homology to behavioral development? To do so for suckling and feeding—to convert this analogous relationship into a homologous one—might require that we identify common embryological roots of these two ingestive behaviors that, through development, differentiate and diverge in the same individual to produce these two parallel systems. However, given that all development necessarily entails differentiation and divergence from a single fertilized egg to specialized cells and tissue, there is a sense in which every organismic feature shares such a deep relationship with every other. Accordingly, a search for deep homology in a developing animal might very well be a trivial one. Once again, we see that the very different meanings of descent when used in developmental and evolutionary contexts seem to limit the value of developmental homology.
This discussion of suckling, feeding, and deep homology also serves to highlight the importance of identifying the appropriate hierarchical or descriptive level for comparing behaviors across the lifespan. Because suckling and feeding are ingestive behaviors that entail movement of nutrients from the mouth to the gut, it is understandable that some would assume that they are developmentally continuous. It was only after detailed analysis of behavior and physiology that the many dissimilarities between them were revealed. As we will now see, similar issues pertain when we consider the appropriate conceptual framework for describing sleep and wakefulness across the lifespan.
In the realm of sleep, our questions are relatively straightforward. First, is infant sleep a developmental precursor of adult sleep? Second, are the parts of sleep—active sleep (aka REM or paradoxical sleep) and quiet sleep (aka non-REM, slow-wave, or delta sleep)—homologous in infants and adults? The philosophical, historical, and methodological background associated with these questions has been reviewed recently (Blumberg & Seelke, 2010). Here I restrict my focus to those aspects of a recent debate about infant sleep that relate to the question of homology and developmental continuity (Blumberg, Karlsson, Seelke, & Mohns, 2005; Frank & Heller, 2003, 2005).
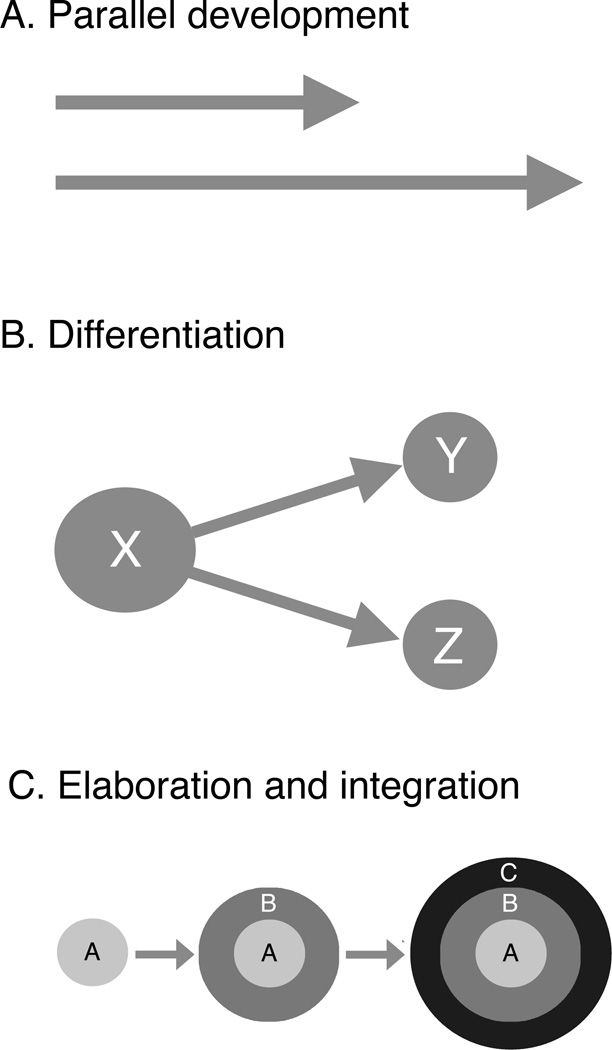
One can imagine several ways to conceptualize the relationship between infant and adult sleep. First, as with suckling and feeding, infant and adult forms of sleep could be mechanistically unrelated, following parallel—perhaps even autonomous—paths across development (Figure 1A). However, as will be described below, there are too many common elements between infant and adult sleep to take such a possibility seriously. Most investigators, it seems, accept that there is at least some developmental continuity within the sleep system.
Figure 1.
Three patterns of development discussed in this paper. (A) Parallel development, as conceptualized by Hall and Williams (1983) for the developmental relations between suckling (short arrow) and feeding (long arrow). Both suckling and feeding are expressed early in development in rats, but suckling disappears at weaning as feeding continues. This is an idealization of the relationship of Hall and Williams who acknowledged the likelihood that some control mechanisms would be shared between the two behavioral systems. (B) Differentiation, as conceptualized by Frank and Heller (2003) for the developmental relations among presleep (X) and adult forms of active (Y) and quiet sleep (Z). According to this view, presleep is a "common precursor" to active and quiet sleep. (C) Elaboration and integration, based on Blumberg and Seelke’s (2010) conceptualization of the development and emergence of sleep-wake components. According to this view, some foundational components and their neural mechanisms are expressed early in development and are retained throughout infancy into adulthood. In this figure, component A is a foundational component and could represent the behavioral manifestations of sleep and wake (e.g., high-amplitude movements indicative of wake and myoclonic twitches indicative of active sleep). Component B could represent fluctuations between high muscle tone (indicative of wake) and low muscle tone or atonia (indicative of sleep). Finally, component C could represent the emergence at P11 of differentiated cortical activity, especially delta activity.
A second possibility is that infant sleep is, by comparison with that of adults, disorganized or undifferentiated (Adrien & Lanfumey, 1984; Frank & Heller, 2003). Adopting this position, Frank and Heller (2003) refer to the early form of sleep as presleep, in which separable states of active and quiet sleep cannot be distinguished, and posit that there comes a point during development when active and quiet sleep emerge (Figure 1B). In their words: “Our argument is that presleep is not a homolog of REM sleep and instead represents a common precursor to REM and NREM sleep. By analogy, embryonic limb buds are still limbs, but they are not hands, fingers or feet” (Frank & Heller, 2005, p. 91, italics added).
There are several aspects of this last statement that deserve emphasis. First, in referring to presleep as a precursor to the adult form of sleep, it seems clear that the authors are endorsing some form of developmental continuity. Second, by comparing sleep to an embryonic limb bud, they are literally adopting a differentiation model of sleep development: accordingly, the amorphous, singular form of sleep they call presleep differentiates into the adult form composed of active and quiet sleep. Finally, leaving aside the issue of whether embryonic limb buds should be considered homologous with the differentiated limb they become (e.g., see D. Moore, in this special section), it is clear that Frank and Heller believe that presleep is not homologous with either active or quiet sleep.
Having described Frank and Heller’s position, we can now turn to their rationale. The first empirical report from these authors employed standard electrophysiological methods to track sleep development in rats at postnatal day 11 (P11) and older (Frank & Heller, 1997). This choice of age is significant because, as they and others have shown (Gramsbergen, 1976; Mirmiran & Corner, 1982), it is at P11 that cortical delta activity first emerges, which means that these authors never directly assessed sleep-wake processes at ages before the onset of delta activity. Regardless, based on their sleep scoring system, Frank and Heller reported that P12 rats exhibited high amounts of delta activity during periods that were otherwise scored as active sleep, and that active and quiet sleep increasingly dissociated over the subsequent week. Similar mixed bouts of “half-activated” active sleep in rats had been reported earlier (Jouvet-Mounier, Astic, & Lacote, 1970).
Not unreasonably, Frank and Heller interpreted their results as supporting their presleep hypothesis. They also interpreted their results as justifying their emphasis on the cortical EEG as a measure possessing special value for documenting the developmental transition from undifferentiated presleep to differentiated adult-like sleep:
An old debate in the field of neonatal sleep research concerns the choice of criteria in assigning vigilance states. Our position on this matter… is straightforward: EEG differentiation is a consistent hallmark of the appearance of states that satisfy multiple behavioral and neurophysiological criteria for sleep. This suggests that organized sleep states appear around this time. This seems reasonable because most scientists agree that mammalian sleep is a brain phenomenon and field potentials like the cortical EEG are measurements of brain activity. Could there be states homologous to EEG-defined REM and NREM sleep before the appearance of differentiated cortical EEGs? Yes, but this has not been conclusively demonstrated. Until this happens, we find it useful to classify states as either behaviorally determined … or electrographically determined….” (Frank & Heller, 2005, pp. 91–92)
Despite Frank and Heller’s focus on the cortical EEG, it is the brainstem that contains many of the critical nuclei necessary for the generation of sleep states, including active sleep (Siegel, 2005b). However, because these authors argue that “executive sleep mechanisms” in the brain are not involved in presleep (Frank & Heller, 2003), their statement above suggests that, beginning around the time of EEG differentiation at P11, the entire brain is reorganizing to effectuate the transition from presleep to sleep. Testing this notion would require detailed analysis of sleep (or presleep) before the emergence of delta activity and an assessment of changes in sleep parameters across the transition to EEG differentiation. If Frank and Heller were correct about the signal importance of the cortical EEG, we should see a momentous transition in brain and behavioral organization in the days before and after P11. By their analogy, this transition should be no less dramatic than the differentiation of a limb bud into a fully realized limb.
Our work in this domain took a very different path and led us to conclude that the brain does indeed modulate behavioral states during the “pre-EEG period” (reviewed in Blumberg & Seelke, 2010). Briefly, we isolated a region within the medial medulla that is necessary for the suppression of skeletal muscle tone (Karlsson & Blumberg, 2005) and linked this area to a host of sleep-and wake-related areas within the mesopontine region, including areas specifically associated with the myoclonic twitching that is a defining feature of active sleep (Karlsson, Gall, Mohns, Seelke, & Blumberg, 2005). Subsequent work implicated specific forebrain regions whose modulatory influence on sleep and wake consolidation increases over early development (Mohns, Karlsson, & Blumberg, 2006).
However, the core of the presleep hypothesis is its characterization of neonatal sleep as undifferentiated and disorganized. We have never found this to be the case in neonatal rats. Instead, we have consistently seen highly organized sleep-wake cycles in which sleep and wake movements are tightly coupled with skeletal muscle activity (recall that standard EEG measures are not available at these early ages for defining sleep states). A typical cycle begins with a period of high muscle tone that is often accompanied by high-amplitude movements (e.g., kicking, stretching) indicative of waking, followed by a transition to low muscle tone accompanied by behavioral quiescence, indicative of quiet sleep. This quiescence is interrupted by the onset of twitching against a background of muscle atonia; this twitching can be detected behaviorally and also electrographically as sharp spikes in the electromyogram (EMG). Although most of our EMG recordings were from the nuchal muscle (which controls head movements), even the extraocular muscles, as early as P3, exhibit similar patterns of muscle tone fluctuation and twitch-related spiking (Seelke, Karlsson, Gall, & Blumberg, 2005). During a period of sleep, waxing and waning of twitching activity is seen until muscle tone abruptly increases and wake behaviors are once again exhibited, thus completing the cycle. This general, organized structure to the sleep-wake cycle is seen soon after birth and, although relatively constant across development, the speed of cycling slows considerably over development as sleep and wake bouts consolidate (Blumberg, Seelke, Lowen, & Karlsson, 2005). Therefore, based on all of the accumulated evidence, if there is a period in rats of undifferentiated and disorganized sleep, it will be found during the fetal period.
We now arrive at the central issue for our discussion of homology: Are the periods of quiet and active sleep that we identified before P11 and, therefore, without the aid of EEG activity homologous to those sub-states that can be defined after P11 with the aid of EEG activity? Are these transitions across P11 smooth or momentous? To answer these questions, we assessed sleep-wake processes at P9 using only behavior and EMG, and at P11 and P13 using behavior, EMG, and EEG (Seelke & Blumberg, 2008). At P11 and P13, we also scored the data without reference to the EEG for comparison with the data at P9.
We found no evidence of momentous organizational change across the P11 transition. When delta activity first emerged at P11, it fit smoothly into the quiet sleep “slot” as defined at P9 using only behavior and EMG, which intervenes between bouts of wake and active sleep. We found no evidence of mixed or half-activated states. Not surprisingly, delta activity helped to provide better estimates of the quantity of quiet sleep, especially when delta activity occasionally occurred between lulls in twitching. We concluded that
the fundamental structure of sleep and wake states is already established at the time when delta activity first emerges. Thus, the absence of delta activity should not lead us to reinterpret the nature of sleep in early infancy. Rather, its presence provides additional confidence in the designations that we are able to make when it is available as a measure of [quiet sleep]. Finally, the smooth integration of delta activity into the existing organizational structure of infant sleep—as evidenced by tonic and phasic EMG activity and associated behaviors—once again shows how, early in infancy, coordinated brainstem activities unify behavioral states from muscle to neocortex. (p. 698)
It was the unifying influence of the brainstem that, in a subsequent chapter, inspired us to suggest that “the states designated as QS and AS before the emergence of delta activity are homologous with those that come later” (Blumberg & Seelke, 2010, pp. 398–399). We went further to suggest that “homologous sleep–wake states… can be identified in the EEG and EMG records. This homology arises because EEG- and EMG-defined states are generated by common brainstem mechanisms” (p. 416). Finally, noting that the EMG and EEG independently convey a lot of information about behavioral state, we went still further to suggest that “sleep and wakefulness are body-wide processes that entail homologous activational states in muscle, spinal cord, brainstem, and forebrain” (p. 417).
By using homology in the statements above, we meant to communicate the simple notion that there are correspondences between (i) those behavioral states that are so readily defined in adults and (ii) those that precede them in early infancy. The correspondences arise from shared foundational components or elements that can be identified in the infant and traced through development. In Figure 1C, a foundational component A is depicted as being present early in development whereas components B and C emerge later. Components A and B could represent, for example, sleep-wake behaviors (e.g., kicking and twitching) and state-dependent fluctuations in muscle tone, respectively. (Because these two components are already integrated around the time of birth in rats, the integration suggested in the figure would occur during the fetal period.) Component C could represent the emergence at P11 of cortical delta activity.
The key notion of Figure 1C is that, through developmental time, new components are elaborated and become integrated with those that precede them; integration of elements suggests overlapping neural mechanisms or neural connectivity. It should be noted that developmental dependencies among components are not indicated in the figure; depending on the system, it may be that one component must develop before another one can (i.e., strong dependency) or that a later component will develop regardless of whether an earlier one does (i.e., non-dependency).
In comparing the models in Figure 1B and 1C, it is important to emphasize what they do and do not have in common. For example, both posit a precursor state early in development, but whereas Figure 1B identifies a common precursor that is qualitatively distinct from what emerges later, Figure 1C identifies common threads coursing through development. We might be tempted to state that Figure 1B rejects continuity (e.g., “differentiation is a discontinuous event”) and that Figure 1C embraces it. But we must be careful in making such claims: clearly the precursor X in Figure 1B can claim a continuity relationship with Y and Z, and one can easily argue that the emergence of components B and C in Figure 1C suggest discontinuities in the system. These may be pointless distinctions: Words such as continuity can be infuriatingly ambiguous, shifting their meaning depending on context and level of analysis. As already mentioned, there is a trivial sense in which all development in an individual is continuous.
The more vital distinction I wish to make between the models depicted in Figures 1B and 1C relates to the different ways they consider sleep components across development. As argued 15 years ago (Blumberg & Lucas, 1996), the individual components comprising sleep states (e.g., muscle atonia, myoclonic twitching, rapid eye movements, cortical delta activity) may have unique developmental trajectories and evolutionary histories. Our work with infant rats over the intervening years suggests that such a focus on components is feasible and justifiable—and that the model depicted in Figure 1C provides a valid framework for dissecting those components. In contrast, with the presleep model depicted in Figure 1B, the developmental appearance at P11 of just one component—cortical delta activity—is thought to signal or trigger the differentiation of presleep into active and quiet sleep. Also, it is not clear what the composition of the undifferentiated presleep state is meant to be; if truly undifferentiated, it should be very difficult to monitor and track any sleep components before P11, which we now know not to be the case.
Our use of homology as a synonym for correspondence was meant only to draw attention to the various components of sleep and their individual trajectories through development. This componential view was captured metaphorically by Corner’s (1985) comparison of sleep to a rope comprising multiple strands; as we go back in developmental time some strands fall away such that only a few remain. The rationale for describing the somewhat “unraveled” state of neonates as sleep rests on the behavioral similarities that we observe across development and the individual components that we can trace through development. This should hardly be controversial; after all, if flies (Shaw, Cirelli, Greenspan, & Tononi, 2000), nematodes (Raizen et al., 2008), and zebrafish (Yokogawa et al., 2007) sleep, certainly newborn rats do. Nonetheless, we should never lose sight of the fact that sleep, no less than wake, is shorthand for describing a complex behavioral, physiological, and neural state of an organism. Accordingly, we should strive to avoid pointless debates about the “essential” features of sleep and focus instead on the developmental processes by which individual sleep components “coalesce, cohere, and self-organize during ontogeny. In other words, sleep is not the product of any single, essential controller but an emergent property of the dynamic interactions among individual components” (Blumberg & Lucas, 1996, p. 4).
A focus on sleep components may have other benefits as well—one that helps us return to the evolutionary notion of homology. To appreciate those benefits, one must first consider that most comparative assessments of sleep rest heavily on measures of sleep duration across vertebrate and invertebrate species (Campbell & Tobler, 1984). Of course, given the wide diversity of sleep components expressed by, for example, a rat and a fly, it must first be accepted that sleep in both species can be compared. And the rationale for such comparisons across species must be similarities in their behavior patterns. (A similar rationale justifies the within-species, across-age comparisons described above in rats.) In addition to sleep duration, investigators have compared mammalian species with regard to the existence of active and quiet sleep, their durations, and their cycle lengths (Tobler, 1995).
Critical to all such analyses of whether a given species “has” active or quiet sleep are the specific criteria that are used and the conditions under which animals are observed. For example, although echidnas exhibit idiosyncratic cortical activity, the presence of vigorous twitching of the eyes, bill, and head led Siegel et al. (1998) to conclude that they express active sleep. Similarly, although active sleep is typically believed to be absent in reptiles, Ayala-Guerrero and Mexicano (2008) argued otherwise based on their observations of the green iguana. In contrast, if cortical activity is considered a necessary feature of active sleep, neither echidnas nor iguanas “have” it.
There is also great diversity in sleep within species depending on ecological and test conditions. For example, some birds exhibit long periods of sleeplessness during long-distance migrations (Rattenborg et al., 2004). In fur seals, the unihemispheric sleep exhibited in the water transitions to the typical pattern of bihemispheric sleep exhibited on land (Lyamin & Mukhametov, 1998). In sloths, sleep durations vary widely depending on whether the subject is observed in the wild or captivity (Rattenborg et al., 2008), a finding that has potentially important implications for across-species comparisons that rely on measures of sleep duration.
Beginning with Zepelin and Rechtschaffen (1974) and continuing to today (Capellini, Barton, McNamara, Preston, & Nunn, 2008; Lesku, Roth, Amlaner, & Lima, 2006; Siegel, 2005a), investigators have compared sleep durations with physiological and life-history variables (e.g., neonatal brain weight, gestation length, metabolic rate, risk of predation) to test hypotheses about the functions of sleep. Like Rattenborg et al. (2008), Cappellini et al. (2008) noted the profound effect of testing conditions on sleep durations and restricted their analyses to studies that used “standardized procedures;” based on a lack of correlation between neonatal and adult brain weight and adult sleep times, they concluded that developmental factors may not account for variation in sleep durations. I do not doubt that such correlational studies linking sleep variables and life-history variables can be very useful for generating hypotheses and even, under some conditions, testing them. But I see no reason why static measures of sleep duration in adult mammals would ever provide much insight into the functions of sleep in early development, which is when sleep predominates (Roffwarg, Muzio, & Dement, 1966) and sleep-related motor activity is so robust (Blumberg, 2010).
What is sorely needed is a greater emphasis on developmental comparative studies that focus less on sleep durations at one point in time (i.e., adulthood) and more on the processes and mechanisms that govern sleep-wake transitions across the lifespan. Sleep (and wake) durations are simply the sum of all of the individual sleep (and wake) bouts, and the statistical properties of these bouts—in infants (Blumberg, Seelke, et al., 2005) and adults (Lo et al., 2002)—provide useful insights into the neural mechanisms that govern sleep-wake cycling. That these bout-analytic methods have been applied effectively to developing wild-type and knockout mice (Blumberg, Coleman, Johnson, & Shaw, 2007) and fetal sheep (Karlsson, Arnardóttir, Robinson, & Blumberg, 2011), suggests that broader application of these methods to other species will open up new opportunities for understanding the evolution of sleep and identifying sleep-related homologous processes and mechanisms. When we appreciate that evolution occurs through the modification of developmental processes (Arthur, 2011; Blumberg, 2009; Gottlieb, 1992; West-Eberhard, 2003), it becomes readily apparent that our understanding of the evolution of sleep will depend on greater comparative knowledge of the developmental trajectories of sleep-wake bout organization.
Just as we should go beyond sleep durations to examine individual sleep bouts, so should we move beyond which species “have” some form of sleep and rather assess, with greater care and precision, individual sleep components. Such a focus on components may lead to a greater understanding of the developmental and evolutionary origins of the neural systems that produce, for example, muscle atonia, myoclonic twitching, ponto-geniculo-occipital (PGO) waves, and cortical delta waves. This is not a radical proposal as there already are several hypotheses that focus exclusively on individual sleep components. For example, the “synaptic homeostasis” hypothesis posits a specific function for the cortical delta waves of quiet sleep (Tononi & Cirelli, 2003). Similarly, Berger (1969) theorized creatively about the role played by rapid eye movements in the development and maintenance of binocularly coordinated eye movements in those species that have them. Other hypotheses have addressed the possible functions of PGO waves (Shaffery, Roffwarg, Speciale, & Marks, 1999) and myoclonic twitching (Blumberg, 2010). It is only a short step from these hypotheses to the realization that the individual components of sleep are likely to have unique developmental and evolutionary histories.
In summary, I have argued here that homology may have little to add to our conceptual toolbox for understanding individual development. Despite my skepticism, I do not believe that this is a settled issue but rather one that demands and deserves continued debate and discussion. Regardless, within the context of renewed interest in developmental evolutionary biology (e.g., Arthur, 2011; West-Eberhard, 2003), homology certainly has a continuing role to play in the identification of evolved developmental processes that are shared across diverse species (Striedter, 1998). Within the realm of sleep, identifying evolutionarily homologous processes and mechanisms may prove essential for moving to a new level of understanding of the developmental and evolutionary origins of this pervasive, abundant, and mysterious aspect of our lives.
Acknowledgments
I thank David Moore and Chris Moore for generously inviting me to participate in their workshop at Dalhousie University in August, 2011. David Moore also provided helpful feedback and encouragement as I prepared this paper. I thank Jeff Alberts, Adele Seelke, Greta Sokoloff, and Trey Todd for many helpful comments. Preparation of this paper was made possible in part by a Career Development Award from the National Institute of Mental Health (MH66424).
References
- Adrien J, Lanfumey L. Neuronal activity of the developing raphe dorsalis: its relation with the states of vigilance. Experimental Brain Research. 1984;(Suppl. 8):67–78. [Google Scholar]
- Arthur W. Evolution: A developmental approach. Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- Ayala-Guerrero F, Mexicano G. Sleep and wakefulness in the green iguanid lizard (Iguana iguana) Comparative Biochemistry and Physiology-Part A: Molecular & Integrative Physiology. 2008;151(3):305–312. doi: 10.1016/j.cbpa.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Berger RJ. Oculomotor control: A possible function of REM sleep. Psychological Review. 1969;76:144–164. doi: 10.1037/h0027235. [DOI] [PubMed] [Google Scholar]
- Blumberg MS. Freaks of nature: What anomalies tell us about development and evolution. New York: Oxford University Press; 2009. [Google Scholar]
- Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Coleman CM, Johnson ED, Shaw C. Developmental divergence of sleep-wake patterns in orexin knockout and wild-type mice. European Journal of Neuroscience. 2007;25:512–518. doi: 10.1111/j.1460-9568.2006.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Karlsson KÆ, Seelke AMH, Mohns EJ. The ontogeny of mammalian sleep: A response to Frank and Heller-2003. Journal of Sleep Research. 2005;14:91–101. doi: 10.1111/j.1365-2869.2004.00430_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Developmental Psychobiology. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. 2010. pp. 391–423. [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, Karlsson KÆ. Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: A review of sleep duration across phylogeny. Neuroscience and Biobehavioral Reviews. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–1776. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner MA. Ontogeny of brain sleep mechanisms. In: McGinty DJ, editor. Brain mechanisms of sleep. New York: Raven Press; 1985. pp. 175–197. [Google Scholar]
- Frank MFM, Heller HC. Development of REM and slow wave sleep in the rat. American Journal of Physiology. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. Journal of Sleep Research. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Unresolved issues in sleep ontogeny: a response to Blumberg et al. Journal of Sleep Research. 2005;14:98–101. doi: 10.1111/j.1365-2869.2004.00430_2.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Individual development and evolution. New York: Oxford University Press; 1992. [Google Scholar]
- Gramsbergen A. The development of the EEG in the rat. Developmental Psychobiology. 1976;9:501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- Hall BK. Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biological Reviews. 2003;78:409–433. doi: 10.1017/s1464793102006097. [DOI] [PubMed] [Google Scholar]
- Hall WG, Williams CL. Suckling isn’t feeding, or is it? A search for developmental constraints. Advances in the Study of Behavior. 1983;13:219–254. [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep, in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Arnardóttir H, Robinson SR, Blumberg MS. Dynamics of sleep-wake cyclicity across the fetal period in sheep (Ovis aries) Developmental Psychobiology. 2011;53:89–95. doi: 10.1002/dev.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3(5):e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesku JA, Roth TC, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. American naturalist. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Lo CC, Amaral LAN, Havlin S, Ivanov PC, Penzel T, Peter JH, Stanley HE. Dynamics of sleep-wake transitions during sleep. Europhysics Letters. 2002;57:625–631. [Google Scholar]
- Lyamin OI, Mukhametov LM. Organization of sleep in the northern fur seal. In: Sokolov VE, Aristov AA, Lisitzjna TU, editors. The northern fur seal. Systematic, morphology, ecology, be-havior. Moscow: Nauka; 1998. pp. 280–302. [Google Scholar]
- Mirmiran M, Corner MA. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Research. 1982;255(1):37–48. doi: 10.1016/0165-3806(82)90074-8. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Karlsson KÆ, Blumberg MS. The preoptic hypothalamus and basal forebrain play opposing roles in the descending modulation of sleep and wakefulness in infant rats. European Journal of Neuroscience. 2006;23:1301–1310. doi: 10.1111/j.1460-9568.2006.04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Mandt BH, Obermeyer WH, Winsauer PJ, Huber R, Wikelski M, Benca RM. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii) PLoS Biology. 2004;2(7):E212. doi: 10.1371/journal.pbio.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattenborg NC, Voiron B, Vyssotski AL, Kays RW, Spoelstra K, Kuemmeth F, Wikelski M. Sleeping outside the box: electroencephalographic measures of sleep in sloths inhabiting a rainforest. Biology Letters. 2008;4:402–405. doi: 10.1098/rsbl.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffery JP, Roffwarg HP, Speciale SG, Marks GA. Ponto-geniculo-occipital-wave suppression amplifies lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Developmental Brain Research. 1999;114(1):109–119. doi: 10.1016/s0165-3806(99)00027-9. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005a;437(7063):1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: W.B. Saunders Company; 2005b. pp. 120–135. [Google Scholar]
- Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Pettigrew JD. Monotremes and the evolution of rapid eye movement sleep. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1998;353(1372):1147–1157. doi: 10.1098/rstb.1998.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. Stepping into the same river twice: Homologues as recurring attractors in epigenetic landscapes. Brain, Beahvior and Evolution. 1998;52:218–231. doi: 10.1159/000006565. [DOI] [PubMed] [Google Scholar]
- Tobler I. Is sleep fundamentally different between mammalian species. Behavioural Brain Research. 1995;69:35–41. doi: 10.1016/0166-4328(95)00025-o. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Research Bulletin. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford: Oxford University Press; 2003. [Google Scholar]
- Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, Rosa F, Mourrain P, Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biology. 2007;5:2379–2397. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain, Behavior, and Evolution. 1974;10:425–470. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]