Abstract
The Kölliker–Fuse nucleus (KFN) in dorsolateral pons has been implicated in many physiological functions via its extensive efferent connections. Here, we combine iontophoretic anterograde tracing with posthypoxia c-Fos immunohistology to map KFN axonal terminations among hypoxia-activated/nonactivated brainstem and spinal structures in rats. Using a set of stringent inclusion/exclusion criteria to align visualized axons across multiple coronal brain sections, we were able to unequivocally trace axonal trajectories over a long rostrocaudal distance perpendicular to the coronal plane. Structures that were both richly innervated by KFN axonal projections and immunopositive to c-Fos included KFN (contralateral side), ventrolateral pontine area, areas ventral to rostral compact/subcompact ambiguus nucleus, caudal (lateral) ambiguus nucleus, nucleus retroambiguus, and commissural–medial subdivisions of solitary tract nucleus. The intertrigeminal nucleus, facial and hypoglossal nuclei, retrotrapezoid nucleus, parafacial region and spinal cord segment 5 were also richly innervated by KFN axonal projections but were only weakly (or not) immunopositive to c-Fos. The most striking finding was that some descending axons from KFN sent out branches to innervate multiple (up to seven) pontomedullary target structures including facial nucleus, trigeminal sensory nucleus, and various parts of ambiguus nucleus and its surrounding areas. The extensive axonal fan-out from single KFN neurons to multiple brainstem and spinal cord structures (“one-to-many relationship”) provides anatomical evidence that KFN may coordinate diverse physiological functions including hypoxic and hypercapnic respiratory responses, respiratory pattern generation and motor output, diving reflex, modulation of upper airways patency, coughing and vomiting abdominal expiratory reflex, as well as cardiovascular regulation and cardiorespiratory coupling.
Keywords: Pneumotaxic center, Pons, Medulla, Hypoxia, Respiratory control, Upper airway resistance
Introduction
In 1923, British physician T. Lumsden (Lumsden 1923) reported that ablation of the rostral pons in vagotamized cats caused extreme prolongation of inspiration—a condition he termed “apneusis”. This seminal study established the rostral pons as the “pneumotaxic center” which is believed to play a critical role in inspiration-to-expiration phase transition or inspiratory “off-switch”. Subsequently, it was found that localized lesions or disruptions of excitatory synaptic transmission at Kölliker–Fuse nucleus (KFN)1,2 in the dorsolateral pons produced similar apneusis effects as reported by Lumsden, while electrical stimulation or glutamate injection at this nucleus provoked inspiratory “off-switch” (Cohen 1971; Dutschmann and Herbert 2006; Stettner et al. 2007; von Euler et al. 1976; von Euler and Trippenbach 1976). Electrophysiological recordings revealed many respiratory-related neurons clustering at the KFN and to a lesser extent in adjacent medial and lateral parabrachial nuclei (Cohen and Shaw 2004; Cohen and Wang 1959; Dick et al. 1994; Ezure and Tanaka 2006; Song et al. 2006). Hence, the KFN/parabrachial complex is generally considered as the anatomic substrate of the Lumsden pneumotaxic center (Alheid et al. 2004; Song and Poon 2004; Song et al. 2006).
Recent studies have revealed that the KFN, besides promoting the inspiratory off-switch, may also modulate a variety of respiratory-related functions such as the hypoxic and hypercapnic respiratory responses (Mizusawa et al. 1995; St John 1975), Hering–Breuer reflex (MacDonald et al. 2007; MacDonald et al. 2009; Siniaia et al. 2000; Song et al. 2011a), pharyngeal and laryngeal modulation of upper airways resistance during breathing and obstructive sleep apnea, vocalization, coughing and sneezing, swallowing, mastication, suckling, and diving reflex (Dutschmann and Herbert 1996, 2006; Dutschmann et al. 2007; Gestreau et al. 2005; Kuna and Remmers 1999), and nonrespiratory-related functions such as cardiovascular regulation and cardiorespiratory coupling, control of water and sodium intake, and anti-nociception (Dick et al. 2009; Gasparini et al. 2009; Guo et al. 2002; Hodge et al. 1986; Lara et al. 1994; Nag and Mokha 2004; Young et al. 1992). In humans, hypoplasia of the KFN/parabrachial complex in utero has been associated with perinatal asphyxia and death (Lavezzi et al. 2004a, b), and severe pathological changes in the KFN/parabrachial complex including KFN has been noted in patients with certain forms of dementia such as Alzheimer disease (Parvizi et al. 1998).
The profound multi-functional significance of the KFN is consistent with its extensive afferent and efferent connections with many respiratory-related structures in the brainstem and spinal cord [reviewed in (Ezure 2004; Song and Poon 2004)], and nonrespiratory midbrain and forebrain structures such as periaqueductal gray, central amygdale nucleus, and lateral hypothalamus (Fulwiler and Saper 1984; Saper and Loewy 1980). In the present report, we will focus on the KFN's efferent descending projections to brainstem respiratory- and nonrespiratory-related structures. The efferent projections from KFN to brainstem have been extensively studied using both anterograde and retrograde tracing methods. Early anterograde tracing studies (Fulwiler and Saper 1984; Gerrits and Holstege 1996; Herbert et al. 1990; Holstege 1988; Holstege and Kuypers 1977; Krukoff et al. 1993; Saper and Loewy 1980; Yokota et al. 2004) established the macropathways. However, since the medullary neurons targeted by the KFN were unidentified, whether those pathways actually terminated at hypoxia-activated or nonactivated structures has remained unclear. Moreover, it is not certain whether those KFN efferent targets were innervated by distinct types of KFN neurons each with single-axonal trajectory (one-to-one axonal fan-out) or the same type of KFN neurons each with multiple collateral trajectories (one-to-many axonal fan-out). This is an important question because a one-to-one relationship would imply a segregation of KFN neuronal functions as suggested by previous studies using antidromic activation of dorsolateral pontine neurons (Ezure and Tanaka 2006), whereas a one-to-many relationship would imply that some KFN neurons may influence multiple physiological functions all at once. In studies that employed retrograde tracing (Ellenberger and Feldman 1990; Gang et al. 1995; Kalia 1981; Nunez-Abades et al. 1993; Smith et al. 1989; Zheng et al. 1998), distinct brainstem and spinal cord respiratory-related structures were selectively mapped for their afferent connections one at a time; however, whether the same retrogradely labeled neurons in KFN also projected to other respiratory- or nonrespiratory-related structures could not be revealed. Although this problem may be alleviated to some extent by multiple retrograde labeling, current technology is limited to retrograde tracing from no more than three concurrent efferent targets.
Here, we combine anterograde tracing with posthypoxia c-Fos immunohistology to examine the terminations of labeled KFN axons among hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. By applying very small amounts of tracer into KFN using iontophoretic injection, and by introducing a set of stringent inclusion/exclusion criteria to align visualized axons across multiple coronal brain sections, we were able to trace the descending projection of individual axons and axonal branches unequivocally for a long rostrocaudal distance to their terminations in the brainstem and spinal cord. Our results reveal a one-to-many axonal fan-out from KFN neurons to multiple hypoxia-activated and nonactivated pontomedullary and spinal structures, including some novel pathways that have not been reported previously.
Materials and methods
Animal preparation
Experiments were conducted on 24 adult male Sprague–Dawley rats (Charles River Laboratory, Wilmington, MA) weighing 290–310 g. All experimental protocols had been reviewed and approved by the MIT Committee on Animal Care in accordance with published guidelines.
After injection with atropine sulfate (0.025 mg, s.c.) to reduce tracheal secretions, Buprenex (0.03 mg/kg, s.c.) for analgesia, the rat was anesthetized with pentobarbital at an initial dosage of 50 mg/kg (i.p.). Surgical anesthesia was affirmed if a pinch with a clamp at the hind paw caused no withdrawal response. Throughout the experiment, the level of anesthesia was assessed every 15 min and a supplemental dose of pentobarbital (1/10 of initial dosage, i.p.) was given when withdrawal response reappeared. Body temperature was kept at 37 ± 0.5°C with a temperature controller (CWE, TC-831, Ardmore, PA).
The animal's head was then fixed in a stereotaxic frame (KOPF 1430, David Kopf Instrument, Tujunga, CA) using ear bars but without breaking the animal's ear drums. Bregma was raised 1.5 mm higher than Lambda, leaving the dorsolateral pons readily accessible from a vertical dorsal approach. All surgical procedures were performed under sterile conditions. A small area of the dorsal scull around Lambda was exposed by cutting the skin and removing overlying tissues. A small hole was drilled on the scull at interaural level on the right side at 2.4 mm lateral to the midline sagittal seam. The brain surface was exposed by making a small “×” cutting on the dura.
Microinjection
Glass micropipettes were fabricated from borosillica pipettes (O.D. 1.0 mm, I.D. 0.5 mm) on a Sutter P-87 puller (Sutter Instrument, Novato, CA). The micropipette tip was pulled to an O.D. of 20–30 μm. Biotin dextran (BDA, M.W. 10,000) was purchased from Invitrogen-Molecular Probe (Carlsbad, CA) and dissolved in artificial cerebrospinal fluid or 0.5 M NaCl to make a 10% solution. The micropipette was back-filled with the BDA solution and inserted with a silver wire that was connected to an electrical stimulator (Master-8, A.M.P.I., Jerusalem, Israel) through an isolator (ISO-Flex, A.M.P.I.), which was used to supply stimulation or iontophoretic currents. The micropipette was inserted stereotaxically into the KFN in the dorsolateral pons [coordinates: 2.45–2.55 mm lateral from the midline, between –0.2 mm (caudal) and +0.2 mm (rostral) to Lambda, and at a depth of 8–8.5 mm from Lambda surface] (Paxinos et al. 1999; Paxinos and Watson 1986). Electrical stimulation (80 Hz, pulse duration 0.1 ms) was delivered through the micropipette to search for an optimal tip position where inhibitory respiratory responses such as bradypnea or apnea could be induced with the lowest stimulation intensity possible (typically 15–30 μA). After such low-threshold loci were identified, microinjections were made iontophoretically or by applying pressure pulses to the micropipette. For iontophoretic injection, positive square-wave current pulses (5 s-on, 5 s-off; intensity 3–5 μA) were applied for 20–30 min. For pressure injection, the micropipette was connected to a pressure microinjector (BH-2, Harvard Apparatus, Holliston, MA) and pressure pulses (5–10 Psig, duration 0.1 s) were applied repeatedly to obtain an injection volume of 20–50 nl, as calculated according to the displacement of the meniscus viewed under a stereoscope. Only one injection was made unilaterally in each animal.
In three control animals the injections (pressure injections) were deliberately made outside of KFN, at lateral parabrachial nucleus (LPBN) [coordinates: 2.4 mm lateral from the midline, 0 mm to Lambda, and at a depth of 7 mm from Lambda surface], a site ~1.0 mm medial to KFN, or trigeminal motor nucleus [coordinates: 2.0 mm lateral from the midline, 0.5 mm caudal to Lambda, and at a depth of 8.5 mm from Lambda surface].
After the injection, the micropipette was left at the injection site for at least 10 min before being withdrawn slowly. Then the dura was moved back to cover the brain surface and the hole on the skull was sealed with bone wax. The wound was subsequently cleaned and sutured. Another dose of Buprenex (0.03 mg/kg, s.c.) was given at 8 h from the initial dosage and t.i.d. for 2–3 postsurgery days until the animal showed no signs of pain. A dose of Meloxicam (1 mg/kg, s.c.) was given at the end of surgery and another on day-1 postsurgery. Ringer's solution (10–20 ml) was given subcutaneously if the rat showed signs of dehydration.
Hypoxia challenge
After a survival period of 10 days, some experimental animals with BDA pressure injections at KFN were challenged with hypoxia to induce c-Fos expression in brainstem neurons, as described previously (Song et al. 2011b). Before the hypoxia challenge, rats were accustomed to handling with daily mock experiments for 3–5 days. For hypoxia challenge, the rat was put into a 7.8-l airtight chamber that was continuously ventilated with humidified 8% O2 (balance N2) at a flow rate of 4–5 l/min. The animal was kept in the chamber for 30 min and then moved back to its daily cage. Rats were kept under room temperature (25°C) throughout the hypoxia challenge and the 2-h posthypoxia period. Rats remained alert and responsive, and no abnormal behavior (such as shivering) was observed. Blood pressure was not monitored, but another study of this laboratory revealed a moderate decrease of about 28 mmHg at the end of 1 h of hypoxia at 8% (Song et al. 2011b).
Immunohistology
Two hours after the hypoxia challenge, the rats were deeply anesthetized with urethane (2.0 g/kg, i.p.) and transcardially perfused with 300 ml of heparinized PBS (0.05 M phosphate buffer saline, pH 7.4) followed by 300 ml of 4°C 4% paraformaldehyde (dissolved in PBS). Rats that were not exposed to hypoxia were also anesthetized and perfused 10 days postsurgery in a similar fashion for histochemical analysis. The brainstems and the C5 segment of cervical spinal cord (where the phrenic motor neurons are located) were immediately removed and postfixed in 4% paraformaldehyde overnight and subsequently cut into sequential coronal sections at 40 or 50 μm thickness on a vibratome or freezing microtome. Brainstem and spinal cord sections from all hypoxia-challenged animals and some of the non-challenged animals were processed for c-Fos immunohistology. Sections were incubated in PBS-T (PBS containing 0.3% Triton X-100) that contained 0.3% H2O2 for 1 h to quench the background peroxidase activity, rinsed, and incubated in rabbit anti-c-Fos polyclonal antibody ([K-25]: sc-253, lot # B2208, Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:4,000 dilution for 24–48 h at 4°C. According to Santa Cruz Biotechnology Inc., this anti-c-Fos antibody was raised against a peptide (amino acids 115–165) mapping within an internal region of c-Fos of human origin, immunoaffinity purified, and fully characterized using Western blot analysis.
For sham control, some sections (1 from every 4 sections) were incubated in a solution that did not contain primary antibodies.
All sections were rinsed and then incubated in biotinconjugated goat anti-rabbit IgG for 1–2 h and then in ABC reagent (standard ABC kit, Vector Laboratories Inc., Burlingame, CA, at 1:200 dilution for 1–2 h) and developed with standard DAB (DAB 0.05%, H2O2 0.0015%, in PBS) method.
Axonal tracing
For systemic tracing of individual axons and axonal branches, brains of some animals with iontophoretic BDA injections were cut into sequential sections at 80 or 100 μm thickness and were processed with ABC-DAB method for visualizing anterograde labeling. To trace stem axons or branches over relatively short distances (1–3 consecutive sections), the selected axon was identified and reconstructed in each section with camera lucida drawings under a 20× or 40× objective. Reconstruction of any selected axon or axonal branch across different sections was performed only if its identity in each section was unambiguous based on its size, location, and direction of projection.
For long distance tracing of individual stem axon, only large isolated stem axons (i.e., without any surrounding axons of comparable size) were selected. In addition, since the descending pathway, once into ventrolateral pons, projected mainly in a rostrocaudal direction perpendicular to the coronal plane, a given stem axon should appear in each coronal section at a locus contiguous to that in the preceding section. If the locus of the selected stem axon shifted significantly in any section or encroached into regions where other axons of comparable size emerged in its vicinity, the tracing was discontinued. These stringent inclusion/exclusion criteria (which take advantage of the peculiar rostrocaudal trajectories of descending KFN axons) ensured the unequivocal identification of the selected stem axon over a large number (20–60) of consecutive 80 or 100 μm Nissl-stained coronal sections. In each section, if an axonal branch arising from the stem axon was identified under a 100× oil objective, the axonal branch and its terminal bifurcations within that section were drawn under the oil objective or a 40× objective. With few exceptions, axonal branches were traced only within the same section they arose; reconstruction across multiple (<3) sections was performed only when the same branch could be unambiguously identified in each section based on the above criteria.
Drawings of all reconstructed axons or axonal branches were scanned into TIF images at a resolution of 600 dpi. Photomicrographs of selected areas were taken with a Sony (Tokyo, Japan) DFW-SX900 CCD digital camera. Scanned images and photomicrographs were inputted into a computer and edited with Photoshop CS2 (Adobe Systems Incorporated, San Jose, CA) to achieve uniform brightness and contrast in group photos or montage photos.
Results
Injection sites
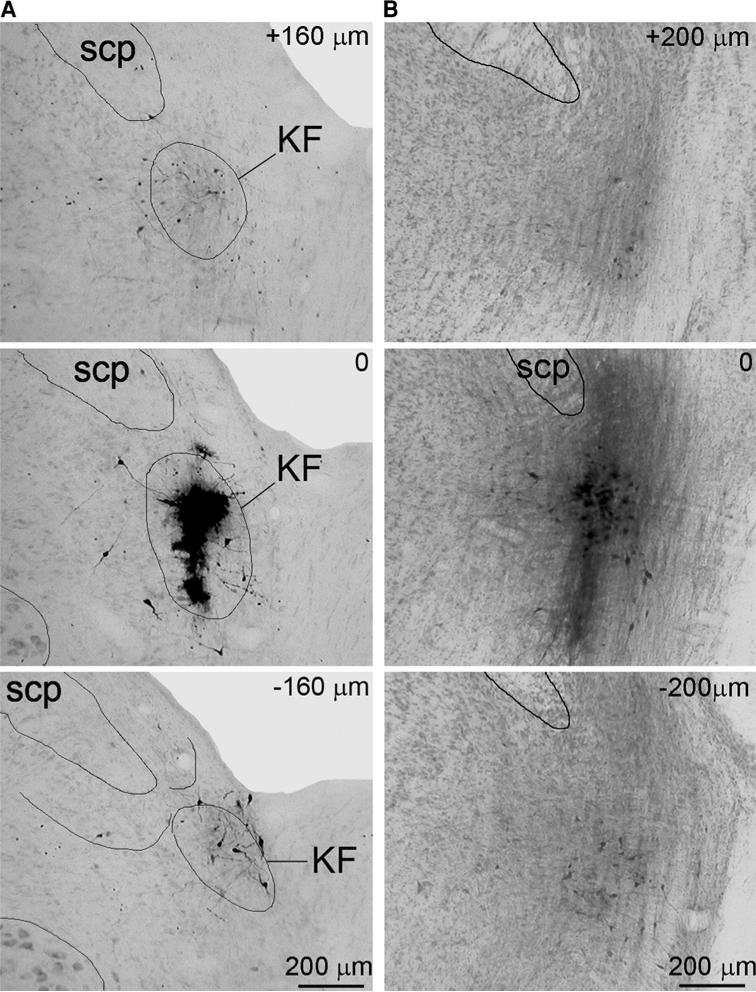
The centers of injections were confirmed to be within the boundaries of KFN in 18 rats. In these animals, electrical stimulation with very low intensity (typically 15–30 μA) at the microinjection loci evoked strong inspiratory inhibition. Iontophoretic injections were performed in seven animals, in which the diffusion of BDA (as visualized by the DAB reaction products) was quite limited and confined mainly within the boundaries of KFN. In the remaining 11 animals pressure injections were performed and the BDA infiltrated the entire KFN in mediolateral dimension, and sometimes diffused slightly into adjacent medial parabrachial nucleus. Slight diffusion along the pipette tract to the external-lateral subnucleus of LPBN was also observed. Photos of the iontophoretic and pressure injection sites in two representative animals are shown in Fig. 1. Despite the differences in injection volumes, methods of injection and extents of BDA infiltration, similar projection and innervation patterns of the labeled axons were observed in all these 18 animals.
Fig. 1.
The BDA injection sites in two animals. a iontophoretic injection; b pressure injection
In three other animals, injections were made at loci where inspiratory inhibition was evoked with relatively larger stimulation intensity (50–70 μA). The centers of injections in these animals were not within the boundaries of the KFN but at the margins or beyond; the KFN was only partially infiltrated by the injection. In these three animals, anterogradely labeled axonal terminals were also observed in pontomedullary respiratory-related structures, but much fewer than when the injections were right at the KFN with comparable injection size. Therefore, the localization of respiratory-related loci with low stimulation threshold was a useful guide for successful microinjection within the KFN. To avoid ambiguity, these three animals were not included in the present report.
In another three control animals in which injections were deliberately made outside the KFN, the injection sites were confirmed to be at the central subnucleus of LPBN (No. 1) [which also modulates the hypoxic and hypercapnic respiratory responses (Song and Poon 2009a, b)], a site medial to KFN (No. 2), or trigeminal motor nucleus (No. 3). In No. 1 and 2, labeled axons projected mainly rostrally toward forebrain, although some terminals were also observed in pontomedullary respiratory-related structures. In No. 3, labeled axons were observed to project ventrolaterally to join the motor root of trigeminal nerve. These control data showed that injections outside the KFN led to very different axonal projection patterns than those being reported here. For brevity, data from these control animals are not described further in this report.
Single KFN axon innervates multiple pontomedullary structures
One of the most striking findings of this study was that some descending axons from KFN sent out discrete branches to innervate multiple structures at different levels of the brainstem all at once (see “Appendix” for a critique of the methodologies). Frequently, stem axons were seen to send out branches or collaterals along their way to innervate neighboring structures in ventrolateral pons and medulla while continuing to project caudally therefrom (Fig. 2a, b). These stem axons, once into ventrolateral medulla, bifurcated repeatedly to innervate multiple segments of the ventrolateral medulla in close proximity to ambiguus nucleus. Some axons were seen to turn dorsomedially passing by the ambiguus nucleus to innervate the solitary tract nucleus (NTS), even crossing the midline into the NTS of contralateral side as well (Fig. 2c, d).
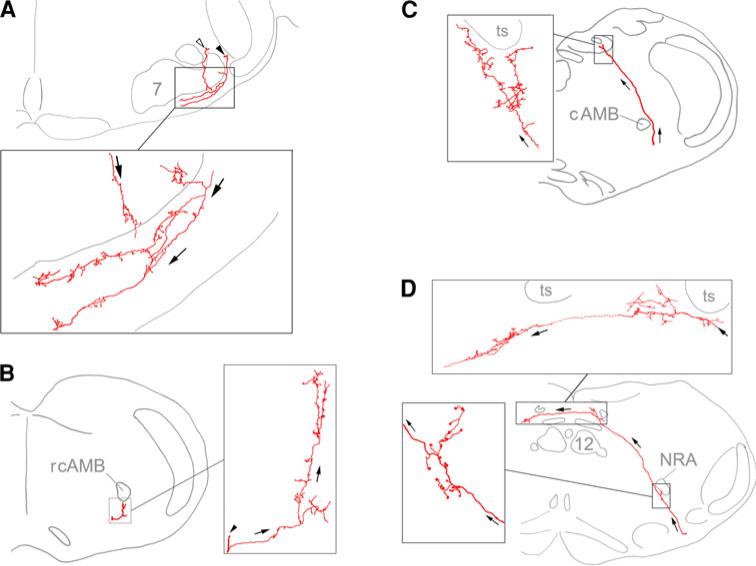
Fig. 2.
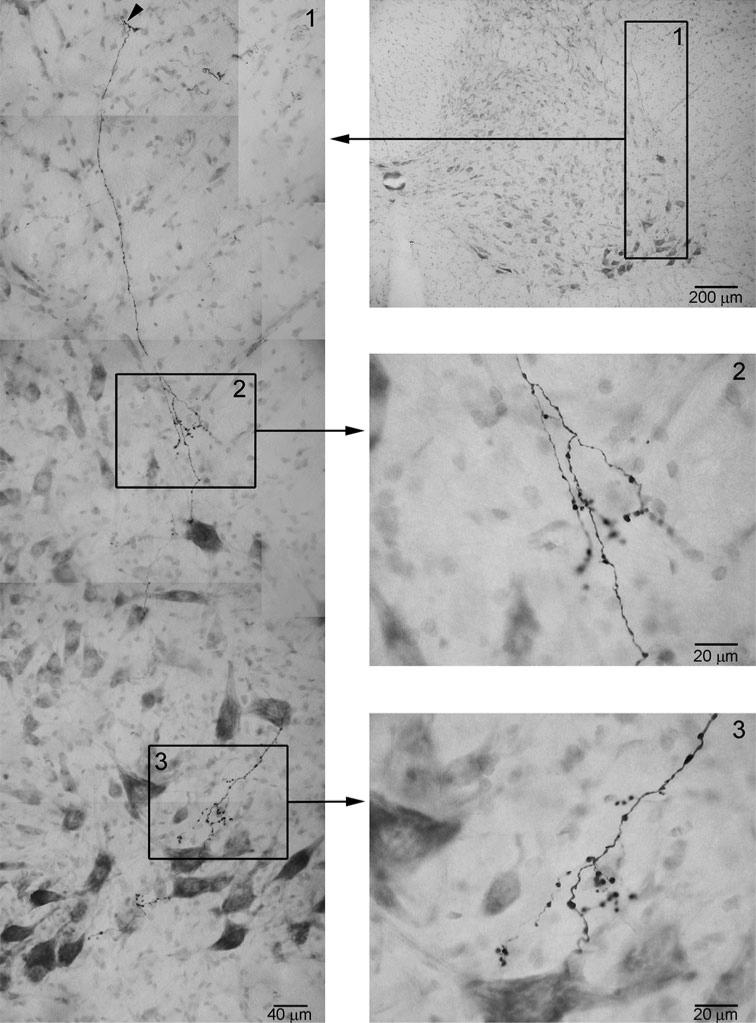
Camera lucida drawings showing the innervations of multiple structures by axonal branches from single descending axon revealed by iontophoretic tracing. a two stem axons (arrowheads) send out branches to innervate retrotrapezoid nucleus in the area ventral to the facial motor nucleus; b a stem axon (arrowhead) in the ventral medulla sends out a branch to innervate the area ventral to rcAMB. c An axon in ventrolateral medulla turns dorsomedially passing by cAMB to innervate ipsilateral ventrolateral NTS; d another axon in the ventrolateral medulla turns dorsomedially to innervate ipsilateral NTS and proceeds to cross the midline to innervate contralateral NTS. Note that this axon also sends out a few branches to innervate the NRA and its neighboring ventral area. In all the camera lucida drawings, the axonal branches and terminals in the square areas were traced within a single section (thus not all terminals are shown in the drawings). The stem axons in c and d projected in coronal plane and could be traced for most parts of their trajectories. Missing segments were found in neighboring sections and reconstructed into the drawings. The stem axons in a and b (arrow heads) projected rostrocaudally and could not be identified unequivocally after several consecutive sections
Tracing of individual axon over multiple coronal sections (15–60 sections) was successfully performed on a total of 10 axons (1–2 axons in each animal that received iontophoretic injection). All of them were relatively large-sized and well separated from neighboring axons. The stem part of these ten axons, once into ventrolateral pons, projected caudally toward medulla perpendicular to the coronal sections and none of them was seen to turn dramatically to other directions. Branches or collaterals arising from these stem axons projected mainly in coronal planes; hence they could be traced for up to 500 μm in 100-μm coronal sections. Although the stringent standards that we used in tracing stem axons ensured unequivocal identification of the same stem axon over many coronal sections, the tracings and reconstructions of axonal branches over two or more consecutive sections were often complicated by the presence of branches from other axons. As a result, axonal branches were only traced within a single section unless they could be clearly identified across sections. Figures 3–5 show the tracings of four representative axons and their branches.
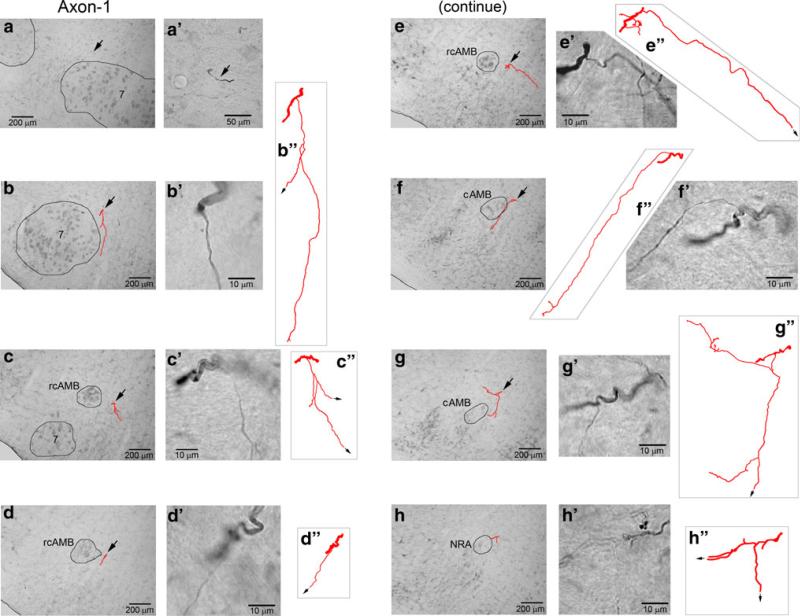
Fig. 3.
Long distance tracing of a stem axon (axon 1) and its branches. This axon was traced from the level of rostral facial nucleus to caudal medulla. At all levels, no stem axon of similar size was observed within 150 μm from this stem axon. Branches were observed to innervate parafacial area (b) and areas neighboring ambiguus nucleus (c–h). All bifurcations were confirmed to arise from the same stem axon under ×100 oil immersion objective and traced within corresponding single 100-μm section
Fig. 5.
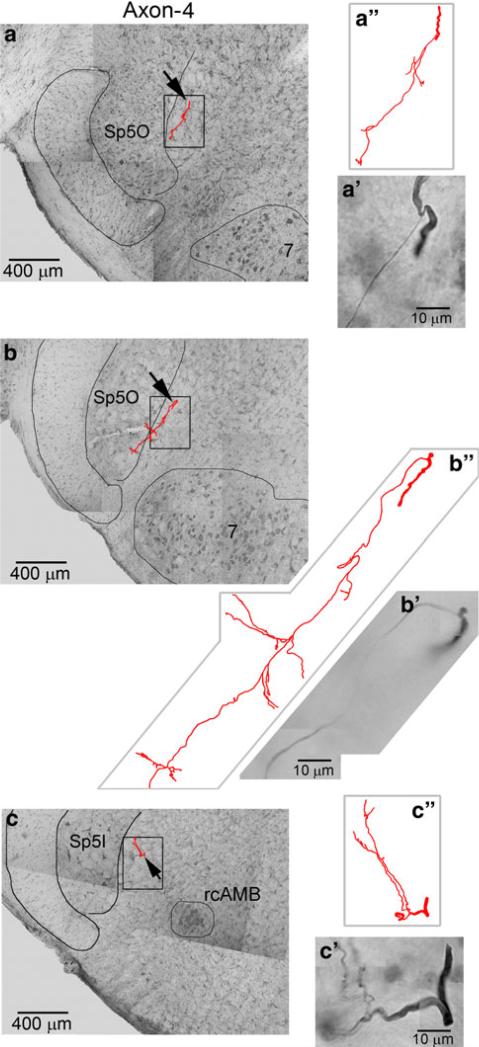
Tracing of a stem axon (axon 4) and its branches. This axon was traced from the level of rostral facial nucleus to the level of rcAMB. In every brain section until the level of rcAMB the same stem axon was identified unequivocally with no other stem axon of similar size within 150 μm from this stem axon. All the three bifurcations shown in this figure were confirmed to arise from the same stem axon under ×100 oil immersion objective and traced within corresponding single 100-μm section
Axon 1 (Fig. 3) was first identified from caudal pons where it became relatively separated from other axons. As can be seen in Fig. 3a′, the stem axon under tracing (black arrow) was well separated from other stem axons. This axon was identified in every section at roughly the same locus as in the preceding section until caudal medulla. A total of seven branches were observed to arise from this axon. They were traced for distances from ~50 to 400 μm within corresponding single 100-μm sections. The first branch innervated caudal facial nucleus and its medial neighboring area (Fig. 3b). The other six branches innervated neighboring areas of ambiguus nucleus at various levels (Fig. 3c–h).
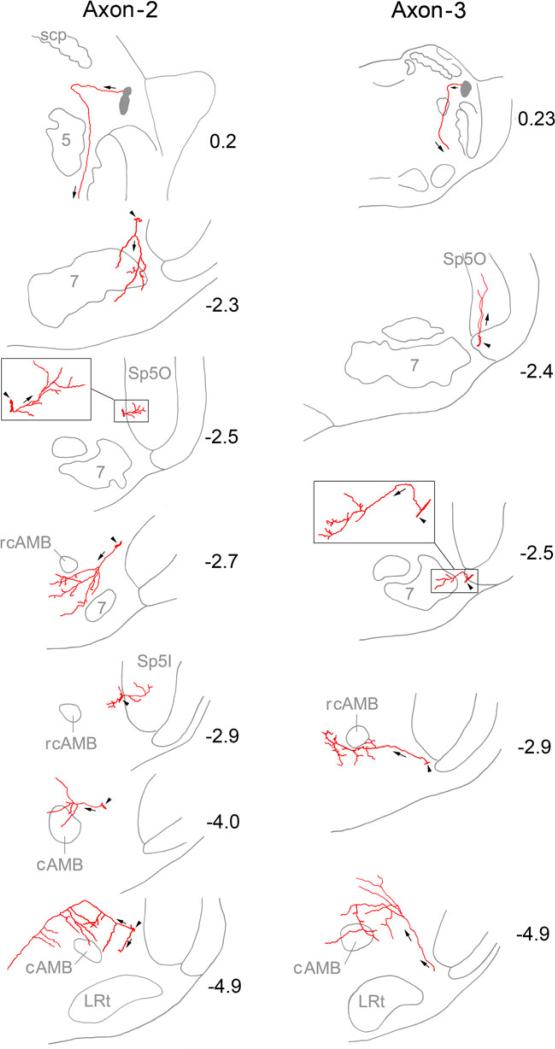
Axons 2 and 3 (Fig. 4), which were observed from different animals, had similar projection and innervation patterns. Both of them were first identified in dorsolateral pons and followed into ventrolateral pons. From here they were identified at roughly the same loci in all consecutive sections. No axon of similar size was observed in their neighborhoods in all sections. Branches or collaterals arising from these axons were observed to innervate the lateral facial nucleus that controls the perioral muscles and vibrissae muscles (Fig. 4 Axon 2 at level –2.3, and Axon 3 at level –2.5), trigeminal sensory nucleus (Fig. 4 Axon 2 at levels –2.5 and –2.9, and Axon 3 at level –2.4), areas neighboring rostral compact ambiguus nucleus (rcAMB) (Fig. 4 Axon 2 at level –2.7 and Axon 3 at level –2.9), and caudal ambiguus nucleus (cAMB, or lateral AMB in Paxinos et al. 1999) itself (Fig. 4 Axon 2 at levels –4.0 and –4.9, and Axon 3 at level –4.9).
Fig. 4.
Long distance tracing and reconstruction of two stem axons (axon 2 and 3) and their branches. Both axons were traced from rostral pons to caudal medulla. At all levels, no stem axon of similar size was observed within 150 μm from the stem axons under tracing. Branches were observed to innervate lateral division of facial nucleus (7 M), Sp5O, Sp5I, and areas neighboring ambiguus nucleus. Numbers beside the drawings indicate the distances (mm) from the interaural line. The axonal branches were identified under ×100 oil immersion objective and traced within corresponding single 80-μm section, with the exceptions at levels –2.7 and –4.9 of axon 3, and levels –2.9 and –4.9 of axon 4 where the branches were traced and reconstructed from two or three consecutive sections
Axon 4 (Fig. 5) was first identified from caudal pons where it became relatively separated from other axons. It was identified in every section at roughly the same locus as in the preceding section until the level of rcAMB. The tracing was discontinued because the axon disappeared in subsequent sections because of the fading of the labeling. Three branches were observed to arise from this axon. They were traced for various distances from ~100 to ~500 μm within corresponding single 100-μm sections. All of them projected toward the trigeminal sensory nucleus.
Besides the above described four axons that were traced for relatively long distances, another six large-diameter axons were traced for shorter distances but at least one branch was identified to arise from each stem axon to innervate neighboring areas, while the stem axons themselves continued to project caudally. Four such stem axons were found to reach spinal cord C1 segment, from which further tracing was not performed (Table 1).
Table 1.
Collateral innervations of multiple structures by single axon arising from KFN
| Confirmed number of branches | Structures innervated | |
|---|---|---|
| 1 | 7 | Parafacial area (1); para-rcAMB areas (3); cAMB/NRA areas (3) |
| 2 | 6 | Parafacial area and facial nucleus (1); para-rcAMB area (1); cAMB areas (2); trigeminal sensory nucleus (2) |
| 3 | 4 | Parafacial area and facial nucleus (1); para-rcAMB area (1); cAMB areas (1); trigeminal sensory nucleus (1) |
| 4 | 3 | Trigeminal sensory nucleus (1); areas medial to trigeminal sensory nucleus (2) |
| 5 | 1 | Para-rcAMB area; (continue into spinal cord) |
| 6 | 1 | Medial and commissural NTS; (continue into spinal cord |
| 7 | 1 | Facial nucleus and retrotrapezoid nucleus; (continue into vl-medulla) |
| 8 | 1 | Facial nucleus and retrotrapezoid nucleus; (continue into vl-medulla) |
| 9 | 2 | Para-rcAMB areas; (continue into spinal cord) |
| 10 | 1 | cAMB area; (continue into spinal cord) |
Number in brackets indicates the number of branches innervating this structure
None of the small to medium-sized axons (0.6–0.8 μm diameter) that comprised 2/3 of all the labeled descending axons was traced unequivocally for sufficient distance. Nevertheless, branches and collaterals were observed to arise from such stem axons (see below).
KFN axonal projections to hypoxia-activated/nonactivated pontomedullary structures
It is well known that many CNS neurons express c-Fos upon strong excitation (Bullitt 1990; Sagar et al. 1988). The expression of proto-oncogen c-fos reaches peak level at 1 h postexcitation and declines back to baseline after 3 h, while the c-Fos protein remains in the neurons for much longer time (Morgan et al. 1987). This phenomenon has been employed extensively for morphological visualization of neuronal excitation. As reported in early studies (Berquin et al. 2000; Bodineau and Larnicol 2001; Erickson and Millhorn 1994; Gozal et al. 1999; Hirooka et al. 1997; Song et al. 2011b; Teppema et al. 1997), hypoxia challenge or activation of carotid sinus nerve evoked significant neuronal expression of c-Fos protein in various brainstem respiratory-related structures such as ventrolateral medullary areas where ventral respiratory neuronal column (VRC) is located (i.e., areas ventral to rcAMB-subcAMB-cAMB), medial and commissural NTS, dorsolateral pontine pneumotaxic center (LPBN and KFN) and ventrolateral pontine areas. In the present study, we used this method to mark hypoxia-activated brainstem neurons that were juxtaposed to terminations of descending KFN axonal projections. Table 2 summarizes the extents of labeled KFN axonal terminals and posthypoxia c-Fos positive neurons and of their overlapping in various respiratory-related pontomedullary structures.
Table 2.
Labeled Kölliker–Fuse axonal terminals, c-Fos immunopositive neurons and their overlappings in various pontomedullary and spinal structures
| Brainstem structures | Labeled terminalsa | c-Fosa | Overlappinga |
|---|---|---|---|
| KF (contralateral) | |||
| Rostral | + | ++ | + |
| Caudal | + | +/– | +/– |
| MPBN | + | +/– | +/– |
| LPBN | |||
| Central | +/– | +++ | – |
| Ext-lat. | ++ | +++ | ++ |
| vl-pons | |||
| ITN | ++ | +/– | +/– |
| vl-pons, lateral (A5) | ++ | ++ | ++ |
| vl-pons, medial | +/– | +/– | +/– |
| Locus coeruleus | – | ++ | – |
| Trigeminal motor nucleus | +/– | – | – |
| Facial motor nucleus/parafacial region | |||
| Facial motor nucleus | +++ | – | – |
| Parafacial region | ++ | +/– | +/– |
| RTN | ++ | +/– | +/– |
| rcAMB/peri-rcAMB and subcAMB/peri-subcAMB | |||
| rcAMB or subcAMB | – | – | – |
| Medial | +/– | + | +/– |
| Ventral (RVL) | +++ | +++ | +++ |
| LPGi | +/– | ++ | +/– |
| cAMB/peri-cAMB (obex level) | |||
| cAMB itself | + | + | + |
| Medial | ++ | ++ | ++ |
| Ventral (RVL/CVL) | +++ | +/– | +/– |
| LRt | + | +++ | + |
| NRA/peri-NRA (caudal to obex) | |||
| NRA | + | + | + |
| Dorsal and medial | + | + | + |
| Ventral (CVL) | + | +++ | + |
| Parapyramidal nucleus | +/– | ++ | +/– |
| Raphe (midline) | |||
| Raphe magnus | + | +/– | +/– |
| Raphe obscurus | +/– | + | +/– |
| Raphe pallidus | +/– | ++ | +/– |
| NTS | |||
| Commissural | ++ | ++ | ++ |
| Medial | ++ | +++ | ++ |
| vl-NTS | |||
| Rostral to obex | ++ | + | + |
| Obex level | ++ | ++ | ++ |
| Dorsal vagal motor nucleus | +/– | +/– | – |
| Hypoglossal nucleus | ++ | – | – |
| Trigeminal sensory nucleus, ventral part | + | +/– | +/– |
| Spinal cord C5 | |||
| Dorsal horn | – | – | – |
| Intermediate | + | – | – |
| Ventral horn | + | – | – |
Innervations of brainstem structures by labeled terminals are dominantly ipsilateral; however, the distribution of c-Fos positive neurons is bilaterally symmetrical. In this table, the innervations of labeled terminals and their overlappings with c-Fos positive neurons are shown for the ipsilateral side only unless otherwise indicated +++, high density; ++, moderate density; +, low density; +/–, occasionally observed; –, not observed AMB ambiguus nucleus, cAMB caudal AMB, rcAMB rostral compact AMB, subcAMB subcompact AMB, NRA nucleus retroambiguus nucleus, CVL caudal ventrolateral reticular nucleus, ITN intertrigeminal nucleus, KF Kölliker–Fuse nucleus, LPBN lateral parabrachial nucleus, LPGi lateral paragigantocellular reticular nucleus, LRT lateral reticular nucleus, MPBN medial parabrachial nucleus, NTS solitary tract nucleus, vl-NTS ventrolateral NTS, RTN retrotrapezoid nucleus; RVL rostral ventrolateral reticular nucleus
Pons
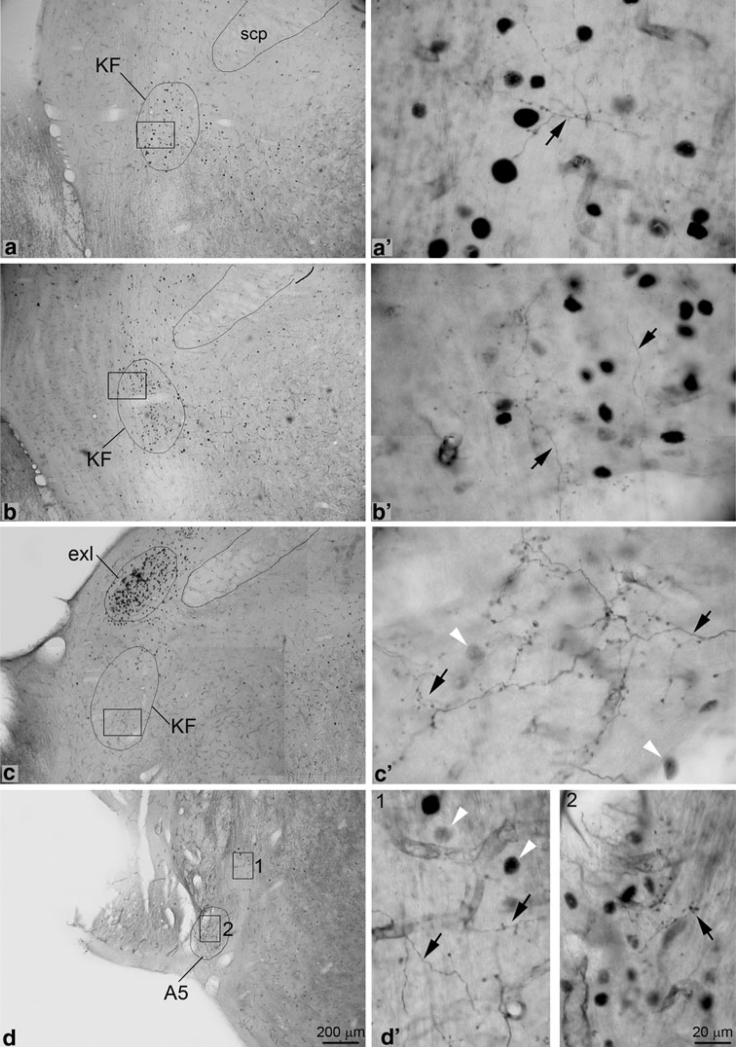
Overlapping of labeled axonal terminals with c-Fos positive neurons was observed in the rostral part of KFN of contralateral side (Fig. 6a, b), the external-lateral subnucleus of LPBN of ipsilateral side, and the ventrolateral pons of both sides (Fig. 6d, showing contralateral side only). The caudal part of the contralateral KFN contained labeled terminals but only a few c-Fos positive neurons (Fig. 6c). The contralateral LPBN contained the highest density of c-Fos positive neurons but only a few labeled terminals in its external-lateral subnucleus. A small but densely packed group of c-Fos positive neurons was observed in the locus coeruleus; however, few labeled terminals were seen in this structure.
Fig. 6.
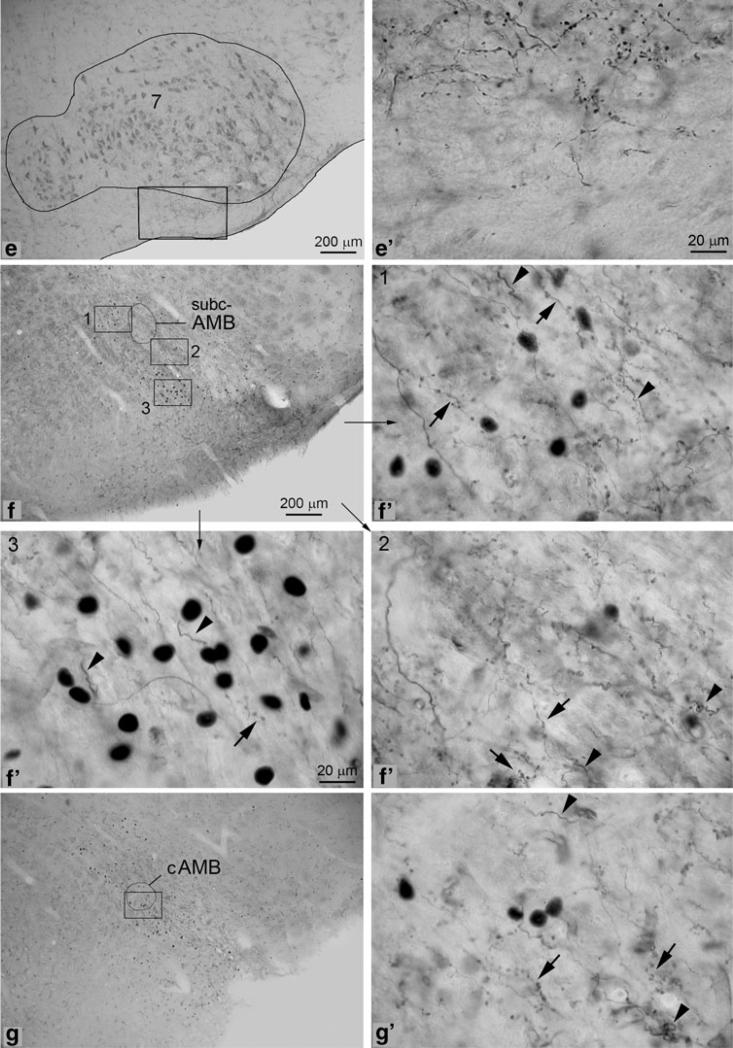
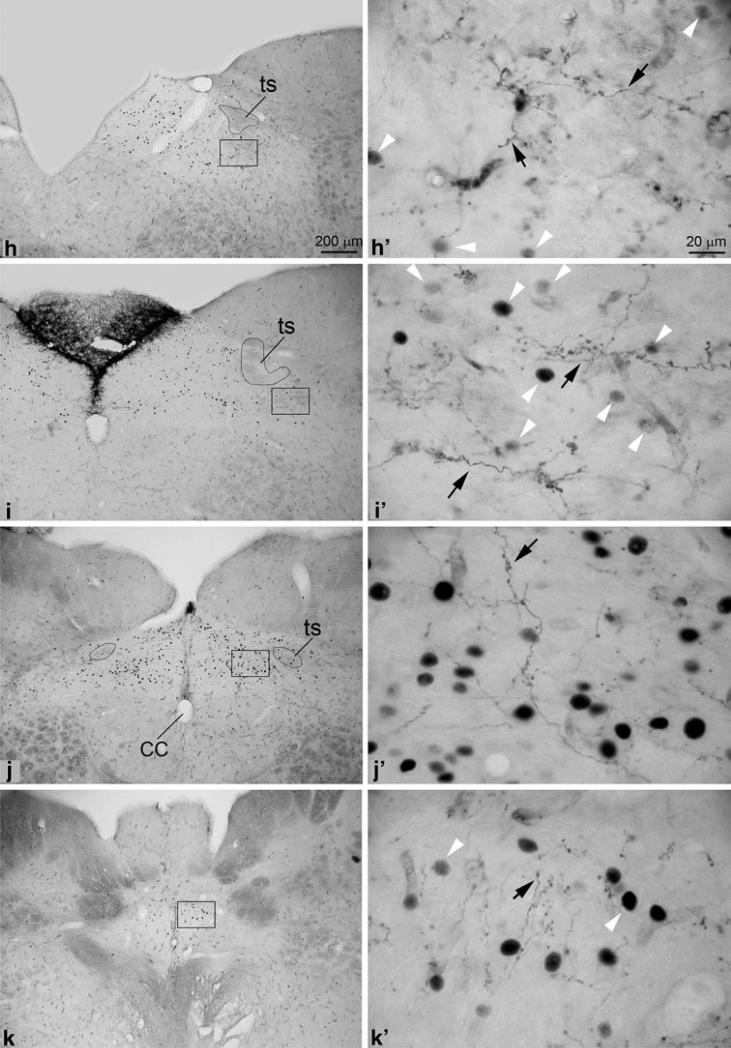
Photomicrographs showing the overlapping of labeled axonalc terminals and c-Fos positive neurons. The latter, as indicated by white arrowheads are darkly labeled in their nuclei. Photos were taken from brainstem sections of the animal shown in Fig. 1b. a–d (a′–d′): Many c-Fos positive neurons are observed in contralateral rostral KFN (a, a′; b, b′). Among the c-Fos positive neurons, a few labeled terminals that come from crossing axons are observed. The contralateral caudal KFN (c, c′) contains only a few c-Fos positive neurons but moderate density of labeled terminals. Also at this level, the external-lateral subnucleus of lateral parabrachial nucleus contains the highest density of c-Fos positive neurons but few labeled terminals. A small but densely packed group of c-Fos positive neurons are observed in the ventrolateral pontine region that corresponded to A5 (d, d′). Many labeled terminals are observed to overlap with them in this region, especially in the ipsilateral side (data not shown). The photo shows the contralateral side, where fewer labeled terminals are observed (square 2). A few c-Fos positive neurons and labeled terminals are also observed in the region that is ventral to the trigeminal motor nucleus (square 1). e–g (e′–g′): The retrotrapezoid nucleus (e, e′) were richly innervated but few c-Fos immunopositive neurons are observed in this structure. At the obex level (f, f′), a small group of c-Fos positive neurons are observed in area medial to subcAMB (square 1), where labeled terminals are also observed. Still at this level, the area immediately ventrolateral to the subcAMB contains the highest density of labeled terminals but only a few c-Fos positive neurons (square 2). Going further ventrally from this area, a large group of c-Fos positive neurons are observed but much fewer labeled terminals to overlap with them (square 3), although many stem axons can be seen in this region. At levels caudal to obex (g, g′), both c-Fos positive neurons and labeled terminals are observed to overlap with each other in and around the cAMB. h–k (h′–k′): In the rostral part of NTS, labeled terminals are only observed in the ventrolateral subnucleus (h, h′). A few c-Fos positive neurons are also observed in this subnucleus. In the mediate part of NTS (around the level of obex), c-Fos positive neurons and labeled terminals are observed to overlap with each other in commissural, medial, and ventrolateral subnuclei (i–j, i′–j′). In caudal part of NTS, c-Fos positive neurons and labeled terminals are only observed to overlap each other in commissural subnucleus (k, k′)
Ventrolateral medulla
The ventrolateral medulla is the major target of KFN descending pathways. In general, the ventrolateral medullary regions that were innervated by labeled terminals constituted a continuous and rostrocaudally extending column (“column of innervations”) starting from the facial nucleus and extending all the way to the level of pyramidal decussation at the medullary-spinal junction, with the density of innervation exhibiting gradual decrease while going caudally. Functionally, this “column of innervations” corresponds to the VRC, which comprises the parafacial/RTN respiratory group, Bötzinger complex, pre-Bötzinger complex, and the classical VRG in association with the ambiguus nucleus. The most rostral part of this column—retrotrapezoid nucleus (RTN) located in the narrow region between the ventral margin of facial motor nucleus and ventral medullary surface. This region was richly innervated by labeled terminals (Fig. 6e) but contained only a few c-Fos immunopositive neurons. Labeled axons or branches were observed to leave the descending pathway (traveling in the region lateral to facial motor nucleus) and extend medially to enter this region. Some axonal branches entered this region from its dorsal aspect after leaving the medial facial subnucleus. Once into this region, labeled axons or axonal branches were seen to bifurcate to generate terminals rich in synaptic buttons. In addition, areas surrounding the caudal part of facial nucleus, especially the region between the caudal end of facial nucleus and the rcAMB [which corresponds to part of the parafacial respiratory group as defined by Onimaru et al. (1987, 2006; Onimaru and Homma 2003)] were richly innervated by labeled terminals. However, since no effort was made to label the neurons of RTN/parafacial respiratory group, whether those labeled axonal branches and terminals actually made synaptic connections with RTN/parafacial respiratory neurons could not be confirmed. Innervations of the contralateral RTN and parafacial areas were very weak or not observed at all.
Moving to rostral medulla, the rcAMB and subcAMB themselves were not (or only weakly) innerved by labeled terminals. No c-Fos positive neuron was observed in these two subnuclei. In contrast, the region that is immediately ventral to rcAMB-subcAMB contained a high density of labeled terminals with rich varicosities or synaptic buttons and moderate density of c-Fos positive neurons (Fig. 6f). This region corresponds to the Bötzinger/pre-Bötzinger complexes. Medial to this region, the lateral paragiganto-cellular nucleus contained many c-Fos positive neurons and a few labeled terminals.
Moving caudally, the trajectory continued into the cAMB and its surrounding areas without interruption. Here the cAMB itself was moderately innervated by labeled terminals and contained a few c-Fos positive neurons. At the levels around the obex, both labeled terminals and c-Fos positive neurons were observed in the area of cAMB and in its surrounding areas (Fig. 6g). At levels caudal to the obex, terminals were still observed in and around the NRA, but the density decreased significantly. A few terminals were observed even at the level of pyramidal decussation in the area corresponding to NRA.
Anterogradely labeled axonal terminals were observed in areas corresponding to the C1 and A1 catecholaminergic neuronal groups (Paxinos et al. 1999), but much less dense. c-Fos immunopositive neurons were also observed in these areas.
Solitary tract nucleus
Rich innervations by labeled terminals were observed at levels around the obex. Labeled axons were found to leave the descending pathway and project dorsomedially to the NTS. Once into NTS, those axons bifurcated repeatedly to generate large number of terminal branches innervating the ventrolateral, medial, and commissural subnuclei. Axonal branches were also found to extend medially crossing the midline into the contralateral medial subnucleus, where they bifurcated repeatedly into many terminals. In addition, the contralateral NTS was also innervated by axons that crossed to the other side of the pons before descending.
A high density of c-Fos positive neurons overlapping with labeled terminals was observed in the medial subnucleus. Overlapping was also found in the commissural and ventrolateral subnuclei, although less numbers of c-Fos positive neurons were observed here (Fig. 6h–k).
Trigeminal sensory nucleus
At all levels from pons to caudal medulla, the trigeminal sensory nucleus was mainly innervated by axonal branches arising from the descending stem axons. Sporadic c-Fos positive neurons were observed in this structure and overlapping with labeled terminals was insignificant.
Raphe nuclei
In contrary to previous studies in cats that showed intense KFN projections to medullary raphe nucleus (Gang et al. 1991; Holstege 1988), we observed that the innervations of medullary raphe were weak in general. While some labeled terminals were confirmed in the raphe magnus, few were observed in raphe pallidus and obscurus. A few c-Fos positive neurons were observed in raphe pallidus and obscurus, but rarely in raphe magnus. Overlapping with labeled terminals was insignificant.
KFN axonal projections to cranial and spinal motoneurons
Facial and hypoglossal motor nuclei
Innervation of facial nucleus was predominantly ipsilateral. In the rostral part of facial nucleus, labeled axonal terminals were observed in its lateral and ventral subnuclei (as well as in P7); only a few terminals were found in the medial subnucleus and none in the dorsal subnucleus. The caudal part of the facial motor nucleus and its immediate surrounding regions were heavily innervated. Large motor neurons were observed to be in close contact with BDA labeled terminals and buttons, indicating the synaptic connections between these labeled terminals and facial motoneurons. No c-Fos positive neuron was observed in the facial motor nucleus.
The dorsal and lateral parts of the ipsilateral hypoglossal nucleus were richly innervated by labeled axons coursing via the ventrolateral medullary pathway. Hypoglossal motoneurons were observed to be in close contact with the BDA labeled axonal terminals and buttons. The contralateral hypoglossal nucleus was only weakly innervated. No c-Fos positive neuron was observed in this nucleus.
Cervical spinal cord C5 segments
In cervical spinal cord segment 5 (C5) from which the phrenic nerve arises, stem axons (medium size, diameter 0.6–0.7 μm) were mostly observed in the dorsal aspect of lateral funiculus. From here axons or branches turned medially to enter the gray matter of Rexed (Rexed 1954) laminae 5–7, then ventrally to enter the ventral horn, terminating in the phrenic nucleus in lamina 9 in proximity of large motor neurons (Fig. 7). A few stem axons were observed in the ventral funiculus. From there axons or branches turned dorsally to enter the ventral horn directly, terminating in lamina 9 in proximity of motor neurons. Innervation of C5 was bilateral with ipsilateral dominance. Axons were also observed to cross to the other side through anterior commissura. Few or no c-Fos positive neurons were observed in C5.
Fig. 7.
Photomicrographs showing the innervation of cervical spinal cord segment C5. Left: montage photo of the square area in right upper photo (square-1), showing the innervation of lamina 7 (square-2) and lamina 9 (square-3) by an axonal branch that arises from a stem axon (black arrowhead) in the dorsal lateral funiculus. Right (mid and bottom photos): large magnification photos of square areas in the left photo, showing the terminals and synaptic buttons in lamina 7 (mid photo) and lamina 9 (bottom photo)
Innervation of dorsal horn laminae 1–2 at the C5 level was rare, but a few terminals were observed in laminae 3–4. A few labeled terminals were also observed in the intermediate zone (laminae 5–7) and lamina 10.
Discussion
The present results reveal descending projections from KFN to brainstem structures known to participate in respiratory rhythm generation (parafacial region and areas ventral to rcAMB-subcAMB), respiratory chemoreflex (vl-pons, medial and commissural NTS, RTN), control of inspiratory motor output and upper airways patency (facial motor nucleus, hypoglossal nucleus, ventrolateral NTS, cAMB and its surrounding regions), control of expiratory motor output (NRA), and cardiovascular regulation (e.g., KFN, vl-pons, medial/commissural NTS). Some (but not all) of these structures were also activated by hypoxia, indicating their possible involvements in the hypoxic chemoreflex. Most strikingly, a single descending axon from KFN could send out branches to innervate multiple pontomedullary structures all at once. This “one-to-many” collateralization property of KFN stands in contrast to those of other brainstem structures, some of which (such as the NTS) may also send neuronal projections to multiple structural targets but often only one at a time (Hermes et al. 2006). These findings (summarized in Fig. 8) shed new light on the multi-functional role of KFN neurons in modulating respiratory and other physiological functions.
Fig. 8.
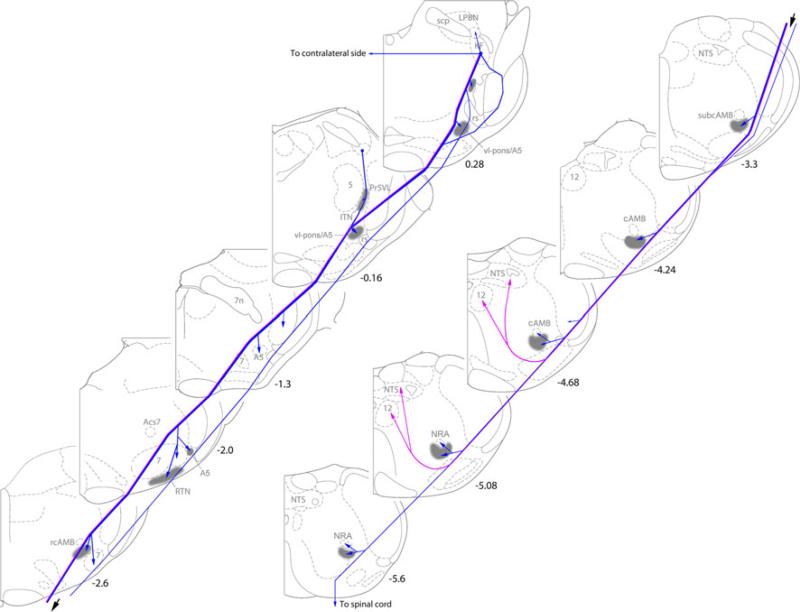
Schematic drawings summarizing the general innervation pattern of descending axonal projections from KFN. Arrows indicate axonal terminations and branch/collateral innervations. Numbers beside the drawings indicate the distances (mm) from the interaural line. Background drawings (halftone) are adapted from Paxinos rat brain atlas (Paxinos et al. 1999; Paxinos and Watson 1986). The descending projection comprised three discrete pathways: the dominant ipsilateral ventrolateral pathway that contained fibers innervating ventrolateral medulla and fibers innervating dorsomedial medulla (pink) (NTS), the accessory rubrospinal pathway, and the contralateral pathway. The rubrospinal pathway cannot be distinguished from the ventrolateral pathway once into medulla in our sections. Structures innervated by these descending pathways include LPBN in dl-pons, ITN, vl-pons/A5, facial motor nucleus and parafacial regions including RTN, areas ventral to rcAMB-subcAMB, cAMB and NRA and their surrounding regions, hypoglossal nucleus, caudal NTS subnuclei (medial, commissural, ventrolateral), and ventral horn of spinal cord C5-6 segments (not included in this figure). The contralateral pathway innervates similar structures but much weaker. 12 hypoglossal nucleus, 5 trigeminal motor nucleus, 7 facial nucleus, 7n facial nerve, Acs7 accessory facial nucleus, A5 A5 group of catecholamine neurons, AMB ambiguus nucleus, LPBN lateral parabrachial nucleus, ITN intertrigeminal nucleus, KF Kölliker–Fuse nucleus, NTS solitary tract nucleus, Pr5VL ventrolateral principal sensory trigeminal nucleus, RTN retrotrapezoid nucleus, rs rubrospinal tract, scp superior cerebellum peduncle, vl-pons ventrolateral pons
“One-to-many” collateralization of KFN neurons: role in synchronizing respiratory activities
Besides respiratory pump muscles (diaphragm and intercostals), respiratory-related activities are also manifested in multiple somatic, cranial and visceral motor nerves such as abdominal, facial, hypoglossal, vagal and recurrent laryngeal, etc. The respiratory activities of all these motor neurons (and muscles) must be synchronized to the same biphasic respiratory rhythm. In addition, these motor neurons (and muscles) are also involved in nonrespiratory somatic movements and must be coordinated with respiratory movements. We observed that KFN neurons projected extensively to these motor neurons and some KFN axons even innervated multiple structures all at once through “one-to-many” collateralization, i.e., they sent out branches to innervate multiple brainstem structures along their pathway in ventrolateral medulla.
As suggested in previous studies, dorsolateral pontine respiratory neurons may play an important role in integrating afferents from pulmonary mechanoreceptors, peripheral/central chemoreceptors, and trigeminal sensory and somatic proprioceptive afferents (Ezure 2004; Song and Poon 2004; Song et al. 2011b; Song et al. 2006). The divergent fan-out of the KFN efferent projections to multiple pontomedullary respiratory-related structures provides a possible mechanism to ensure that the entire respiratory motonetwork be synchronized. A good example is the early-expiratory (postinspiratory) neurons in the dorsolateral pons (Ezure and Tanaka 2006; Poon and Song 2004; Song et al. 2006), which have been shown to exhibit fast activation-adaptation response to pulmonary mechanoafferents and respiratory phase–gated excitation response to hypoxia (Poon and Song 2004). The present findings support the hypothesis that postinspiratory neurons in the KFN may be involved in synchronizing the termination of inspiration or promoting the inspiratory-to-expiratory off-switch in all inspiratory-related premotor/motor neurons and pre-Bötzinger inspiratory pacemaker neurons (Wittmeier et al. 2008).
Interestingly, KFN axons that exhibited “one-to-many” collateralization property had large stem diameters (1.0–1.8 μm) thus high conduction velocities. This anatomical feature enabled them to transmit pontine pneumotaxic information to brainstem and spinal cord with the shortest possible delays (see “Appendix” for a critique of the microinjection and axonal tracing techniques). This is necessary since certain respiratory activities such as inspiratory off-switching is finished within tens of milliseconds.
The “one-to-many” innervation pattern appears to be unique to axonal projections to the ventrolateral respiratory column – no axon was observed to send out branches to innervate both ventrolateral medulla and NTS (dorsomedial medulla). In a previous study (Chamberlin and Saper 1994), double-labeled neurons were observed in dl-pons after two retrograde tracers were simultaneously injected into rostral and caudal ventrolateral medulla, but few after retrograde tracers injections into NTS and ventrolateral medulla. In the present study, all the ten axons that we traced did not send branches to NTS. Presumably, the KFN projections to NTS or ventrolateral medulla are of different functional modalities, hence few cross-innervations existed. For example, KFN projections to NTS may modulate peripheral chemoreflex, baroreflex or Hering–Breuer reflex, whereas projections to ventrolateral medulla may participate in the generation and shaping of respiratory pattern and the synchronization of respiratory motor activities.
In particular, laryngeal constrictor motoneurons exhibit postinspiratory (or early-expiratory) activity and are located in caudal AMB, while the laryngeal dilator motoneurons exhibit inspiratory activity and are located in rostral ambiguus (rcAMB and subcAMB) (Davis and Nail 1984; Hinrichsen and Ryan 1981; Shiba et al. 2007; Sun et al. 2008). We found that the rcAMB-subcAMB was rarely innervated (if at all) by axonal projections from KFN, whereas the caudal AMB was well innervated. These findings afford anatomical evidence in support of recent report that the KFN facilitated laryngeal postinspiratory muscle contraction, providing a braking effect for early-expiratory airflow during breathing (Dutschmann and Herbert 2006). The NRA contains premotor neurons that control the thoracic and abdominal expiratory muscles. Subramanian and Holstege (2009) reported that chemical stimulations delivered to different subdivisions of this structure activated different expiratory muscles without causing any changes in inspiratory activity. The present and previous studies (Gerrits and Holstege 1996) revealed rich innervations of NRA from KFN and lateral parabrachial nucleus, indicating a possible role for KFN and lateral parabrachial nucleus in modulating active expiration (e.g., during heavy exercise, hypoxia, hypercapnia, vocalization, etc.) and reflexes (e.g., vomiting, coughing, sneezing, swallowing, etc.) that recruit expiratory muscles such as external intercostals and abdominal muscles.
Similarly, upper airways resistance is controlled by motor neurons in hypoglossal motor nucleus, facial motor nucleus and ambiguus nucleus, all of which exhibit respiratory-related activities. For example, pre-inspiratory/inspiratory activities of the hypoglossal nerve cause the tongue to move forward, enlarging the pharyngeal diameter to facilitate airflow during inspiration (Ryan and Bradley 2005), whereas respiratory-related activities of the facial nerve cause the dilation of nasal airway (Hwang et al. 1988; Strohl 1985). The heavy innervations of the hypoglossal motor nucleus and facial motor nucleus by axonal projections from KFN indicated that the KFN may participate in the control of upper airways resistance via the hypoglossal and facial nerves. This hypothesis is supported by previous findings that the hypoglossal motor nucleus received axonal projections from pontine inspiratory and expiratory–inspiratory neurons (Ezure and Tanaka 2006), and that hypoglossal nerve discharge was depressed and its pre-inspiratory component was eliminated following removal of the rostral pons (Smith et al. 2007). Since hypoglossal pre-inspiratory/inspiratory activity is critical in maintaining pharyngeal airway patency especially during sleep, malfunctioning of the KFN neurons could increase the risk of obstructive sleep apnea (Dutschmann et al. 2007) and may potentially disrupt a variety of oropharyngeal functions such as during vocalization, coughing/sneezing, swallowing, mastication and suckling (Gestreau et al. 2005). In addition, the facial motor nucleus (its lateral subnucleus) controls the movements of vibrissae in rats. When the rats were sniffing around to explore their environment, the whisking movements of vibrissae were synchronized with respiration (Welker 1964). The KFN innervations of lateral facial nucleus might play a role in this synchronization.
Role of KFN in respiratory pattern generation
The basic respiratory rhythm is thought to be generated through interactions between two groups of pacemaker-like neurons that set the inspiratory and expiratory rhythm (Wittmeier et al. 2008). The inspiratory pacemaker-like neurons are localized in the pre-Bötzinger complex (Smith et al. 1991). The anatomical loci of the expiratory pacemaker neurons are not very well defined but were recorded in areas surrounding the facial motor nucleus (RTN and other areas ventrolateral to facial motor nucleus) and have been collectively referred to as the parafacial respiratory group (Onimaru et al. 1987; Onimaru and Homma 2003). An intermediate phase of the respiratory rhythm, the postinspiratory phase, is thought to be gated by the KFN (Dutschmann and Herbert 2006; Stettner et al. 2007) and is mediated by neurons in the Bötzinger complex (Burke et al. 2010). In the present study we observed rich KFN axonal innervations in areas corresponding to the pre-Bötzinger complex, Bötzinger complex and parafacial respiratory group and RTN; similar innervations of the RTN have also been reported by Rosin et al. (2006) in rats and Li and Song (2001) in rabbits. These findings provide anatomical support for the notion that pontine neurons play an important role in the generation and shaping of the three-phase respiratory pattern (Chamberlin 2004; Cohen and Shaw 2004; Dutschmann and Herbert 2006; Smith et al. 2007; Song and Poon 2004; Song et al. 2010).
Interaction of KFN with other pontine respiratory-related nuclei
The existence of local respiratory neuronal circuits within the pons has long been suggested. For example, multielectrode array recordings have revealed excitatory connections between respiratory modulated neurons in dorsolateral pons (Yu et al. 2006). Such functional local connections are supported by the present anatomical finding of axonal branches and terminals rich in synaptic buttons in ipsilateral medial parabrachial nucleus and external-lateral subnucleus of LPBN. In addition, the present study revealed innervations of the contralateral KFN, indicating interactions between bilateral KFN neurons.
Our data show that descending axons from KFN passed through intertrigeminal nucleus and ventrolateral pons en route to the medulla. Both these structures have been established as accessory pneumotaxic structures (Chamberlin and Saper 1994; Jodkowski et al. 1994, 1997). We found that many descending axons that arose from KFN sent out branches to innervate intertrigeminal nucleus and ventrolateral pons. This anatomic finding, together with the previous report that many ventrolateral pontine neurons were orthodromically or antidromically activated by electrical stimulations at the dorsolateral pons (Dawid Milner et al. 2003), suggest direct connections between KFN and ventrolateral pons.
Role of KFN in hypoxic respiratory and cardiovascular control
The present study revealed an identical distribution pattern of c-Fos immunopositive neurons in brainstem following hypoxia challenge as previous reports (Bodineau and Larnicol 2001; Erickson and Millhorn 1994; Song et al. 2011b; Teppema et al. 1997). Axonal terminals that were anterogradely labeled from KFN were observed to overlap with c-Fos immunopositive neurons mainly in KFN, ventrolateral pons, ipsilateral NTS subnuclei (commissural, medial, ventrolateral), and ventrolateral medullary areas immediately ventral to AMB. All these structures are established participants of the hypoxic respiratory reflex: the NTS subnuclei being the second-order relay station of peripheral chemoreceptors (Finley and Katz 1992), areas ventral to rcAMB-subcAMB [the pre-Bötzinger complex (Smith et al. 1991), which is considered to be chemosensitive itself (Solomon et al. 2000)] being the locations of the putative inspiratory pacemakers, and the ventrolateral pons region mediating the hypoxic expiratory short-term depression or posthypoxia frequency decline (Coles and Dick 1996). In addition, our recent study has demonstrated that the dorsolateral pons itself receives axonal projections from hypoxia-excited neurons in the NTS and ventrolateral medulla. Some of these structures such as the commissural/medial NTS and ventrolateral/dorsolateral pons are also established participants of cardiovascular regulation (Guyenet 2006). The present findings provide direct anatomical support for previous reports that the pneumotaxic center modulates the hypoxic cardiorespiratory response (Mizusawa et al. 1995; Song et al. 2011b; St John 1975).
In control (nonhypoxia-challenged) animals c-Fos immunopositive neurons were confined mainly in nonrespiratory-related structures and rarely in respiratory-related structures (except the LPBN). It should be pointed out that some respiratory neurons may not express c-Fos even under strong excitatory respiratory stress (e.g., no c-Fos immunopositive neurons were observed in structures where respiratory motor neurons clustered, such as hypoglossal nucleus, rcAMB-subcAMB itself, spinal cord C5 ventral horn). On the other hand, hypoxia may evoke c-Fos expressions in nonrespiratory neurons secondary to other resultant physiological effects such as hypothermia, hypotension, and CNS depression. However, activation of the peripheral chemo- and baroreceptor afferents by electrical stimulation of carotid sinus nerve in rats produced similar c-Fos expression in brainstem respiratory-related structures as hypoxia, presumably without other side effects of hypoxia (Erickson and Millhorn 1994). Thus it is highly likely that the c-Fos expressions following hypoxia challenge were primarily due to the activation of central pathways mediating the peripheral cardiorespiratory chemoreflex and secondarily, baroreflex.
Many c-Fos immunopositive neurons in NTS and VLM were catecholaminergic neurons (immunopositive to tyrosine hydroxylase). In one study in rats (Erickson and Millhorn 1994) it was reported that up to 68% of hypoxia/CSN stimulation evoked c-Fos immunopositive neurons in NTS and 89% in VLM were catecholaminergic. In another study in rabbits, 27% of hypoxia-evoked c-Fos immunopositive neurons in NTS and about half in VLM were catecholaminergic (Hirooka et al. 1997), and approximately one quarter of those c-Fos positive neurons in caudal and intermediate VLM projected to rostral VLM pressor region (Hirooka et al. 1997). In addition, the VLM catecholaminergic neurons were also induced to express c-Fos by hypotension (Li and Dampney 1994; Potts et al. 1997). These previous findings indicated that some hypoxia-excited NTS and VLM neurons were involved in hypoxic cardiovascular reflex. In the present study we observed both anterogradely labeled axonal terminals and c-Fos immunopositive neurons in the VLM catecholaminergic area, although much less dense than that in areas corresponding to ventral respiratory column. Previous studies have shown that electrical or chemical stimulations at loci within the KFN elicited increases in arterial blood pressure in addition to inspiratory inhibition (Dawid Milner et al. 2003; Lara et al. 1994). Indeed, many neurons in the dorsolateral pons including the KFN display modulations by both arterial pulse pressure and respiratory activity and such neurons could contribute to cardiorespiratory coupling (Dick et al. 2009). Therefore, inputs from the KFN to the VLM catecholaminergic neurons are likely to exert influences on cardiovascular functions.
Acknowledgments
This work was supported by National Institutes of Health grants HL067966, HL072849, HL079503, and HL093225.
Abbreviations
- 5
Trigeminal motor nucleus
- 7
Facial motor nucleus
- 7n
Facial nerve
- 12
Hypoglossal motor nucleus
- A5
A5 noradrenergic cells
- AMB
Ambiguus nucleus
- rcAMB
Ambiguus nucleus, rostral compact part
- subcAMB
Ambiguus nucleus, subcompact part
- cAMB
Caudal ambiguus nucleus
- BDA
Biotin dextran
- CSN
Carotid sinus nerve
- CVL
Caudal ventrolateral reticular nucleus
- ITN
Intertrigeminal nucleus
- KFN
Kölliker–Fuse nucleus
- LPBN
Lateral parabrachial nucleus
- LPGi
Lateral paragigantocellular reticular nucleus
- LRt
Lateral reticular nucleus
- NRA
Nucleus retroambiguus
- NTS
Nucleus of solitary tract
- vl-NTS
Ventrolateral nucleus of solitary tract
- dl-pons
Dorsolateral pons
- vl-pons
Ventrolateral pons
- P7
Parafacial area
- Pr5VL
Ventrolateral principal sensory trigeminal nucleus
- RTN
Retrotrapezoid nucleus
- RVL
Rostral ventrolateral reticular nucleus
- scp
Superior cerebellum peduncle
- Sp5O
Trigeminal sensory nucleus, oral
- Sp5I
Trigeminal sensory nucleus, interpolar
- ts
Solitary tract
- VLM
Ventrolateral medulla
- VRC
Ventral respiratory neuronal column
Appendix: Critique of methodologies
Microinjection
BDA is a proven sensitive and reliable anterograde tracer. With sufficient postinjection survival time (1–2 weeks), even very fine axonal terminals can be sufficiently filled for microscopic observation and long distance tracing. Retrograde labeling has also been reported but only after large injections. In the present study, labeled neurons were observed in areas surrounding the injection site (Fig. 1a) but rarely in other regions. However, BDA is not functionally specific and the KFN is functionally heterogeneous, i.e., besides the well-known pneumotaxic function, KFN is also known to participate in cardiovascular control, anti-nociception, and control of feeding and drinking. To increase the likelihood of labeling neurons that were respiratory-related, we first mapped the KFN with electrical microstimulation for loci that produced respiratory inhibition with the lowest stimulation intensity. Although such electrical microstimulation could activate neurons far from the tip of the stimulating electrode as a result of direct axonal activation (Histed et al. 2009), previous studies have shown that electrical or chemical microstimulation (with glutamate) of neurons in this structure cause similar profound changes in respiratory patterns (Chamberlin and Saper 1992; Cohen 1971; Dutschmann and Herbert 2006; Haji et al. 1998). Thus we believe that the electrical microstimulation-evoked respiratory inhibition in the present study was due to activation of KFN neurons in the vicinity of the electrode tip. Hence, BDA was injected only into such low-threshold regions. With this functionally guided method, we found that the centers of injections in all experimental animals were within the boundaries of KFN and the diffusion of the injectant into neighboring areas was quite limited in most cases. In contrast, failure to identify such low-threshold regions resulted in misplaced injections in three animals. Data from misplaced injections were not included in this report.
In light of the above precautions, we believe that the labeled axons were predominantly from respiratory-related KFN neurons because: (1) the KFN contains the highest density of respiratory-related neurons that send axons to ventrolateral pons and medulla (Ezure and Tanaka 2006; Song et al. 2006); (2) ascending projections from medullary respiratory-related structures predominantly terminate at this structure (Gaytan et al. 1997; Kalia 1977); (3) the highly selective termination of labeled terminals in well-established brainstem respiratory-related structures with c-Fos positive response to hypoxia as demonstrated in this study; (4) BDA injections at control sites neighboring the KFN resulted in very different projection/innervation patterns of the labeled axons. Although nonspecific labeling of axons of other functional modalities (e.g., cardiovascular) from KFN and a minority of axons from neighboring medial and lateral parabrachial nuclei or A7 region (especially for the A7 region) cannot be ruled out especially with pressure injection, useful information about the multi-functional role of the KFN can still be derived from this study.
Axonal tracing
Traditionally, retrograde tracing with a combination of fluorescent tracers of two or three different colors is commonly used to reveal the collateral innervations of a single neuron or axon. However, current fluorescent imaging techniques cannot differentiate the labeling of one neuron by more than three fluorescent tracers. As a result, no more than three innervations can be revealed at a time. In addition, this technique requires 2–3 microinjections at different brain structures, which is rather traumatic to the animal. Because of potential spread of the injectants into neighboring areas, distinct innervations of adjacent target structures cannot be reliably resolved by this method. When more than three structures or when two or more neighboring structures are innervated by branches from such an axon, single-axon anterograde tracing is the only currently available method to reveal these multiple innervations.
On the other hand, long distance anterograde tracing of individual axons over multiple brain sections is generally a challenging task because each section may contain many labeled axons that are difficult to align across consecutive sections. This difficulty has bedeviled early studies using pressure injection, each of which could potentially label hundreds of descending fibers even for a structure with sparse neuronal density such as KFN. To circumvent this difficult, such tracing was performed only in animals with iontophoretic injection in the present study. The number of descending axons labeled after iontophoretic injection varied greatly from five to over one hundred per animal depending on the duration and current intensity of the iontophoresis and the tip diameter of the micropipette. To avoid any ambiguity in the tracing, stem axons that were selected for long distance tracing and reported herein were all relatively large (1.0–2.3 μm diameter) and isolated, i.e., without any neighboring axons of comparable size in each and every section. Such large stem axons typically stood out readily among much smaller axonal branches and were readily identified in coronal sections since they traveled rostrocaudally throughout much of their trajectories from ventrolateral pons to caudal medulla and spinal cord. If the identified axon shifted significantly from its preceding coordinates on the coronal plane or if other axons of comparable size emerged in any section, the tracing was abandoned. These stringent inclusion/exclusion criteria ensured the unequivocal matching of the same stem axon over consecutive brain sections.
In contrast, thinner branches or collaterals arising from such stem axons in any section were typically traced only within the same section to avoid ambiguity; reconstruction of those branches from two or more consecutive sections was performed only when no neighboring fibers with similar diameter and direction of projection were observed in each section. For this reason, some branches could not be traced to their ultimate targets. Fortuitously, unlike the stem axons that projected lengthwise rostrocaudally, such branches or collaterals tended to be much shorter and confined to the coronal planes, with the extensions in sagittal planes rarely spanning beyond three consecutive sections.
With the above precautions, only 1–2 axons of relatively large diameters met our stringent inclusion/exclusion criteria and were traced for various distances in each animal (totally 10 axons in 7 animals). Many of the large-diameter axons could not be traced for a long distance because they followed a more zigzag path that was not always perpendicular to the coronal plane, or simply because there were multiple large-diameter axons in their proximity that confounded the tracing. On the other hand, none of the medium-sized axons was successfully traced for sufficient distance. Nevertheless, branches and collaterals were still observed arising from such axons. In light of these observations, we believe the collateral innervations of multiple target structures might be common (if not universal) among descending axons of both medium and large diameters although many of them might not reach the caudal medulla.
Footnotes
As a historical note, the “Kölliker–Fuse nucleus” as originally described by Kölliker and Fuse (Fuse 1913; Kölliker 1896) in humans and a variety of animal species was subsequently found to actually represent the pedunculopontine tegmental nucleus (Rye et al. 1987). The name “Kölliker–Fuse nucleus” was erroneously given to the dorsolateral pontine structure that bears this name today by Berman in his cat atlas (Berman 1968). Notwithstanding, this mistake has been perpetuated and the “Kölliker–Fuse nucleus” (KFN) in a similar atlas for rat (Paxinos et al. 1999; Paxinos and Watson 1986) is used here to describe the cell group we are studying.
Rudolph Albert von Kölliker coined the term axon in 1896 (Lopez-Munoz and Alamo 2009).
References
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143(2–3):105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Berman A. The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin Press; Madison: 1968. p. 35. [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857(1–2):30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Larnicol N. Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience. 2001;108(4):643–653. doi: 10.1016/s0306-4522(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296(4):517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Burke PG, Abbott SB, McMullan S, Goodchild AK, Pilowsky PM. Somatostatin selectively ablates postinspiratory activity after injection into the Botzinger complex. Neuroscience. 2010;167(2):528–539. doi: 10.1016/j.neuroscience.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143(2–3):115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326(2):245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14(11 Pt 1):6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol. 1971;217(1):133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol. 2004;143(2–3):127–140. doi: 10.1016/j.resp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Wang SC. Respiratory neuronal activity in pons of cat. J Neurophysiol. 1959;22(1):33–50. doi: 10.1152/jn.1959.22.1.33. [DOI] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for posthypoxic frequency decline in rats. J Physiol. 1996;497(Pt 1):79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Nail BS. On the location and size of laryngeal motoneurons in the cat and rabbit. J Comp Neurol. 1984;230(1):13–32. doi: 10.1002/cne.902300103. [DOI] [PubMed] [Google Scholar]
- Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S. A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res. 2003;982(1):108–118. doi: 10.1016/s0006-8993(03)03005-1. [DOI] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res. 1994;636(2):259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol. 2009;168(1–2):76–85. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport. 1996;7(8):1432–1436. doi: 10.1097/00001756-199605310-00022. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24(4):1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159(2):232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513(1):35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348(2):161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143(2–3):167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuro-science. 2006;141(2):1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572(1–2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319(3):229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Fuse G. In: Arbeiten aus dem Hirnanatomischen Institut in Zurich. von Monakow C, editor. JF Bergmann Verlag; Wiesbaden: 1913. pp. 211–253. [Google Scholar]
- Gang S, Mizuguchi A, Aoki M. Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir Physiol. 1991;85(3):329–339. doi: 10.1016/0034-5687(91)90072-q. [DOI] [PubMed] [Google Scholar]
- Gang S, Sato Y, Kohama I, Aoki M. Afferent projections to the Botzinger complex from the upper cervical cord and other respiratory related structures in the brainstem in cats: retrograde WGA-HRP tracing. J Auton Nerv Syst. 1995;56(1–2):1–7. doi: 10.1016/0165-1838(95)00049-x. [DOI] [PubMed] [Google Scholar]
- Gasparini S, de Luca LAJ, Colombari DS, de Paula PM, Barbosa SP, Menani JV. Adrenergic mechanisms of the Kolliker-Fuse/ A7 area on the control of water and sodium intake. Neuroscience. 2009;164(2):370–379. doi: 10.1016/j.neuroscience.2009.08.048. [DOI] [PubMed] [Google Scholar]
- Gaytan SP, Calero F, Nunez-Abades PA, Morillo AM, Pasaro R. Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res Bull. 1997;42(4):323–334. doi: 10.1016/s0361-9230(96)00292-4. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373(2):173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147(2–3):159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett. 1999;262(2):93–96. doi: 10.1016/s0304-3940(99)00065-8. [DOI] [PubMed] [Google Scholar]
- Guo ZL, Li P, Longhurst JC. Central pathways in the pons and midbrain involved in cardiac sympathoexcitatory reflexes in cats. Neuroscience. 2002;113(2):435–447. doi: 10.1016/s0306-4522(02)00173-2. [DOI] [PubMed] [Google Scholar]
- Guyenet P. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Yamazaki H, Takeda R. NMDA receptor-mediated inspiratory off-switching in pneumotaxic-disconnected cats. Neurosci Res. 1998;32(4):323–331. doi: 10.1016/s0168-0102(98)00103-5. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293(4):540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198(2):539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Ryan AT. Localization of laryngeal motoneurons in the rat: morphologic evidence for dual innervation? Exp Neurol. 1981;74(2):341–355. doi: 10.1016/0014-4886(81)90174-6. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80(4):1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63(4):508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CJJ, Apkarian AV, Stevens RT. Inhibition of dorsal-horn cell responses by stimulation of the Kolliker-Fuse nucleus. J Neurosurg. 1986;65(6):825–833. doi: 10.3171/jns.1986.65.6.0825. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical evidence for a strong ventral parabrachial projection to nucleus raphe magnus and adjacent tegmental field. Brain Res. 1988;447(1):154–158. doi: 10.1016/0006-8993(88)90977-8. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG. Propriobulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. I. An anterograde degeneration study in the cat. Brain. 1977;100(2):239–264. doi: 10.1093/brain/100.2.239. [DOI] [PubMed] [Google Scholar]
- Hwang JC, Chien CT, St John WM. Characterization of respiratory-related activity of the facial nerve. Respir Physiol. 1988;73(2):175–187. doi: 10.1016/0034-5687(88)90065-5. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. A ‘pneumotaxic centre’ in rats. Neurosci Lett. 1994;172(1–2):67–72. doi: 10.1016/0304-3940(94)90664-5. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82(2):377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- Kalia M. Neuroanatomical organization of the respiratory centers. Federation Proc. 1977;36:2405–2411. [PubMed] [Google Scholar]
- Kalia MP. Anatomical organization of central respiratory neurons. Annu Rev Physiol. 1981;43:105–120. doi: 10.1146/annurev.ph.43.030181.000541. [DOI] [PubMed] [Google Scholar]
- Kölliker A. Handbuch der Gewebelehre des Menschen. W.Englmann; Leipzig: 1896. p. 874. [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30(1–2):163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol. 1999;117(2–3):85–95. doi: 10.1016/s0034-5687(99)00058-4. [DOI] [PubMed] [Google Scholar]
- Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol. 1994;477(Pt 2):321–329. doi: 10.1113/jphysiol.1994.sp020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Rossi L, Matturri L. Cytoarchitectural organization of the parabrachial/Kolliker-Fuse complex in man. Brain Dev. 2004a;26(5):316–320. doi: 10.1016/j.braindev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Rossi L, Matturri L. Hypoplasia of the parabrachial/kolliker-fuse complex in perinatal death. Biol Neonate. 2004b;86(2):92–97. doi: 10.1159/000078310. [DOI] [PubMed] [Google Scholar]
- Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61(3):613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Song G. Afferent projection to the retrotrapezoid nucleus from respiratory related structures in the brainstem of rabbit–a retrograde CB-HRP tracing study. Sheng Li Xue Bao. 2001;53(5):401–404. [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Historical evolution of the neurotransmission concept. J Neural Transm. 2009;116(5):515–533. doi: 10.1007/s00702-009-0213-1. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J Physiol London. 1923;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SM, Song G, Poon CS. Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS ONE. 2007;2(9):e865. doi: 10.1371/journal.pone.0000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SM, Tin C, Song G, Poon CS. Use-dependent learning and memory of the Hering-Breuer inflation reflex in rats. Exp Physiol. 2009;94(2):269–278. doi: 10.1113/expphysiol.2008.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489(Pt 3):877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS. Estrogen attenuates antinociception produced by stimulation of Kolliker-Fuse nucleus in the rat. Eur J Neurosci. 2004;20(11):3203–3207. doi: 10.1111/j.1460-9568.2004.03775.x. [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Morillo AM, Pasaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst. 1993;42(2):99–118. doi: 10.1016/0165-1838(93)90042-s. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23(4):1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett. 1987;78(2):151–155. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96(1):55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Damasio A. Severe pathological changes of parabrachial nucleus in Alzheimer's disease. Neuroreport. 1998;9(18):4151–4154. doi: 10.1097/00001756-199812210-00028. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Carrive P, Wang H, Wang P. Chemoarchitectonic atlas of the rat brainstem. Academic Press; San Diego: 1999. [Google Scholar]
- Poon C, Song G. Distinct responses of respiratory neurons in rostrolateral pons to vagal stimulation and brief hypoxia. Soc Neurosci Abs. 2004;145:4. [Google Scholar]
- Potts PD, Polson JW, Hirooka Y, Dampney RA. Effects of sinoaortic denervation on Fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neuroscience. 1997;77(2):503–520. doi: 10.1016/s0306-4522(96)00459-9. [DOI] [PubMed] [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499(1):64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99(6):2440–2450. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259(4):483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197(2):291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci. 2007;27(19):5156–5162. doi: 10.1523/JNEUROSCI.0001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniaia MS, Young DL, Poon CS. Habituation and desensitization of the Hering-Breuer reflex in rat. J Physiol. 2000;523(Pt 2):479–491. doi: 10.1111/j.1469-7793.2000.t01-1-00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281(1):69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98(6):3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Pre-Botzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol. 2000;83(5):2854–2868. doi: 10.1152/jn.2000.83.5.2854. [DOI] [PubMed] [Google Scholar]
- Song G, Poon C. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143(2–3):281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009a;165(1):9–12. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2009b;165(1):1–8. doi: 10.1016/j.resp.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26(1):300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS. Bilateral lesions of pontine Kolliker-Fuse nuclei provoke apnea instead of apneusis in anesthetized adult rats. Adv Exp Med Biol. 2010;669:185–188. doi: 10.1007/978-1-4419-5692-7_37. [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Giacometti E, Poon CS. Habituation without NMDA receptor-dependent desensitization of Hering-Breuer apnea reflex in a Mecp2+/- Mutant Mouse Model of Rett syndrome. Front Integr Neurosci. 2011a;5:6. doi: 10.3389/fnint.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience. 2011b;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. Differing responses to hypercapnia and hypoxia following pneumotaxic center ablation. Respir Physiol. 1975;23(1):1–9. doi: 10.1016/0034-5687(75)90066-3. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579(Pt 3):863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl K. Respiratory activation of the facial nerve and alar muscles in anaesthetized dogs. J Physiol. 1985;363:351–362. doi: 10.1113/jphysiol.1985.sp015715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J Neurosci. 2009;29(12):3824–3832. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QJ, Berkowitz RG, Pilowsky PM. GABA A mediated inhibition and postinspiratory pattern of laryngeal constrictor motoneurons in rat. Respir Physiol Neurobiol. 2008;162(1):41–47. doi: 10.1016/j.resp.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388(2):169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- von Euler C, Trippenbach T. Excitability changes of the inspiratory “off-switch” mechanism tested by electrical stimulation in nucleus parabrachialis in the cat. Acta Physiol Scand. 1976;97(2):175–188. doi: 10.1111/j.1748-1716.1976.tb10250.x. [DOI] [PubMed] [Google Scholar]
- von Euler C, Marttila I, Remmers JE, Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976;96(3):324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]
- Welker W. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA. 2008;105(46):18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kolliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res. 2004;995(1):118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]
- Young RF, Tronnier V, Rinaldi PC. Chronic stimulation of the Kolliker-Fuse nucleus region for relief of intractable pain in humans. J Neurosurg. 1992;76(6):979–985. doi: 10.3171/jns.1992.76.6.0979. [DOI] [PubMed] [Google Scholar]
- Yu Y, MacDonald SM, Song G, Poon CS. Multielectrode recording and mapping of neuronal microcircuits in pontine pneumotaxic center. Soc Neurosci Abs. 2006;308:305. [Google Scholar]
- Zheng Y, Riche D, Rekling JC, Foutz AS, Denavit-Saubie M. Brainstem neurons projecting to the rostral ventral respiratory group (VRG) in the medulla oblongata of the rat revealed by co-application of NMDA and biocytin. Brain Res. 1998;782(1–2):113–125. doi: 10.1016/s0006-8993(97)01251-1. [DOI] [PubMed] [Google Scholar]