Abstract
Background
Patients with DM are at risk for atrioventricular block and left ventricular (LV) dysfunction. Non-invasive detection of diffuse myocardial fibrosis may improve disease management in this population.
Objective
Our aim was to define functional and post-contrast myocardial T1 time cardiac magnetic resonance (CMR) characteristics in myotonic muscular dystrophy (DM) patients.
Methods
Thirty-three DM patients (24 with type 1 and 9 with type 2) and 13 healthy volunteers underwent CMR for assessment of LV indices and evaluation of diffuse myocardial fibrosis by T1 mapping. The association of myocardial T1 time to ECG abnormalities and LV indices were examined among DM patients.
Results
DM patients had lower end-diastolic volume index (68.9 vs. 60.3 ml/m2, p=0.045), cardiac index (2.7 vs. 2.33 L/min/m2, p=0.005) and shorter myocardial T1time (394.5 vs. 441.4 ms, p<0.0001), compared to control subjects. Among DM patients, there was a positive association between higher T1 time and LV mass index (2.2 ms longer per gm/m2, p=0.006), LV end-diastolic volume index (1.3 ms longer per ml/m2, p=0.026), filtered QRS duration (1.2 ms longer per unit, p=0.005) and low-amplitude (<40mcV) late-potential duration (0.9 ms longer per unit, p=0.01). Using multivariate random effects regression, each 10 ms increase in myocardial T1 time of type 1 DM patients was independently associated with 1.3 ms increase in longitudinal PR and QRS intervals during follow-up.
Conclusion
DM is associated with structural alterations on CMR. Post-contrast myocardial T1 time was shorter in DM patients than controls likely reflecting the presence of diffuse myocardial fibrosis.
Keywords: Myotonic muscular dystrophy, MRI, T1 mapping, ventricular function
INTRODUCTION
Myotonic muscular dystrophy (DM) is a genetic multisystem disorder characterized by skeletal muscle weakness and myotonia. Two types of DM have been defined: Type 1 (DM-1, Steinert’s disease) and type 2 (DM-2, Proximal myotonic myopathy). DM -1 is the most common adult onset muscular dystrophy whereas, DM- 2 is less frequent, tends to have a milder phenotype and later onset of symptoms.1 Cardiac conduction deficits, mild to moderate ventricular dysfunction and sudden death can occur in both DM-1 and DM-2.2 Histopathologically, patchy interstitial fibrosis, myocyte hypertrophy and degeneration, fatty infiltration and lymphocytes have been shown in myocardium, sinoatrial and atrioventricular nodes of DM patients.3
Cardiac magnetic resonance (CMR) imaging is a well established method for assessment of left ventricular (LV) function.2 Additionally, the contrast enhanced CMR T1 mapping technique has been applied to non-invasively quantify diffuse interstitial fibrosis.4 Post contrast CMR images demonstrate retention of gadolinium and thus lower T1 times in the presence of fibrosis. 4, 5 Other histopathologic processes such as edema, fatty infiltration, which coexist in DM patients, can also influence myocardial T1 time. The relationship of myocardial T1 time to ECG or structural abnormalities is unknown.
Thus, it is possible that detection of left ventricular (LV) dysfunction and diffuse myocardial fibrosis may improve the quantification of cardiac involvement and prediction of arrhythmia risk in DM. We sought to define functional and post contrast myocardial T1 time CMR characteristics in patients with DM.
MATERIALS AND METHODS
Patients and Controls
The protocol was reviewed and approved by the Johns Hopkins Institutional Review Board. The patient population included 33 patients with DM (24 with type 1 and 9 with type 2) diagnosed by genetic testing (55 %) or by clinical examination in subjects who had a first degree family member with genetically proven DM (45 %). Patients with clinical findings of DM but negative genotype were not included. All consecutive DM patients who were referred to the electrophysiology service for arrhythmia risk stratification, without history of atrioventricular block, resuscitated sudden death or contraindications to CMR were enrolled in the study. All patients underwent CMR and standard 12-lead ECG. Standard ECG was repeated during routine and symptom prompted follow-up visits. The median follow-up time was 705.5 days [interquartile range (IQR): 408.8 – 1124.5 days] and the median number of follow-up visits was 2 (IQR: 1–5.3 visits/patient). Twenty-three of 33 DM patients underwent signal averaged ECG with Frank orthogonal leads at a sampling rate of 1 kHz/channel and enough QRS complexes to reduce noise level to <1 mcV (PC ECG 1200; Norav Medical Ltd., Thornhill, Ontario, Canada). Thirteen healthy volunteers underwent CMR as a control group under a separate IRB approved protocol and after providing informed consent. None of the volunteers had a history of cardiovascular and/or other systemic disease. Estimated glomerular filtration rate (eGFR) was determined from serum creatinine using the Modification of Diet in Renal Disease Study Equation.
Cardiac Magnetic Resonance Imaging
CMR was performed with a 1.5 Tesla magnetic resonance scanner (Avanto; Siemens Medical Systems, Erlangen, Germany) using anterior and posterior surface coils for signal reception. Cine images were acquired in two-chamber, four-chamber and short axis planes during breath holding using an ECG triggered steady state free precession pulse sequence (TR/TE: 2.5–3.8 msec/1.1–1.2 msec; flip angle: 60–81°; spatial resolution: less than 1.56 × 1.56 × 8 mm; slice gap: 2 mm; temporal resolution: 20–45 msec). Patients and healthy volunteers then received 0.15–0.2 mmol/kg intravenous gadopentetate dimeglumine (n=29) (Magnevist; Bayer Healthcare Pharmaceuticals, New Jersey, USA) or gadodiamide (n=17) (Omniscan; Amersham Health/General Electric Healthcare, Waukesha, Wisconsin, USA). In order to measure T1 time, an inversion recovery prepared Look-Locker steady state free precession pulse sequence was acquired 7–15 minutes after gadolinium injection in the four-chamber plane (FOV = 380×262–326 mm, matrix size = 192×72–90, slice thickness = 8mm, TR/TE = 2.5/1.1ms, phase interval = 23–25ms, flip angle = 50°, 14–56 phases acquired every other R-R interval). Delayed gadolinium enhanced images were acquired immediately after the Look Locker sequence with an ECG triggered, inversion recovery (IR) prepared segmented spoiled gradient recalled echo (GRE) pulse sequence (TR/TE: ≤ 6.9/≤ 4.1 msec; flip angle: 25°; spatial resolution: better than 2.1 × 2.1 × 8 mm; slice gap: 2 mm) with images obtained in the short axis, two and four chamber planes. Inversion time was individually adjusted to null the signal from normal myocardium.
Image Analysis
CMR studies were evaluated and quantified by a single reader who was blinded to the subjects’ ECG and other clinical information. LV mass, volumes, functional parameters and right ventricular (RV) volumes and ejection fraction were determined from short axis cine images covering the heart from base to apex throughout the cardiac cycle using the MASS research software (MASS V2010-EXP, Leiden University Medical Center, Leiden, The Netherlands). LV endocardial and epicardial contours and RV endocardial contours were traced manually at both end-diastole and end-systole. Papillary muscles were included in the LV and RV volume and excluded from LV mass measurements. LV mass and volumes were indexed to body surface area. LV ejection fraction (EF) was calculated as LV stroke volume divided by LV end-diastolic volume multiplied by 100.
Left atrial (LA) and right atrial (RA) area and length measurements were done at atrial diastole using 2-chamber (2ch) and 4-chamber (4ch) cine images according to published methods to obtain atrial volumes 6. LA volume calculated using biplane area-length formula: Volume= 0.848 x (area4ch x area2ch)/[(length4ch+length2ch)/2]. RA volume calculated using the monoplane area-length formula: volume= 0.848 x (area4ch)2/length4ch.
Delayed enhancement images were visually evaluated for possible gadolinium enhancement. For evaluation of diffuse fibrosis with myocardial T1 mapping, left ventricular endocardial and epicardial borders were traced semi-automatically for all phases in the Look Locker sequence. Pixel by pixel fit was performed to a three parameter model (A−Bexp[−TI/T1*]) to obtain myocardial T1 as T1=(B/A−1)T1*. Only pixels where the χ2 test for goodness of fit 7 was significant with level of significance α = 0.05 were included in the average myocardial T1 value. Mean average myocardial T1 values from DM patients and control subjects were corrected to obtain the equivalent T1 value based on standard relaxation rates of gadopentetate dimeglumine and gadodiamide and normalized to a contrast dose of 0.2 mmol/kg, post contrast delay time of 11 min and eGFR of ≅ 90mL/min/1.73m2 as previously described.8 As an alternative indexation method, we obtained skeletal muscle T1 values and calculated the myocardial to skeletal muscle T1 ratio.
Inter-reader variability of post contrast myocardial T1 values was examined by reading 10% of cases (n=6) by two readers and intra-class correlation was 0.997 (95% confidence interval (CI); 0.980–0.999). Those MRIs were also re-read by one reader with an intra-reader intra-class correlation of 0.999 (95% CI; 0.994–0.999). The mean difference for was 0.74ms (95% CI; 12.1ms- −10.6 ms) and 2.15 ms (8.1 ms- −3.83ms) for inter- and intra-reader in Bland-Altman analysis.
Statistics
Continuous variables are summarized as mean ± standard deviation (SD). Categorical and dichotomous variables are presented as percentages. The unpaired Student’s t test was used to compare continuous variables and the chi-square test was used for categorical variables. Univariate linear regression models were used to evaluate the association of average myocardial T1 time with LV indices, CTG repeats size, standard and signal-averaged ECG findings. Multivariate random effects regression models of panel data clustered by patient and adjusted for heart rate and other potential confounders were used to measure the association of myocardial T1 time with time-dependent surface ECG PR and QRS interval progression in DM-1 patients. Statistical analyses were performed using Stata 9.0 for Windows (StataCorp, College Station, Texas).
RESULTS
Comparison of CMR results between Patients and Controls
Baseline Characteristics
Baseline characteristics of DM patients (n=24 DM-1; n=9 DM-2) and control subjects (n=13) are presented in Table 1. The mean age of DM patients was 46.3 years, 46 % of patients were men, 72.7 % had DM-1 and 27.3 % had DM- 2. The mean body mass index (BMI) was 25 kg/m2 and mean eGFR was 106.5 mL/min/1.73 m2. Among DM patients, QRS duration was 113.9 ± 29.9 on standard ECG and 110.6 ± 23.5 on signal averaged ECG. Baseline characteristics of DM-1 patients were similar to DM-2 patients with exception of the following: DM-1 patients were younger (42 vs. 57 years old; p=0.007) and had lower cardiac index (2.2 vs. 2.6 L/min/m2; p=0.02) than DM-2 patients. The mean age of control subjects was 38.1 years, 54% of whom were men. DM-2 patients were older than control subjects (57 vs. 46 years old). There was no significant difference in gender, BMI, or eGFR between either type of DM patients and control subjects.
Table 1.
Baseline Characteristics and Left Ventricular Cine MRI results of DM Patients and Controls
| DM-1 N=24 Mean±SD or % (n) |
DM-2 N=9 Mean±SD or % (n) |
Whole DM Patients N=33* Mean±SD or % (n) |
Healthy Volunteers N=13** Mean±SD or % (n) |
|
|---|---|---|---|---|
|
Demographics
|
||||
| Age (years) | 42.2 ± 14.4 ¶ | 57.2 ± 9.2‡ | 46.3 ± 14.7 | 38.1 ± 11.1 |
| Gender (male) | 50 (12) | 67 (6) | 46 (15) | 54 (7) |
| BMI (kg/m2) | 25.0 ± 5.7 | 24.9 ± 3.4 | 25.0 ± 5.1 | 27.8 ± 5.5 |
| GFR (mL/min/1.73m2) | 106.5 ± 33.1 | 107.0 ± 7.1 | 106.5 ± 31.3 | 99.0 ± 32.7 |
|
Left Ventricular Parameters
|
||||
| LV mass index (g/m2) | 56.8 ± 12.8 | 62.7 ± 12.9 | 58.6 ± 12.9 | 58.9 ± 5.4 |
| LV end-diastolic volume index (ml/m2) | 58.1 ± 17.8 § | 65.7 ± 16.6 | 60.3 ± 17.6 δ | 68.9 ± 9.7 |
| LV end-systolic volume index (ml/m2) | 23.9 ± 11.9 | 26.0 ± 11.5 | 24.5 ± 11.7 | 25.7 ± 5.9 |
| Mass/Volume Ratio | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.87 ± 0.2 |
| Stroke volume index (ml/m2) | 34.2 ± 8.9§ | 39.7 ± 6.1 | 35.8 ± 8.4δ | 43.2 ± 5.2 |
| Cardiac index (L/min/m2) | 2.2 ± 0.6¶§ | 2.6 ± 0.3 | 2.33 ± 0.6δ | 2.7 ± 0.3 |
| Ejection Fraction (%) | 59.6 ± 8.4 | 61.7 ± 6.6 | 60.2 ± 7.9 | 63.0 ± 5.1 |
| Ejection Fraction <55% (yes) | 31.8 (7) § | 11.1 (1) | 25.8 (8)δ | 0 (0) |
|
Right Ventricular Parameters
|
||||
| RV end-diastolic volume index (ml/m2) | 62.9 ± 12.9§ | 72.8 ± 14.9 | 65.8 ±14.0 | 72.0±9.7 |
| RV end-systolic volume index (ml/m2) | 28.7 ± 7.3 | 33.0 ± 10.8 | 29.9 ± 8.5 | 30.6 ± 4.6 |
| RV Stroke volume index (ml/m2) | 34.2 ±8.6§ | 39.8 ±6.4 | 35.8 ± 8.3δ | 41.4 ±5.9 |
| RV Ejection Fraction (%) | 54.3 ±7.3 | 55.4 ±5.8 | 54.6 ± 6.8 | 57.5 ± 3.0 |
|
Atrial Volumes
|
||||
| Left atrial volume index (ml/m2) | 29.9 ± 6.7 | 38.1 ± 13.2 | 32.3 ± 9.6 | 34.1 ± 4.9 |
| Right atrial volume index (ml/m2) | 23.4 ± 6.5§ | 32.9 ± 11.5 | 26.0 ± 9.1δ | 34.9 ± 7.5 |
|
ECG
|
||||
| Standard ECG QRS duration (msec) | 114.8 ± 30.6 | 111.6 ± 29.4 | 113.9 ± 29.9 | 94.5 ± 17.8 |
| Digital ECG filtered QRS duration (msec) | 110.7 ± 21.2 | 114.4 ± 35.1 | 110.6 ± 23.5 | NA |
| Terminal (40msec) root mean square voltage (mcV) | 26.6 ±21.7 | 34.4 ± 32.8 | 33.3 ± 26.6 | NA |
| Low-amplitude (<40mcV) late- potential duration (msec) | 40.4 ± 20.0 | 56.6 ± 47.7 | 41.9 ± 30.3 | NA |
Of 33 myotonic muscular dystrophy patients, 13 had GFR value (n=17 in DM-1 and n=2 in DM-2), 31 had technically adequate MRI data for LV and RV function (n=22 in DM-1 and n=9 in DM-2), 27 had technically adequate MRI data for LA volume measurement (n=19 in DM-1 and n=8 in DM-2), and 29 had technically adequate MRI data for RA volume measurement (n=21 in DM-1 and n=8 in DM-2).
Of 13 healthy volunteers, 8 had standard ECG P-value is based on Chi-Square test for categorical variables and t-test for continuous variables.
P<0.05 DM-1 vs. DM2;
P<0.05 DM-1 vs. healthy volunteers;
P<0.05 DM-2 vs. healthy volunteers;
P<0.05 Whole DM patients vs. healthy volunteers
Cine CMR
Thirty-one of 33 DM patients had technically adequate quality on cine CMR images. The LV mass index and end-diastolic volume index were 58.6 ± 12.9 g/m2 and 60.3 ± 17.6 ml/m2, respectively. The mean stroke volume index was 35.8 ± 8.4 ml/m2, the cardiac index was 2.33 ± 0.6 L/min/m2, and the ejection fraction was 60.2 ± 7.9 %. Eight of 33 (26%) DM patients had an ejection fraction lower than 55% (Table 1). Compared to control subjects, DM-1 patients had significantly lower end-diastolic volume index, stroke volume index, and cardiac index. LV mass, volumes and ejection fraction of DM-2 patients were similar to control subjects. Mass to volume ratio, an index of ventricular remodeling, tended to be greater in both DM-1 and DM-2 patients. There were no significant differences between DM patients and control subjects for LV mass index, and LV end-systolic volume index. Although the mean ejection fraction was not significantly different between DM patients and controls; the number of patients with low ejection fraction (<55%) was significantly higher in the DM-1 group (Table 1).
In DM patients, the RV end-diastolic volume index and stroke volume index were 65.8 ± 14.0 ml/m2 and 35.8 ± 8.3 ml/m2, respectively. RV ejection fraction was 54.6 ± 6.8 %. Left and right atrial volume indexes were 32.3 ± 9.6 ml/m2 and 26.0 ± 9.1 ml/m2. Compared to control subjects, DM-1 patients had significantly lower RV end diastolic volume index, RV stroke volume index and right atrial volume index (Table 1).
Delayed Gadolinium Enhanced CMR and Myocardial T1 Time
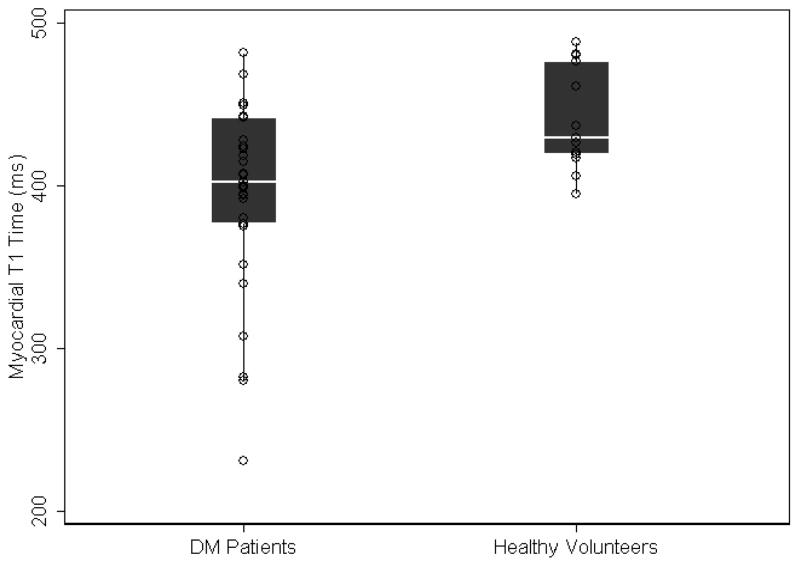
There was no evidence of focal late gadolinium enhanced scar in either the DM patients or the control subjects. However, the mean myocardial T1 time of DM patients was significantly shorter than control subjects (394.5 ± 57.6 ms vs. 441.4 ± 32.0 ms, respectively; p<0.0001)(Figure 1). Additionally, the myocardium/skeletal muscle T1 ratio was also shorter in DM patients compared to controls (0.67 ± 0.08 vs. 0.76 ± 0.08, respectively; p=0.002). This suggests greater accumulation of gadolinium in the myocardium relative to skeletal muscle.
Figure 1.
Scatter and box-plot overlay diagram showing variation in the post-contrast myocardial T1 time between healthy volunteers and myotonic dystrophy patients. The box-plots display the median and the 25th to 75th percentile range (center white line and solid black box), the lower and upper adjacent values (thin lines) and data points (dots).
Univariate Association of Myocardial T1 Time with LV Indices, CTG Repeats Size, and Measures of Conduction Abnormalities in Patients with DM
LV Indices
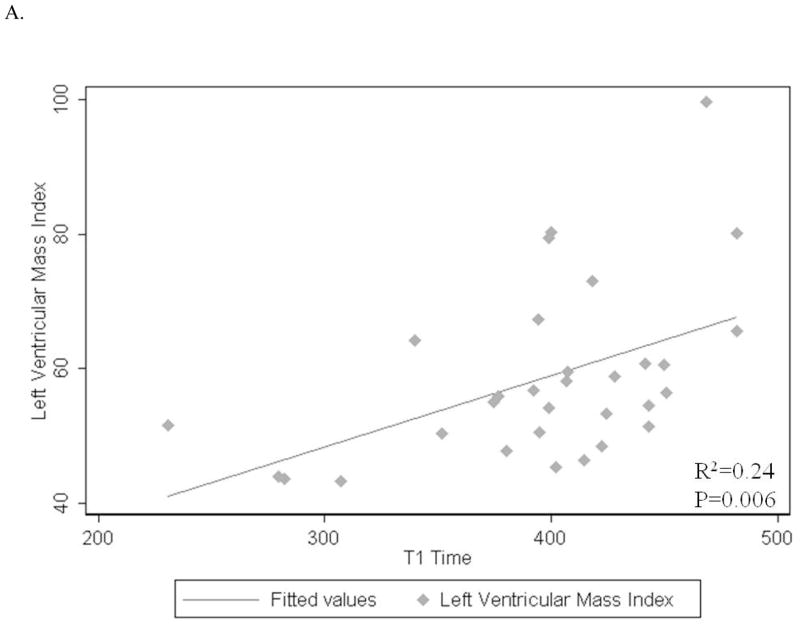
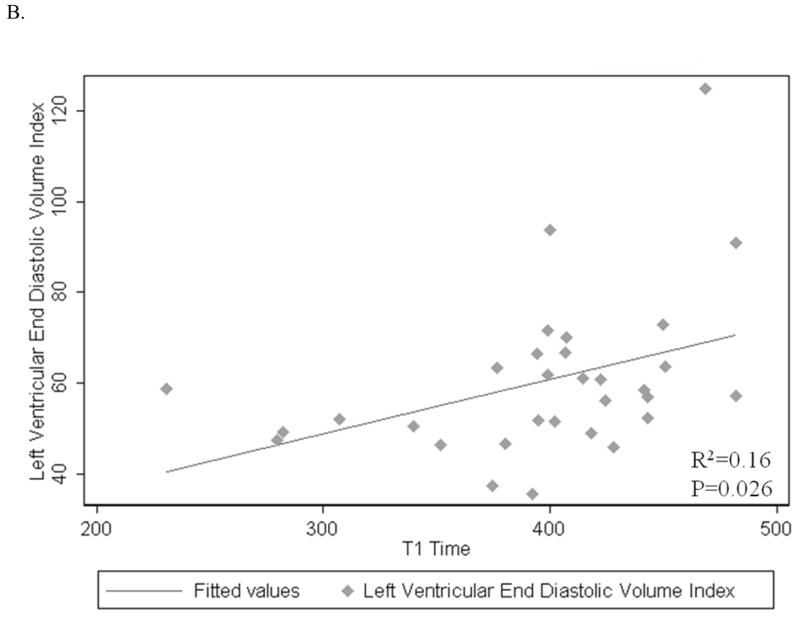
The mean myocardial T1 time was on average 2.2 ms longer per each 1g/m2 increase in LV mass index (p=0.006) and 1.3 ms longer per each 1ml/m2 increase in LV end-diastolic volume index (p=0.026) (Figure 2A, B). There was no significant association between the mean myocardial T1 time and stroke volume index, cardiac index and ejection fraction (p=0.14, 0.80, 0.08; respectively) (Table 2).
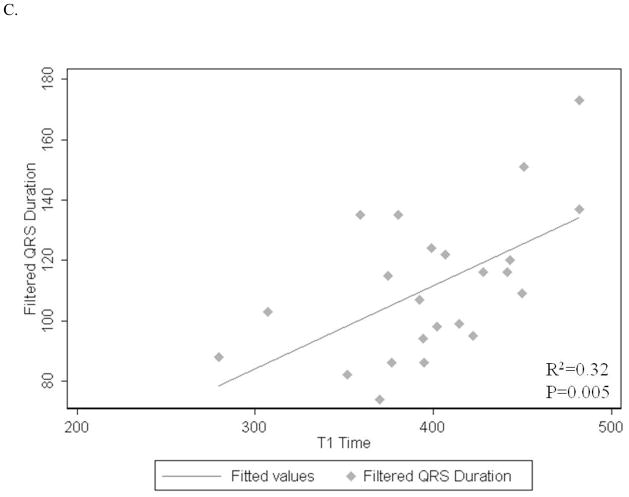
Figure 2.
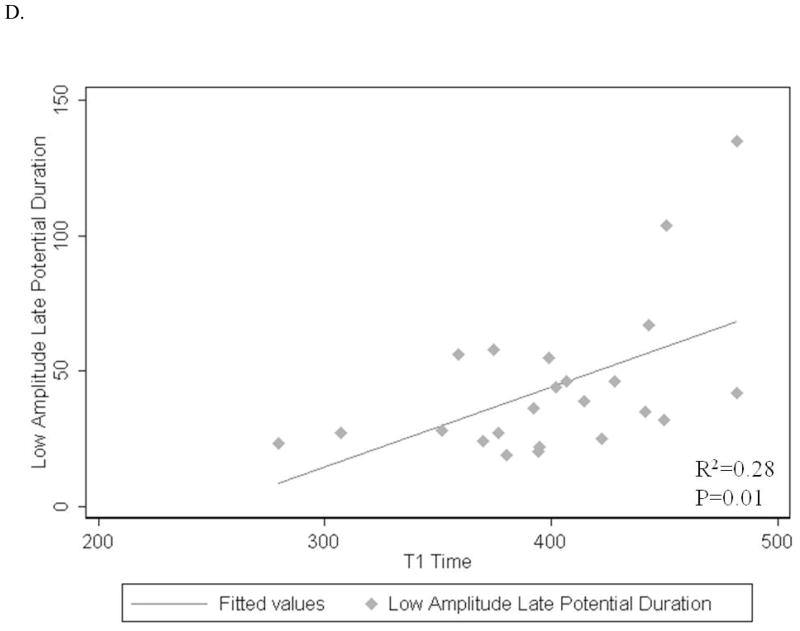
A–D: Associations of post contrast myocardial T1 time with LV end diastolic mass index (A), end diastolic volume index (B), digital ECG filtered QRS duration (C) and low-amplitude (<40mcV) late-potential duration (D).
Table 2.
Univariate Linear Regression Models Showing Associations of Mean Myocardial T1 Time
| Variable | R-squared | Regression Coefficient (ms) | 95% CI | p |
|---|---|---|---|---|
| CTG repeats (yes vs. no) | 0.10 | −0.09 | −0.2-0.1 | 0.196 |
| Digital ECG filtered QRS duration (per 1 msec) | 0.32 | 1.2 | 0.4-1.9 | 0.005 |
| Terminal (40msec) root mean square voltage (per 1 mcV) | 0.14 | −0.8 | −1.6-0.1 | 0.079 |
| Low-amplitude (<40mcV) late-potential duration (per 1 msec) | 0.28 | 0.9 | 0.3-1.6 | 0.01 |
| LV mass index (per 1 g/m2) | 0.24 | 2.2 | 0.7-3.7 | 0.006 |
| LV end-diastolic volume index (per 1 ml/m2) | 0.16 | 1.3 | 0.2-2.5 | 0.026 |
| Stroke volume index (per 1 ml/m2) | 0.07 | 1.9 | −0.6-4.5 | 0.138 |
| Cardiac index (per 1 L/min/m2) | 0.01 | −4.9 | −45.4-35.5 | 0.803 |
| Ejection Fraction (%) | 0.11 | −2.4 | −5.1-0.3 | 0.075 |
CTG-repeats size
There was no significant association between the mean myocardial T1 time and CTG-repeats size in DM-1 patients (p=0.20)
Measures of Conduction Abnormalities
Signal averaged filtered QRS duration and low-amplitude (<40mcV) late-potential duration showed positive associations with mean myocardial T1 time (1.2 ms longer, p=0.005 and 0.9 ms longer, p=0.01; respectively) (Figure 2C, D). The mean myocardial T1 time was not significantly associated with terminal (40msec) root mean square voltage on signal-averaged ECG (p=0.08) (Table 2).
Multivariate Analysis of the Association of Myocardial T1 Time with Longitudinal Changes in PR and QRS Duration
Time-dependent surface ECG progression in PR and QRS intervals of DM-1 patients was derived from repeat surface ECG measures with a median follow-up duration of 385 days (IQR: 16 to 979 days). Table 3 lists predictor variables in the random effects linear regression model of time-dependent changes in PR and QRS intervals. Patient age, number of CTG repeats, and paroxysmal atrial flutter or fibrillation, were independently associated with PR and QRS prolongation during follow-up. Decreased left ventricular ejection fraction was associated with greater QRS (but not PR) prolongation during follow-up. After adjustment for the above covariates, myocardial T1 time remained independently associated with both PR and QRS interval progression during long-term follow-up.
Table 3.
Predictors of Longitudinal PR and QRS Interval Changes (after Adjusting for Heart rate) in the Multivariate Random Effects Regression Model
|
|
PR Interval
|
QRS Interval
|
||
|---|---|---|---|---|
| Variable (unit) | Regression Coefficient | P | Regression Coefficient | P |
|
|
|
|
||
| Time | +4.8 ms/1000 days | 0.029 | NS | |
| Age | +6.5 ms/10 years | 0.015 | +5.9 ms/10 years | 0.008 |
| Number of CTG repeats | +5.3 ms/100 repeats | 0.002 | +8.3 ms/100 repeats | <0.001 |
| Paroxysmal atrial fibrillation or flutter | +65.1 ms | <0.001 | +34.8 ms | <0.001 |
| Left Ventricular Ejection Fraction | NS | −14.3 ms/10% increase | 0.001 | |
| Myocardial T1 Time | +1.3 ms/10 ms | 0.035 | +1.3 ms/10 ms | 0.007 |
DISCUSSION
To the best of our knowledge, the current study is the first to assess diffuse myocardial fibrosis in DM patients. Post-contrast myocardial T1 values of DM patients were significantly lower than control subjects, suggesting the presence of diffuse myocardial fibrosis. The current study also demonstrated that LV end-diastolic volume index, stroke volume index and cardiac index were significantly lower in DM patients compared to controls.
Macroscopic Cardiac Structure and Function
Alterations in cardiac structure and function of patients with DM have been previously reported using echocardiography 9–11 and MRI 12. Left ventricular hypertrophy 9, 10, 12, wall motion abnormality 9, 11, LV dilatation 9, 10 and systolic dysfunction 9, 11, 12 were observed in prior studies. However, two recent studies, did not observe a difference in LV mass, end diastolic and end-systolic volume, and ejection fraction of DM patients compared to control subjects.13, 14 Such discrepancies may be due to variations in disease severity among study samples. The current study was in line with previous studies by showing no significant difference in LV mass and ejection fraction in DM patients compared to control subjects. In contrast to previous studies, however, LV volumes (end diastolic and end systolic and stroke volume) and cardiac output were lower in DM patients compared to control subjects. Additionally, patients with DM-1 had significantly lower right atrial and ventricular end-diastolic volumes and reduced right ventricular stroke volume compared to controls. Many of our patients had evidence of cardiac involvement by ECG (PR intervals>200 ms and QRS intervals>120 ms). The severity of cardiac involvement, however, was usually mild. Only 8 of 33 patients (25%) had an EF < 55% and none had clinical symptoms of heart failure.
Diffuse Fibrosis
The pathologic features underlying cardiac findings in DM patients appear to involve myocyte hypertrophy, interstitial fibrosis, lymphocytes, and/or fatty infiltration of the conduction system and myocardium.15 Invasive electroanatomic voltage mapping has revealed widespread reduced electrogram voltage amplitude involving the interatrial septum, anterolateral atrial wall, and right ventricular outflow tracts of patients with DM-1.16 A prior MRI study has also revealed evidence of fatty infiltration and edema/inflammation in addition to fibrosis in DM patients with severe disease and advanced conduction disturbance.12 Late gadolinium enhanced MRI is a well-established technique to assess focal myocardial scar (dense myocardial fibrosis). In this technique, normal myocardial signal is suppressed by an inversion recovery pulse and focal myocardial scar is seen as hyper-intense areas due to gadolinium retention.17 The inversion recovery technique, however, may suppress diffuse myocardial fibrosis despite substantial retention of gadolinium, because the signal intensity variation compared to normal tissue is minimal in these areas. The myocardial T1 mapping technique can detect diffuse myocardial fibrosis non-invasively by quantitating the variability in myocardial T1 times. Diffuse myocardial fibrosis causes shortening in T1 times due to retention of gadolinium based contrast in increased interstitial spaces. Previous studies have utilized T1 mapping to quantify diffuse fibrosis in patients with heart failure, aortic regurgitation, adult congenital heart disease, non ischemic cardiomyopathy.4, 5, 18, 19 Importantly, the post contrast myocardial T1 time is sensitive to MRI acquisition parameters (i.e., contrast dose, delay time of MRI scan) and physiologic parameters (i.e., eGFR). However, those factors can be corrected to a standardized value of dose, MRI delay time and eGFR value for inter-patient comparison as performed in the current study.8 Histopathologic processes other than diffuse myocardial fibrosis such as fatty infiltration, edema, amyloid protein deposition and iron deposition also influence the myocardial T1 time.
In contrast to patients with Becker and Duchenne muscular dystrophy, those with DM-1 and 2, tend not to have cohesive myocardial fibrosis.20 In the current study, DM patients had no focal myocardial scar in late gadolinium enhanced MRI images. However, T1 mapping revealed significantly shorter values in DM patients compared to control subjects. This observation is most likely due to the presence of diffuse myocardial fibrosis in DM patients. On the other hand, within the group of DM patients, those with evidence of more severe cardiac involvement (higher LV end diastolic mass, volume, or conduction delays on filtered ECG) had longer myocardial T1 times. Fatty infiltration, myocardial edema and inflammation in DM patients may lengthen myocardial T1 times. We have measured T1 values of subcutaneous fat in DM patients and observed that it shows a large variation from 266 ms to 568 ms; fat T1 values can be higher than post contrast myocardial T1 values in some cases. The amount of fatty infiltration is also important. In pathology specimens of DM patients, fatty infiltration was reported to be approximately 5%. We expect that 5% fatty infiltration can increase the post contrast myocardial T1 value by approximately 10 ms. However, the presence of fatty infiltration in DM patients with evidence of more severe cardiac involvement is unlikely to fully explain the observed increase in post contrast myocardial T1 times of those with greater conduction disease. We speculate that, similar to patients with acute myocardial infarction21, myocardial edema and/or inflammation may also contribute to longer myocardial T1 times in patients with severe DM 22. De Ambroggi et al. has shown myocardial T2 hyperintensities in DM patients which suggests presence of edema/inflammation. 12 Unfortunately T2 weighted images were not included in our study protocol. This finding needs to be assessed in further studies.
Limitations
The average myocardial T1 values were obtained from a single plane assuming that diffuse fibrosis/fibro-fatty infiltration affects the whole myocardium evenly. This assumption seems reasonable since fibrosis was diffuse in histopathologic specimens obtained from DM patients.15 Due to associated complications, myocardial biopsies could not ethically be obtained to confirm the presence of fibro-fatty myocardial infiltration in our cases. A more recent Modified lock-locker sequence (MOLLI) is likely to provide more consistent and reproducible results for T1 mapping.23 This sequence was not available at the time of CMR scans of the current study. However, our experience shows an excellent correlation between the two sequences. We cannot clearly establish, at present, whether changes in myocardial T1 time might be predictive of specific clinical outcomes. Additionally, due to lack of standard or signal averaged ECG data in healthy volunteers, the association of T1 time with QRS duration and late potentials could not be assessed in that subset.
Conclusions
LV end-diastolic volume, stroke volume and cardiac output were significantly lower in DM patients compared to controls. Post-contrast myocardial T1 times of DM patients were significantly shorter than control subjects. On the other hand, DM patients with evidence of more severe cardiac involvement had longer myocardial T1 times. These findings suggest a) the early presence of diffuse myocardial fibrosis in patients with DM, and b) greater fat deposition, edema and/or inflammatory infiltration in advanced disease states. Based upon our results, the T1 mapping technique will likely be useful for assessment of cardiac involvement in DM patients, rather than for diagnosis or for ruling out DM in normal subjects. The utility of our findings for risk stratification is unknown and warrants further study.
Acknowledgments
Funding Sources: The study was funded by National Institutes of Health Grant (K23-HL089333) and the PJ Schafer Memorial Research Award to Dr. Nazarian, and the intramural NIH research program.
LIST OF ABBREVIATIONS
- CMR
Cardiac magnetic resonance
- DM
Myotonic muscular dystrophy
- DM-1
Myotonic muscular dystrophy type 1 (Steinert’s disease)
- DM-2
Myotonic muscular dystrophy type 2 (Proximal myotonic myopathy)
- eGFR
Estimated glomerular filtration rate
Footnotes
Disclosures: Dr. van der Geest is consultant for Medis medical imaging systems. Dr. Nazarian has received honoraria for lectures (not speakers’ bureau) from St Jude Medical, Biotronic, and Boston Scientific Inc. He is on the MRI advisory panel (unpaid) for Medtronic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turner C, Hilton-Jones D. The myotonic dystrophies: diagnosis and management. J Neurol Neurosurg Psychiatry. 2010;81(4):358–67. doi: 10.1136/jnnp.2008.158261. [DOI] [PubMed] [Google Scholar]
- 2.Hermans MC, Pinto YM, Merkies IS, de Die-Smulders CE, Crijns HJ, Faber CG. Hereditary muscular dystrophies and the heart. Neuromuscul Disord. 2010;20(8):479–92. doi: 10.1016/j.nmd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Strain JE, Grose RM, Factor SM, Fisher JD. Results of endomyocardial biopsy in patients with spontaneous ventricular tachycardia but without apparent structural heart disease. Circulation. 1983;68(6):1171–81. doi: 10.1161/01.cir.68.6.1171. [DOI] [PubMed] [Google Scholar]
- 4.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52(19):1574–80. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol. 2006;187(6):W630–5. doi: 10.2214/AJR.05.1264. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol. 2010;106(9):1345–50. doi: 10.1016/j.amjcard.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JR. An introduction to error analysis. Sausalito, CA: University Science Books; 1997. [Google Scholar]
- 8.Gai N, Turkbey EB, Nazarian S, et al. T(1) mapping of the gadolinium-enhanced myocardium: Adjustment for factors affecting interpatient comparison. Magn Reson Med. 2011;65(5):1407–15. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakta D, Lowe MR, Groh WJ. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am Heart J. 2004;147(2):224–7. doi: 10.1016/j.ahj.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Finsterer J, Gharehbaghi-Schnell E, Stollberger C, Fheodoroff K, Seiser A. Relation of cardiac abnormalities and CTG-repeat size in myotonic dystrophy. Clin Genet. 2001;59(5):350–5. doi: 10.1034/j.1399-0004.2001.590509.x. [DOI] [PubMed] [Google Scholar]
- 11.Tokgozoglu LS, Ashizawa T, Pacifico A, Armstrong RM, Epstein HF, Zoghbi WA. Cardiac involvement in a large kindred with myotonic dystrophy. Quantitative assessment and relation to size of CTG repeat expansion. Jama. 1995;274(10):813–9. [PubMed] [Google Scholar]
- 12.De Ambroggi L, Raisaro A, Marchiano V, Radice S, Meola G. Cardiac involvement in patients with myotonic dystrophy: characteristic features of magnetic resonance imaging. Eur Heart J. 1995;16(7):1007–10. doi: 10.1093/oxfordjournals.eurheartj.a061011. [DOI] [PubMed] [Google Scholar]
- 13.Di Cori A, Bongiorni MG, Zucchelli G, et al. Early left ventricular structural myocardial alterations and their relationship with functional and electrical properties of the heart in myotonic dystrophy type 1. J Am Soc Echocardiogr. 2009;22(10):1173–9. doi: 10.1016/j.echo.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Ozyigit T, Ozben B, Oflaz H, Serdaroglu P. Evaluation of biventricular functions with tissue Doppler imaging in patients with myotonic dystrophy. Clin Cardiol. 2010;33(3):126–31. doi: 10.1002/clc.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen HH, Wolfe JT, 3rd, Holmes DR, Jr, Edwards WD. Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J Am Coll Cardiol. 1988;11(3):662–71. doi: 10.1016/0735-1097(88)91547-1. [DOI] [PubMed] [Google Scholar]
- 16.Dello Russo A, Pelargonio G, Parisi Q, et al. Widespread electroanatomic alterations of right cardiac chambers in patients with myotonic dystrophy type 1. J Cardiovasc Electrophysiol. 2006;17(1):34–40. doi: 10.1111/j.1540-8167.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 18.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3(6):727–34. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sueyoshi E, Sakamoto I, Uetani M. Contrast-enhanced myocardial inversion time at the null point for detection of left ventricular myocardial fibrosis in patients with dilated and hypertrophic cardiomyopathy: a pilot study. AJR Am J Roentgenol. 2010;194(4):W293–8. doi: 10.2214/AJR.09.3414. [DOI] [PubMed] [Google Scholar]
- 20.Silva MC, Meira ZM, Gurgel Giannetti J, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49(18):1874–9. doi: 10.1016/j.jacc.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 21.Messroghli DR, Walters K, Plein S, et al. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58(1):34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira V, Piechnik SK, Dall’armellina E, et al. T1-mapping accurately detects acute myocardial edema: a comparison to T2-weighted cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2012;14 (Suppl 1):290. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26(4):1081–6. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]