Abstract
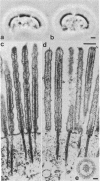
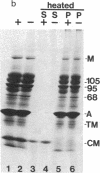
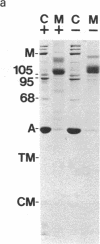
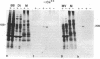
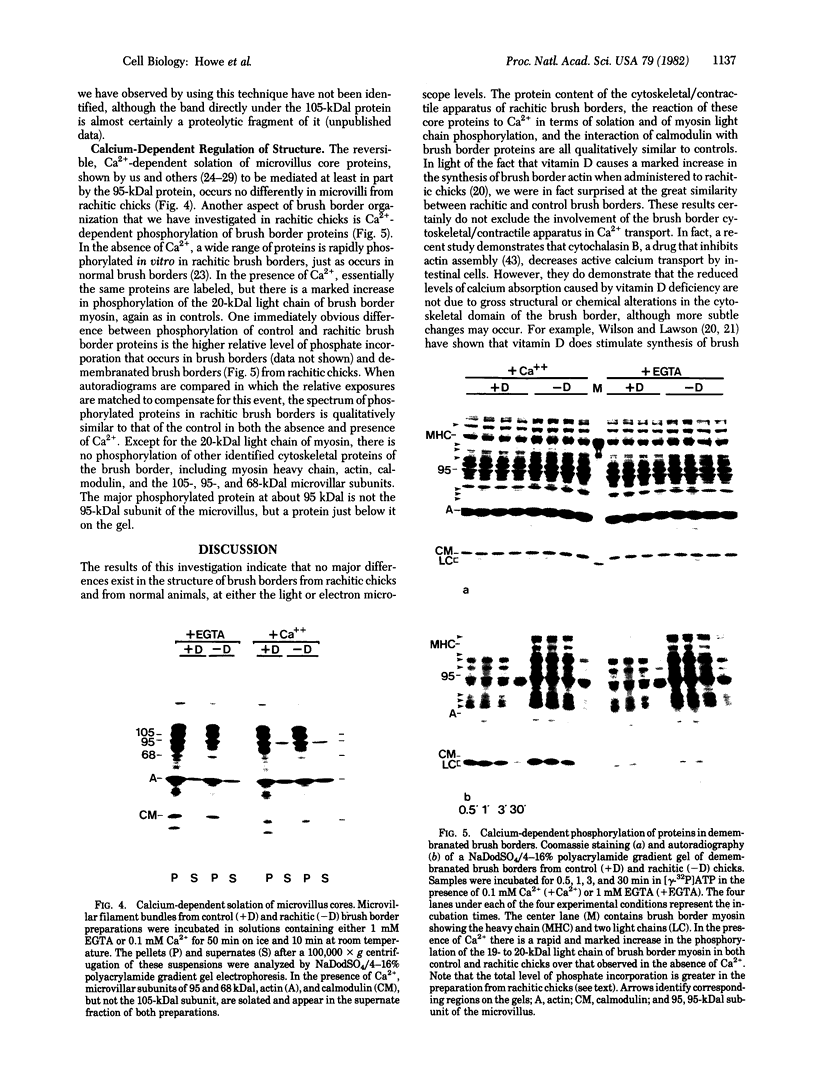
We have investigated several structural aspects of the intestinal epithelial brush border from rachitic chicks. At both the light and electron microscope levels, rachitic brush borders are indistinguishable from controls. Although several of the prominent periodic acid-Schiff-positive proteins of the brush border membrane have slightly slower mobilities on sodium dodecyl sulfate/polyacrylamide gels than do corresponding proteins from control brush borders, the major components of the microvillus core, including subunits of 105, 95, and 68 kilodaltons, actin, and calmodulin, are not detectably different. As assayed by a 125I-labeled calmodulin gel overlay technique, the same calmodulin-binding proteins are present in rachitic and control brush borders. Two proteins, the 105-kilodalton subunit of the microvillus core and an approximately 30-kilodalton membrane protein, bind calmodulin in a calcium-independent manner. Four cytoskeletal proteins (250, 190, 180, and 150 kilodaltons) and one membrane protein (35 kilodaltons) bind calmodulin only in the presence of calcium. Calcium-dependent solation of microvillus core proteins and calcium-dependent phosphorylation of the 20-kilodalton light chain of brush border myosin both occur as in controls. Our results show that rachintic chicks have brush borders that are quite similar to controls with respect to their ultrastructural organization, constituent contractile proteins, and calcium-dependent regulation of contractility and microvillus core structure. Therefore, the decreased absorption of calcium by intestinal epithelial cells in rachitic chicks is probably not due to gross structural or chemical differences in the brush border cytoskeleton.
Keywords: calcium, cytoskeleton, intestine, microvillus, vitamin D
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Brown H. O., Levine M. L., Lipkin M. Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol. 1963 Nov;205(5):868–872. doi: 10.1152/ajplegacy.1963.205.5.868. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Jemiolo D. K., Kretsinger R. H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Jun 26;623(2):257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. The binding of radio-iodinated calmodulin to proteins on denaturing gels. Ann N Y Acad Sci. 1980;356:73–74. doi: 10.1111/j.1749-6632.1980.tb29600.x. [DOI] [PubMed] [Google Scholar]
- Corradino R. A. 1,25-Dihydroxycholecalciferol: inhibition of action in organ-cultured intestine by actinomycin D and alpha-amanitin. Nature. 1973 May 4;243(5401):41–43. doi: 10.1038/243041a0. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Powell L. D. Regulation of actin polymerization by villin, a 95,000 dalton cytoskeletal component of intestinal brush borders. Cell. 1980 Dec;22(3):739–746. doi: 10.1016/0092-8674(80)90550-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feher J. J., Wasserman R. H. Intestinal calcium-binding protein and calcium absorption in cortisol-treated chicks: effects of vitamin D3 and 1,25-dihydroxyvitamin D3. Endocrinology. 1979 Feb;104(2):547–551. doi: 10.1210/endo-104-2-547. [DOI] [PubMed] [Google Scholar]
- Franceschi R. T., DeLuca H. F. Characterization of 1,25-dihydroxyvitamin D3-dependent calcium uptake in cultured embryonic chick duodenum. J Biol Chem. 1981 Apr 25;256(8):3840–3847. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J Biol Chem. 1980 Nov 25;255(22):10551–10554. [PubMed] [Google Scholar]
- Haussler M., Nagode L. A., Rasmussen H. Induction of intestinal brush border alkaline phosphatase by vitamin D and identity with ca-ATPase. Nature. 1970 Dec 19;228(5277):1199–1201. doi: 10.1038/2281199a0. [DOI] [PubMed] [Google Scholar]
- Hitchcock-DeGregori S. E. Actin assembly. Nature. 1980 Dec 4;288(5790):437–438. doi: 10.1038/288437a0. [DOI] [PubMed] [Google Scholar]
- Howe C. L., Mooseker M. S., Graves T. A. Brush-border calmodulin. A major component of the isolated microvillus core. J Cell Biol. 1980 Jun;85(3):916–923. doi: 10.1083/jcb.85.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jande S. S., Brewer L. M. Effects of vitamin D3 on duodenal absorptive cells of chicks. An electron microscopic study. Z Anat Entwicklungsgesch. 1974;144(3):249–265. doi: 10.1007/BF00522810. [DOI] [PubMed] [Google Scholar]
- Kowarski S., Schachter D. Intestinal membrane calcium-binding protein. Vitamin D-dependent membrane component of the intestinal calcium transport mechanism. J Biol Chem. 1980 Nov 25;255(22):10834–10840. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin D. L., Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):819–823. doi: 10.1016/0006-291x(69)90697-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- Max E. E., Goodman D. B., Rasmussen H. Purification and characterization of chick intestine brush border membrane. Effects of 1alpha(OH) vitamin D3 treatment. Biochim Biophys Acta. 1978 Aug 4;511(2):224–239. doi: 10.1016/0005-2736(78)90316-4. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. Brush border motility. Microvillar contraction in triton-treated brush borders isolated from intestinal epithelium. J Cell Biol. 1976 Nov;71(2):417–433. doi: 10.1083/jcb.71.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Graves T. A., Wharton K. A., Falco N., Howe C. L. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol. 1980 Dec;87(3 Pt 1):809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Pollard T. D., Fujiwara K. Characterization and localization of myosin in the brush border of intestinal epithelial cells. J Cell Biol. 1978 Nov;79(2 Pt 1):444–453. doi: 10.1083/jcb.79.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally M. H., Powell L. D., Craig S. W. Reconstitution and regulation of actin gel-sol transformation with purified filamin and villin. J Biol Chem. 1981 Mar 10;256(5):2083–2086. [PubMed] [Google Scholar]
- O'Doherty P. J. 1,25-Dihydroxyvitamin D3 increases the activity of the intestinal phosphatidylcholine deacylation-reacylation cycle. Lipids. 1979 Jan;14(1):75–77. doi: 10.1007/BF02533571. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Fontaine O., Max E. E., Goodman D. B. The effect of 1alpha-hydroxyvitamin D3 administration on calcium transport in chick intestine brush border membrane vesicles. J Biol Chem. 1979 Apr 25;254(8):2993–2999. [PubMed] [Google Scholar]
- Richman P. G., Klee C. B. Interaction of 125I-labeled Ca2+-dependent regulator protein with cyclic nucleotide phosphodiesterase and its inhibitory protein. J Biol Chem. 1978 Sep 25;253(18):6323–6326. [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Intestinal absorption of phosphate in the chick: effect of vitamin D and other parameters. J Nutr. 1973 Apr;103(4):586–599. doi: 10.1093/jn/103.4.586. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E. 1,25-Dihydroxyvitamin D stimulation of specific membrane proteins in chick intestine. Biochim Biophys Acta. 1977 May 26;497(3):805–811. doi: 10.1016/0304-4165(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E. Incorporation of [3H]leucine into an actin-like protein in response to 1,25-dihydroxycholecalciferol in chick intestinal brush borders. Biochem J. 1978 Aug 1;173(2):627–631. doi: 10.1042/bj1730627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. G., Norman A. W. Studies on the mechanism of action of calciferol. VIII. The effects of dietary vitamin D and the polyene antibiotic, filipin, in vitro, on the intestinal cellular uptake of calcium. J Biol Chem. 1975 Apr 10;250(7):2411–2419. [PubMed] [Google Scholar]