Abstract
Background
Computer simulations have predicted that the balance of various electrogenic sarcolemmal ion currents may control the amplitude and phase of beat-to-beat alternans of membrane potential (Vm). However, experimental evidence for the mechanism by which alternans of calcium transients produces alternation of Vm (Vm-ALT) is lacking.
Objective
We sought to provide experimental evidence that Ca-to-Vm coupling during alternans is determined by the balanced influence of two Ca-sensitive electrogenic sarcolemmal ionic currents, INCX and ICa.
Methods and Results
Vm-ALT and Ca-ALT were measured simultaneously from isolated guinea pig myocytes (n=41) using perforated patch and Indo-1AM fluorescence, respectively. There were three study groups: 1) Control, 2) INCX predominance created by adenoviral-induced NCX overexpression, and 3) ICa predominance created by INCX inhibition (SEA-0400) or enhanced ICa (As2O3). During alternans, 14 of 14 control myocytes demonstrated positive Ca-to-Vm coupling, consistent with INCX, but not ICa as the major electrogenic current in modulating action potential duration. Positive Ca-to-Vm coupling was maintained during INCX predominance in 8 of 8 experiments with concurrent increase in Ca-to-Vm gain (p<0.05), reaffirming the role of increased forward mode electrogenic INCX. Conversely, ICa predominance produced negative Ca-to-Vm coupling in 14 of 19 myocytes (p<0.05) and decreased Ca-to-Vm gain compared to control (p<0.05). Furthermore, computer simulation demonstrated that Ca-to-Vm coupling changes from negative to positive was due to a shift from ICa to INCX predominance with increasing pacing rate.
Conclusion
These data provide the first direct experimental evidence that coupling in phase and magnitude of Ca-ALT to Vm-ALT is strongly determined by relative balance of prominence of INCX versus ICa currents.
Keywords: alternans, repolarization, action potentials, intracellular calcium
INTRODUCTION
Microvolt-level T wave alternans is a sensitive marker of vulnerability to ventricular arrhythmias in patients 1, 2. T-wave alternans of the surface ECG arises from beat to beat alternation of action potential duration (Vm-ALT) at the single cell level. Under this paradigm beat-to-beat alternation of the calcium transient (Ca-ALT) causes beat-to-beat alternans in action potential shape and duration (Vm-ALT)3–5. This concept was supported by our previous findings showing close correspondence between myocytes exhibiting depressed expression or function of calcium cycling proteins and their susceptibility to Vm-ALT3. Therefore determining the mechanism by which electrogenic ionic currents transform Ca-ALT to Vm-ALT is critical to understanding how cardiac alternans promotes electrophysiological heterogeneities and cardiac arrhythmias. Previously computer simulations have predicted that the balance of various electrogenic sarcolemmal ion currents may control the amplitude and phase of beat-to-beat alternans of membrane potential 5–8. However, to our knowledge these theoretical predictions have not been tested experimentally. We hypothesized that Ca-to-Vm coupling during alternans (i.e., the relationship between alternating calcium transients and the corresponding phase and amplitude of action potential alternans) is determined by the balanced influence of two Ca-sensitive electrogenic sarcolemmal ionic currents, INCX and ICa. This hypothesis is based on established sensitivity of these currents to cytoplasmic calcium concentration. During Ca-ALT, a large calcium release is expected to promote forward mode INCX, hence prolonging APD, whereas this will be opposed by calcium induced inactivation of ICa, which shortens APD. Therefore, the relative predominance of each current would determine how Ca-ALT is coupled with respect to gain and phase to Vm-ALT. We used complementary and selective approaches to modify INCX or ICa function and hence to examine Ca-to-Vm coupling sign and Ca-to-Vm gain under conditions of INCX vs. ICa predominance. Our data supported the hypothesis that Ca-to-Vm coupling is determined by a competing balance of INCX (positive Ca-to-Vm coupling) and ICa (negative Ca-to-Vm coupling), and demonstrated that INCX is the major electrogenic mechanisms of Vm-ALT. These findings also have implications for disease states where the balance of ion channel expression is altered.
METHODS
Study Design
Myocytes were divided into three groups to investigate the competing balanced influence of two Ca-sensitive electrogenic sarcolemmal ionic currents, INCX and ICa on Ca-to-Vm coupling during alternans: 1) Control, 2) INCX predominance, and 3) ICa predominance. INCX predominance was achieved by In-vivo NCX gene transfer using a modified cross-clamping method9. Western blot from in vitro NCX overexpression showed that NCX protein expression was indeed increased by 3.8 ± 2.9 folds compared to control (n=3) and previously Ranu et al demonstrated that overexpression of NCX increases INCX, with no change in ICa.10 ICa predominance was achieved by INCX inhibition or ICa enhancement. One uM of the selective INCX inhibitor SEA0400 (Taisho Pharmaceutical Co, Ltd, Saitama, Japan) was used to achieve 80% INCX inhibition with no change in ICa 11, 12. ICa was increased with As2O3 13. Myocytes were studied after 4 hours incubation with 3 uM of As2O3. The procedure increased ICa density by about 100% with no significant effect on INCX under our experimental condition (see supplemental data).
Vm-ALT and Ca-ALT recordings
As described in supplementary material, Vm-ALT and Ca-ALT were measured simultaneously from isolated guinea pig myocytes (n=41) using perforated patch and Indo-1AM fluorescence, respectively. All experiments were performed at 32°C. Vm-ALT amplitude was measured by calculating the ratio of the difference in action potential duration (APD90) to the average APD90 for two consecutive beats. Ca-ALT amplitude was measured by calculating the ratio of the difference in Ca transient amplitude to the average Ca transient amplitude for two consecutive beats. Ca-to-Vm coupling was determined from the coincident phase of Vm-ALT to Ca-ALT (i.e. positive vs. negative Ca-to-Vm coupling) and Ca-to-Vm gain was calculated as the ratio of Vm-ALT to Ca-ALT.
ICa recordings
ICa were elicited from a holding potential of −40 mV with depolarizing voltage pulses to 0 mV for 140 ms. Stimulation protocol, solutions, and temperature were the same as for V-ALT recordings.
Computer simulations
Computer simulations were performed using a guinea pig ventricular myocyte model that was constructed by combining mathematical formulations of selected sarcolemmal currents from 14 together with a mathematical model of Ca handling from 15 (see supplementary material for the detail) that produces CaT alternans. To study how the pacing rate and the balance of INCX and ICa influence Ca-to-Vm gain, we performed simulations for the control case and ICa predominance. The latter case was simulated by decreasing the maximum strength of INCX by 80%. Simulations were also carried out using the rabbit ventricular myocyte model of Mahajan et al15 in order to demonstrate the robustness of electrogenic mechanisms underlying Ca-to-Vm coupling in different mammalian species (shown in the supplemental material).
Statistical analysis
Statistical analysis of data was performed using Sigmastat (SPSS Inc., Chicago, Illinois, USA). Statistical differences were assessed with One way ANOVA. A p<0.05 was considered statistically significant. Results were expressed as mean ± standard error of the mean (SEM).
RESULTS
Effect of INCX versus ICa predominance on calcium to voltage coupling during alternans
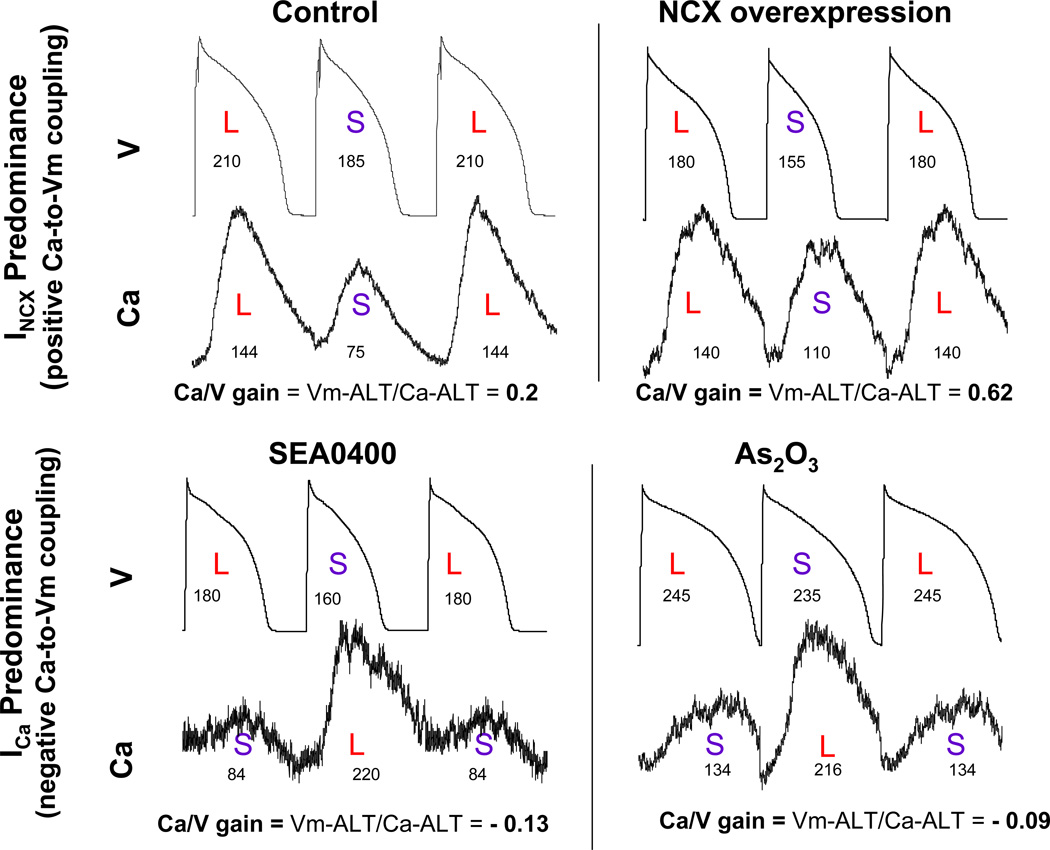
To examine the influence of INCX and ICa on Ca-to-Vm coupling during alternans, action potentials (V) and calcium transients (Ca) alternans were simultaneously recorded as shown in Figure 1. In the control myocyte (top left) Ca-to-Vm coupling was positive; i.e., large Ca transient amplitude was coupled with long APD whereas small Ca transient amplitude was coupled with short APD on subsequent beat, consistent with INCX, but not ICa as the major electrogenic current. Consistent with this observation, with NCX overexpression (top right) positive Ca-to-Vm coupling was maintained with a concurrent increase in Ca-to-Vm gain, i.e. the ratio of Vm-ALT to Ca-ALT was larger than in control myocyte (0.62 vs 0.2 in these examples), confirming increased forward mode electrogenic INCX. In contrast, ICa predominance induced by inhibiting INCX with SEA0400 (1 uM) (bottom left) or increasing ICa with As2O3 (bottom right) produced negative Ca-to-Vm coupling at 240 bpm, i.e small Ca transient amplitude was coupled with long APD whereas large Ca transient amplitude was coupled with short APD on subsequent beat. The gain is small and negative (−0.13 and −0.09 respectively in these examples). These results are summarized in the Table. All control myocytes (14 of 14) demonstrated positive Ca-to-Vm coupling, consistent with INCX, but not ICa as the major electrogenic current. Positive Ca-to-Vm coupling was maintained with NCX overexpression in 8 of 8 myocytes with concurrent increase in Ca-to-Vm gain, confirming increased electrogenic INCX. Conversely, ICa predominance produced negative Ca-to-Vm coupling in 10 of 11 myocytes with inhibited NCX and 4 of 8 myocytes with increased ICa. The bottom panel in Figure 1 shows the negative Ca-to-Vm coupling examples. These data provide direct experimental support to our hypothesis that coupling in phase and magnitude of Ca-ALT to Vm-ALT is strongly determined by relative balance of prominence of INCX versus ICa currents.
Figure 1. Ca-to-Vm Coupling during alternans is determined by prominence of INCX versus ICa.
In control myocyte (top left) Ca-to-Vm coupling was positive; i.e., large Ca transient amplitude was coupled with long APD whereas small Ca transient amplitude was coupled with short APD on subsequent beat. Consistent with this observation, in NCX overexpression myocyte (top right) positive Ca-to-Vm coupling was maintained with concurrent increase in Ca-to-Vm gain, i.e. the ratio of Vm-ALT to Ca-ALT was larger than in control myocyte (0.62 vs 0.2). ICa predominance created by inhibiting INCX with SEA0400 (1 uM) (bottom left) or increasing ICa with As2O3 (bottom right) produced negative Ca-to-Vm coupling at 240 bpm, i.e small Ca transient amplitude was coupled with long APD whereas large Ca transient amplitude was coupled with short APD on subsequent beat. The numbers under action potentials are APD90 (ms) and the numbers under Ca transients are the amplitude of Ca transients (nM).
Table.
Effect of INCX vs ICa predominance on Ca-to-Vm coupling.
| Predominance | Group | Positive Ca-to-Vm Coupling |
Negative Ca-to-Vm Coupling |
|---|---|---|---|
| INCX | Control | 14 | 0 |
| Increased INCX | 8 | 0 | |
| ICa | Decreased INCX | 1 | 10 |
| Increased ICa | 4 | 4 | |
Effect of INCX versus ICa predominance on Ca-to-Vm gain during alternans
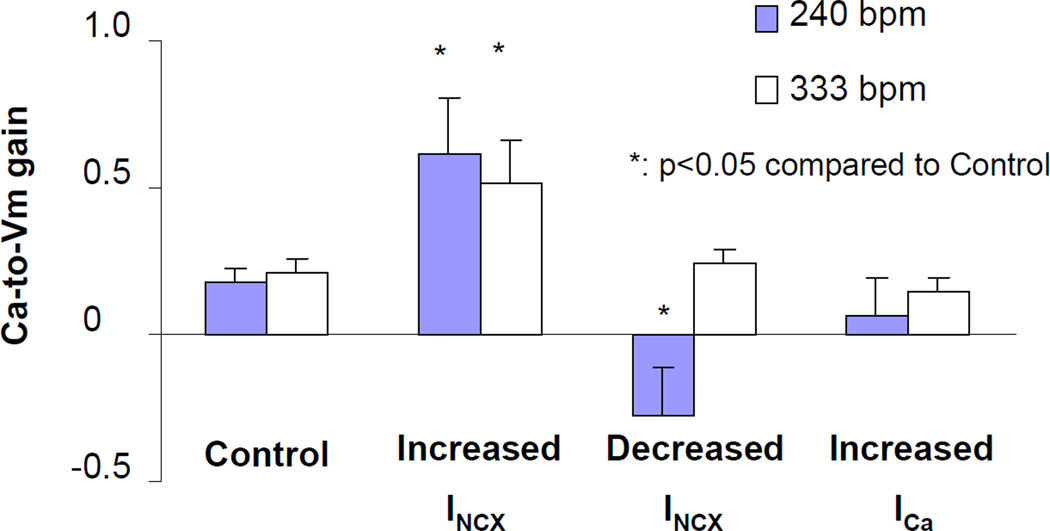
To specifically examine the balanced influence of INCX and ICa on Ca-to-Vm gain during alternans, the ratio of Vm-ALT to Ca-ALT was calculated and plotted in Figure 2. The figure showed that increased INCX predominance by overexpressing NCX increased Ca-to-Vm gain (n=8, p< 0.05 compared to control n=14) indicating that relative availability of forward mode INCX is an important driving force for Vm-ALT. Conversely, at pacing rate of 240 bpm, enhancing ICa predominance by inhibiting INCX with SEA0400 decreased Ca-to-Vm gain significantly (n=11, p< 0.05 compared to control n=14). There was a trend towards increasing ICa with As2O3 also decreased Ca-to-Vm gain, but not significantly (n=8, p=0.09 compared to control n=14). Therefore, Ca-to-Vm gain is strongly influenced by the balance of electrogenic INCX / ICa currents. However, at faster pacing rate (333 bpm) there was no difference in Ca-to-Vm gain between control and enhancing ICa predominance groups.
Figure 2. Increasing INCX predominance increases Ca-to-Vm gain whereas increasing ICa predominance decreases Ca-to-Vm gain.
Ca-to-Vm gain was calculated as the ratio of Vm-ALT to Ca-ALT. The bar graph showed that increasing INCX predominance by overexpressing NCX increased Ca-to-Vm gain significantly (n=8, p< 0.05 compared to control n=14). At pacing rate of 240 bpm increasing ICa predominance by inhibiting INCX with SEA0400 decreased Ca-to-Vm gain significantly (n=11, p< 0.05 compared to control n=14). There was a trend that increasing ICa with As2O3 also decreased Ca-to-Vm gain, but not significantly (n=8, p=0.09 compared to control). However, at faster pacing rate (333 bpm) there was no difference in Ca-to-Vm gain between control and enhancing ICa predominance groups.
Effect of pacing rate on Ca-to-Vm coupling during alternans
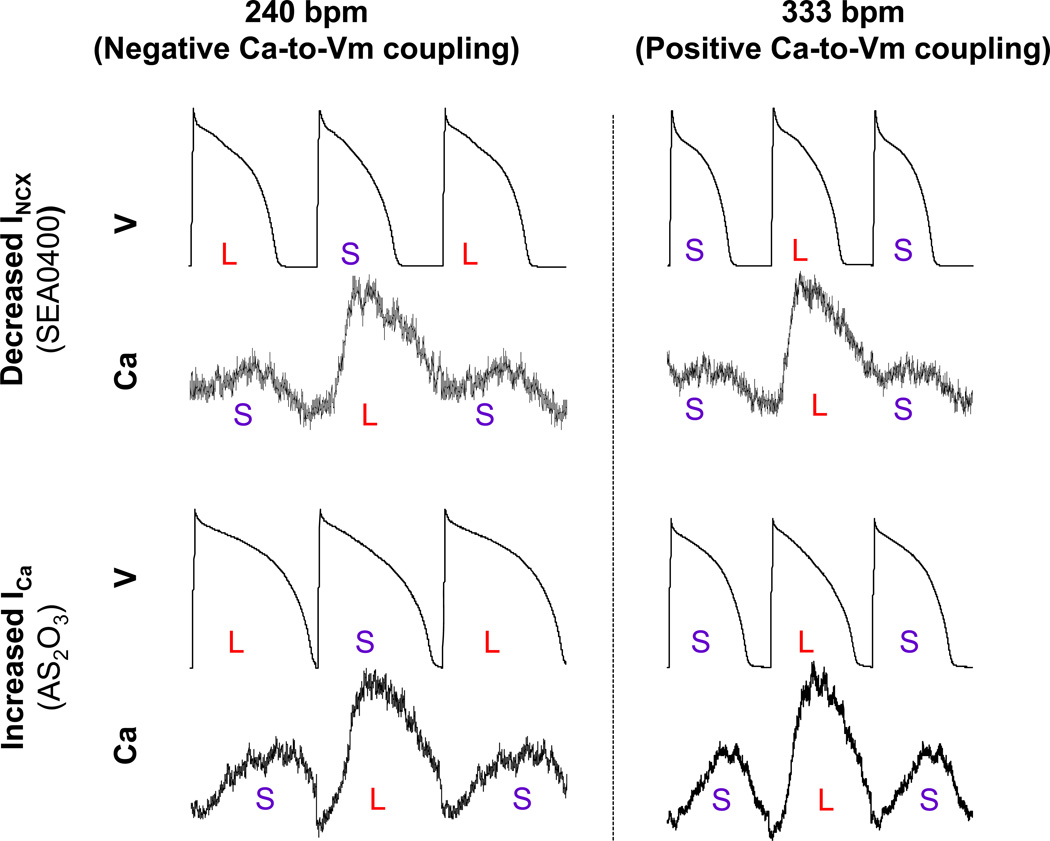
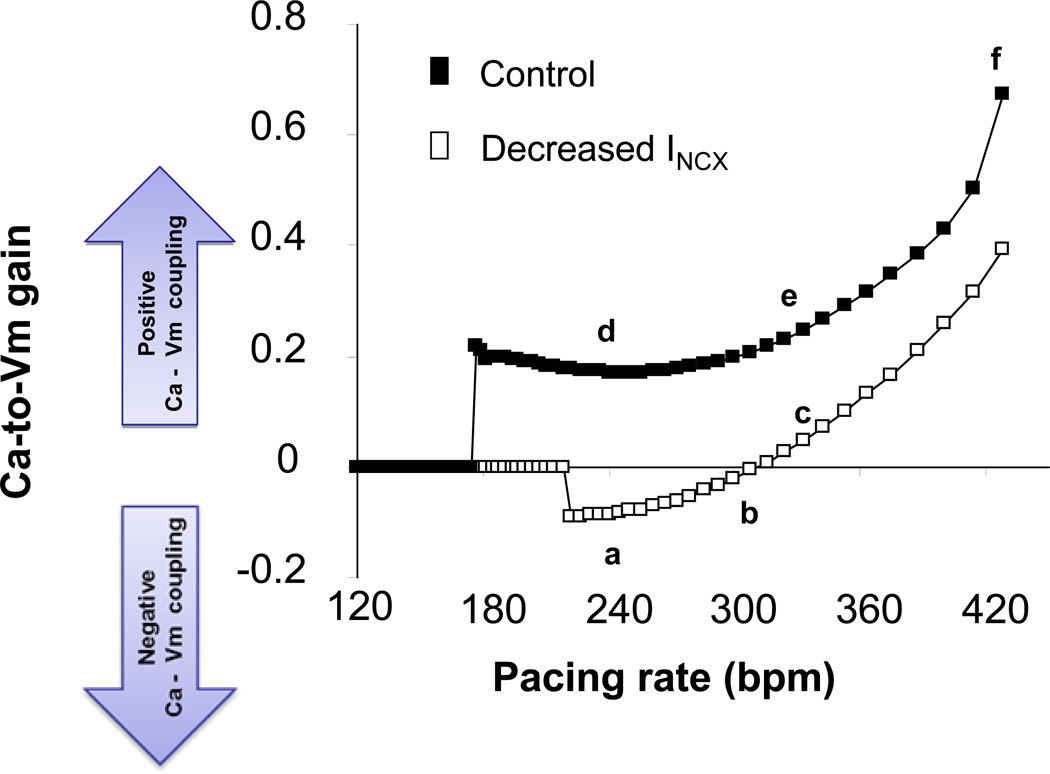
Since current density of ICa but not INCX is influenced by recovery from time-dependent inactivation, increasing pacing rate is expected to reduce ICa current relative to INCX. Hence, we varied pacing rate to shift the relative contribution of ICa versus INCX as a means of further testing how the balance of these currents influences the transduction of Ca-ALT to Vm-ALT. Unlike control conditions, only under conditions of ICa preponderance (Figure 3, left panels) we observed negative Ca-to-Vm coupling. However, at faster pacing rates (Figure 3, right panels) Ca-to-Vm coupling became positive suggesting that the balance of INCX and ICa was shifted in such a way that ICa may no longer be the predominant electrogenic current in modulating action potential duration. This was further explored in the computer simulation shown in figure 4 where rate-dependence of Ca-to-Vm gain is plotted under control versus conditions of ICa preponderance (decreased INCX). Consistent with our experimental results, Ca-to-Vm coupling was always positive in controls (Figure 4 filled rectangles), and Ca-to-Vm coupling increased as heart rate increased, consistent with rate-dependent inactivation of ICa but not INCX (i.e. further enhancing INCX preponderance). Correspondingly, negative Ca-to-Vm coupling was observed only after shifting to conditions of ICa preponderance (Figure 4 open rectangles). However, as heart rate increased there was a reversion to positive Vm-to-Ca coupling, completely mirroring our experimental results (Figure 3).
Figure 3. As pacing rate further increased Ca-to-Vm coupling changed from negative coupling to positive coupling in ICa predominant myocytes.
With SEA0400 inhibiting INCX (top panel) or with As2O3 increasing ICa (bottom panel) Ca-to-Vm coupling was negative at pacing rate of 240 bpm (left). However, as pacing rate increased to 333 bpm Ca-to-Vm coupling changed to positive (right).
Figure 4. Computer simulations showing that the gain of Ca-to-Vm coupling changed from negative to positive as pacing rate increased.
In control alternans occurred when pacing rate reached 180 bpm or faster. Ca-to-Vm coupling was positive. Inhibiting INCX (by 80%) Ca-to-Vm coupling was negative at pacing rate between 220 and 290 bpm. As pacing rate increased to >300 bpm, Ca-to-Vm coupling changed to positive.
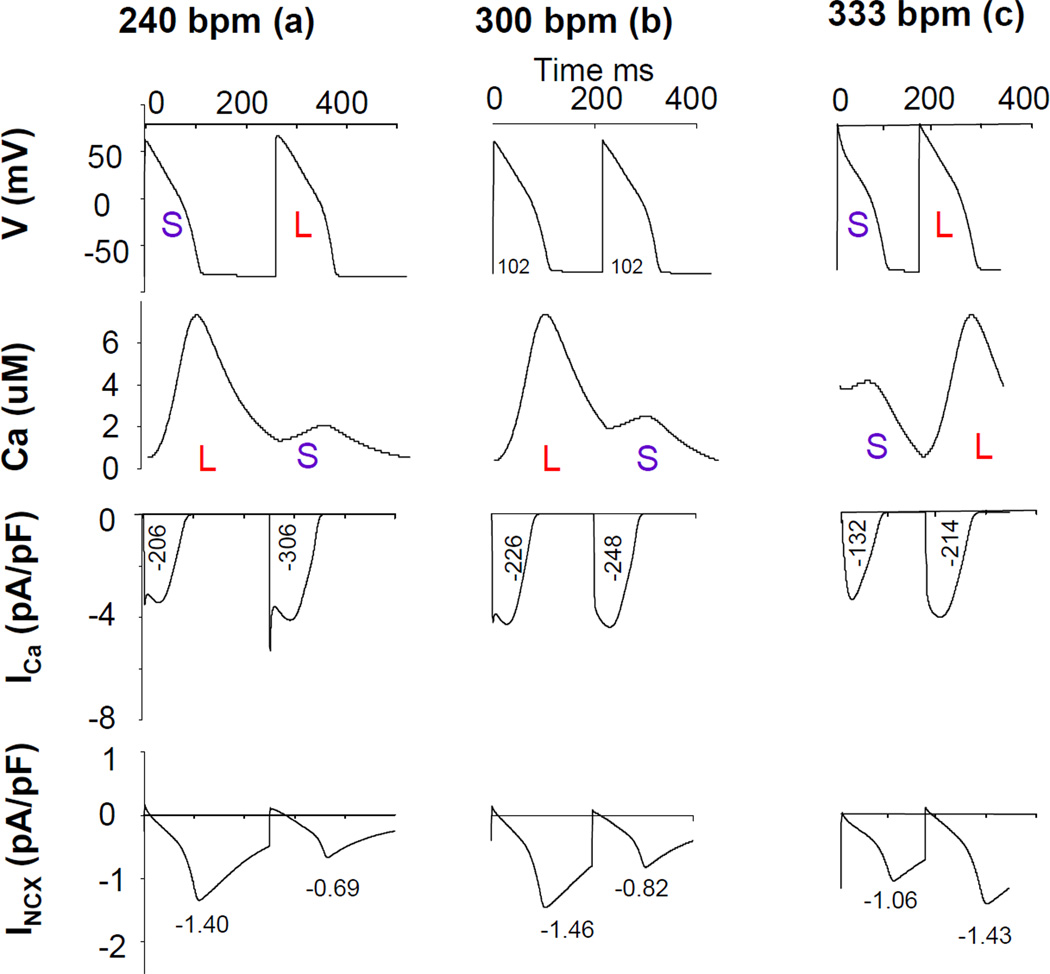
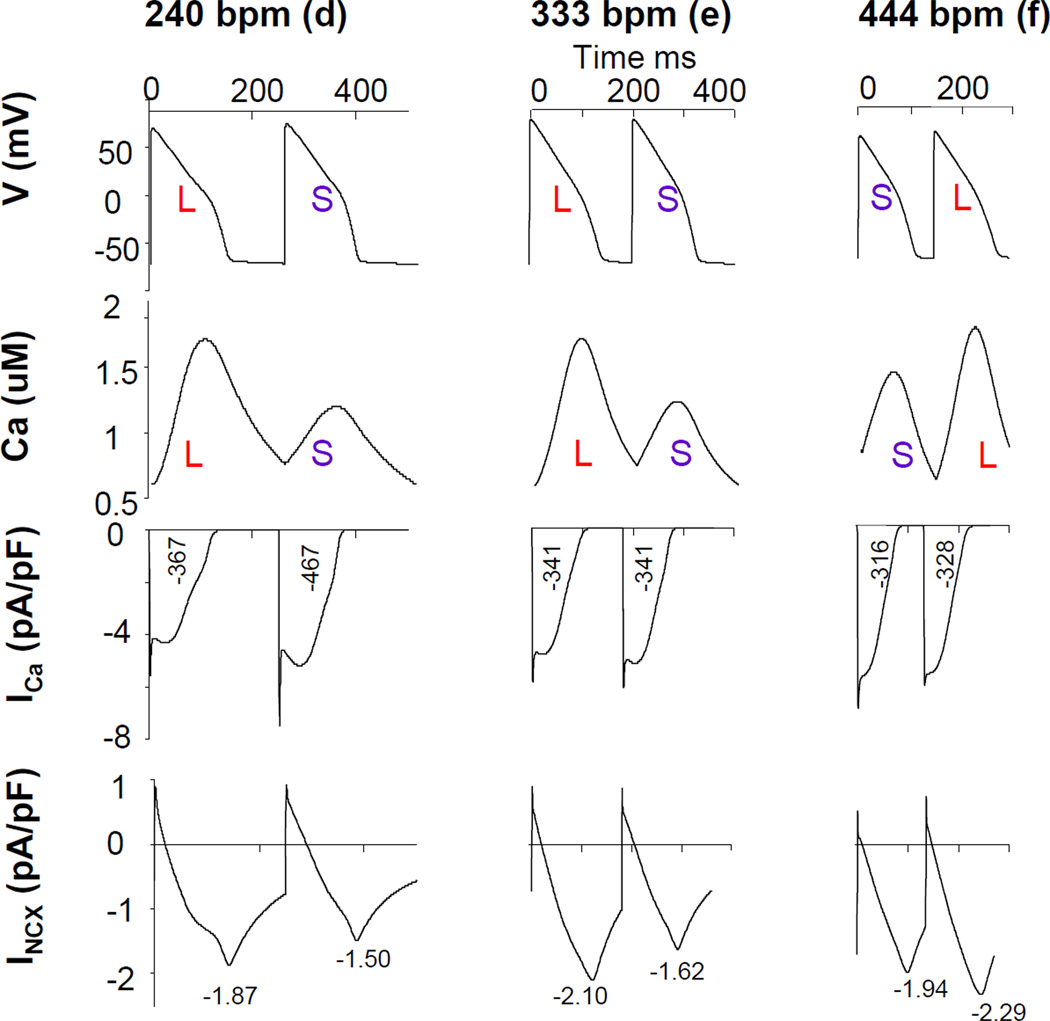
To further explore the mechanism for dynamic shifts from negative to positive Vm-to-Ca coupling, we examined the traces of action potential, Ca transient, ICa and INCX at pacing rate of 240 bpm (left), 300 bpm (middle), and 333 bpm (right) in a condition where INCX is inhibited by 80%. The results of those computer simulations are shown in Figure 5 (a, b, c). Since action potential duration is influenced by the total amount of Ca influx through ICa and the peak INCX current density, we report for each current time trace numerical values for the time integrated value of ICa during the action potential (in (pA/pF)*ms), and the peak INCX value. Ca-to-Vm coupling was negative with inhibited INCX at a pacing rate of 240 bpm (left). As pacing rate increased to 333 bpm, Ca-to-Vm coupling became positive (right). However, at pacing rate of 300 bpm, there was large Ca-ALT but virtually no Vm-ALT. At a pacing rate of 240 bpm, ICa amplitude was large because of reduced Ca-dependent inactivation of this current when the Ca T amplitude is small, consistent with ICa predominance producing negative Ca-to-Vm coupling. As pacing rate increased to 333 bpm, ICa predominance was reduced due to shortening of APD but peak INCX magnitude increased due to increase Ca loading. Interestingly, at a pacing rate of 300 bpm, a smaller Ca T still corresponded to a larger ICa. However the difference of time-averaged ICa values between two beats were smaller than those at 240 bpm, thereby decreasing the contribution of ICa to Vm-ALT. In addition, average INCX was increased at 300 bpm compared to 240 bpm, thereby increasing the contribution of INCX to Vm-ALT. At 300 bpm, the effect of Ca-ALT on INCX and ICa was almost completely balanced so that Vm-ALT were absent despite the presence of Ca-ALT. In this situation, CaT-ALT are “hidden” insofar that their existence could not be inferred from electrical measurement alone. For pacing rate smaller (larger) than 300 bpm, Ca-to-Vm coupling was negative (positive). Therefore as pacing rate increased, the change of Ca-to-Vm coupling from negative to positive was due to a shift from ICa to INCX predominance. However, for control experiments shown in Figure 6, a small Ca T induced a small peak INCX. Thus APD shortened although ICa increased due to reduced Ca induced ICa inactivation at 240 bpm. As pacing rate increased to 444 bpm, INCX magnitude further increased due to its Ca dependence but APD was too short for ICa to dominate. Ca-to-Vm coupling was positive at all pacing rates, consistent with INCX, but not ICa, being the major electrogenic current producing Vm-ALT from Ca-ALT.
Figure 5. Balanced influence of INCX and ICa on Ca-to-Vm coupling in inhibiting INCX condition.
The figure illustrated the traces of action potential, Ca transient, ICa and INCX at pacing rate of 240 bpm (left), 300 bpm (middle), and 333 bpm (right) in inhibiting INCX (by 80%) as shown in Figure 4 (a, b, c) in computer simulation. The numbers under ICa curves came from the time integration of currents ((pA/pF)*ms), and the numbers under INCX was the peak current density. Ca-to-Vm coupling was negative with inhibited INCX at pacing rate of 240 bpm (left). As pacing rate increased to 333 bpm, Ca-to-Vm coupling was positive (right). However, at pacing rate of 300 bpm, there was large Ca-ALT but virtually no Vm-ALT.
Figure 6. Relationship between Ca-to-Vm coupling and electrogenic currents in control.
The figure illustrated the traces of action potential, Ca transient, ICa and INCX at pacing rate of 240 bpm (left), 333 bpm (middle), and 444 bpm (right) in a control as shown in Figure 4 (d, e, f) in computer simulation. Ca-to-Vm coupling were positive at all pacing rate.
Checking the relationship between ICa and CaT for both control and during INCX inhibition we found that small CaT were associated with large ICa at slower pacing rates while at faster pacing rates, small CaT were associated with small ICa. However, Ca-to-Vm coupling changes from negative to positive as pacing rate increased occurred only with INCX inhibition.
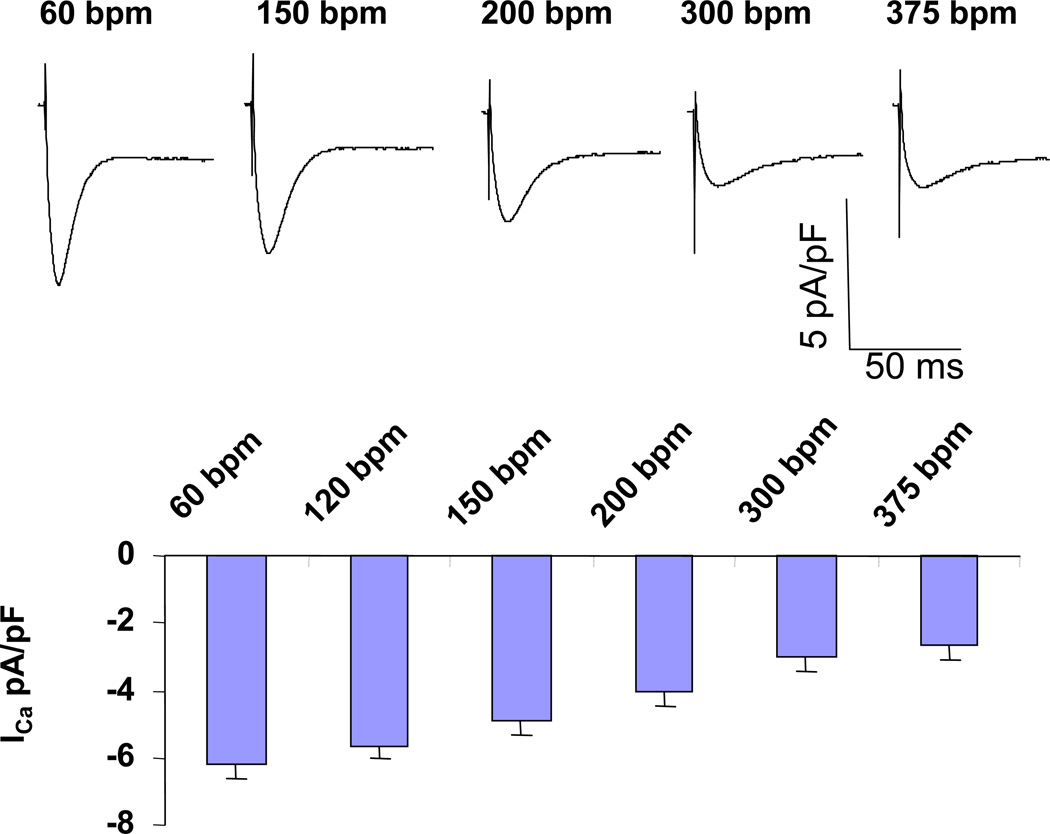
These two figures also demonstrated the rate dependence of ICa and INCX. At control condition, average ICa between two consecutive beats decreased as pacing rate increased (417(pA/pF)*ms, 341(pA/pF)*ms, and 322(pA/pF)*ms at 240bpm, 333bpm, and 444bpm respectively) whereas average INCX between two consecutive beats increased as pacing rate increased (−1.685pA/pF, −1.86pA/pF, and −2.115pA/pF at 240bpm, 333bpm, and 444bpm respectively). At inhibiting INCX (by 80%) condition, average ICa between two consecutive beats decreased as pacing rate increased (256(pA/pF)*ms, 237(pA/pF)*ms, and 173(pA/pF)*ms at 240bpm, 300bpm, and 330bpm respectively) whereas average INCX between two consecutive beats increased as pacing rate increased (−1.045pA/pF, −1.14pA/pF, and −1.245pA/pF at 240bpm, 300bpm, and 330bpm respectively). Figure 7 (top panel) shows representative traces of ICa at different pacing rate at patch-clamp experiments in real myocytes. As pacing rate increased ICa density decreased, confirming the results of computer simulations.
Figure 7. ICa density decreased as pacing rate increased at fast pacing rate.
Top panel illustrated representative traces of ICa at different pacing rate. As pacing rate increased ICa density decreased. Summary data from 10 myocytes was shown in bottom panel.
These data further support that as pacing rate increased the balance of ICa to INCX shift towards INCX predominance even under inhibiting INCX condition. Taken together, these finding reaffirmed that small shifts in the balance of ICa and INCX can influence Ca-to-Vm coupling during cardiac alternans.
DISCUSSION
In the present report, we provide new experimental evidence supporting an important role for a fine balance between electrogenic INCX/ICa currents in cardiac alternans. In particular, we find that INCX predominance produces positive Ca-to-Vm coupling whereas ICa predominance produces negative Ca-to-Vm coupling. When the effect of CaT-ALT on these two currents is perfectly balanced, CaT-ALT does not produce Vm-ALT (zero gain). In addition, the sign of Ca-to-Vm coupling can change with pacing rate due to the fact that ICa and INCX have different dependences. Combined with the theoretical framework provided by the computer simulations, our findings help to shed light on complex mechanisms controlling the relationship between cytosolic calcium and membrane voltage under highly dynamic conditions such as alternans which ultimately influence the spatial organization of repolarization in the heart.
Electrogenic mechanisms for transducing Ca-ALT to Vm-ALT
The central aim of this work was to establish experimental evidence for the mechanism by which the myocyte generates Vm-ALT from Ca-ALT. Elucidating this relationship is critical to understanding how arrhythmogenic Vm-ALT arises under normal and pathological conditions. There are several important electrogenic feedback mechanisms by which Ca-ALT can be transduced to Vm-ALT including; 1. SR calcium release enhances inactivation of sarcolemmal ICa, shown as negative feedback because it results in lowering membrane voltage (shortening APD), and 2. SR calcium release enhances calcium extrusion by INCX, shown as positive feedback because it increases membrane voltage (lengthens APD because electrogenic INCX drives 3 sodium into the cell for every one calcium extruded). It has been observed that the large Ca transient during beat-to-beat (large-small) Ca-ALT is accompanied by a short APD in some species (or certain experimental conditions) 16, 17, while in other species by a prolonged APD 18, 19. It was suggested that the INCX is responsible for prolongation of APD during large Ca transient, while Ca-dependent inactivation of ICa is the mechanism of APD shortening 7, 18.
Our data suggest that INCX is the most preponderant electrogenic mechanism for Vm-ALT, based on the fact that we consistently observed positive Ca-to-Vm coupling in normal myocytes in this (Figure 1) and prior studies. 4, 20 In other words, under normal conditions, Ca-ALT and Vm-ALT are in phase. The opposite would be expected if ICa was the electrogenic mechanism. However, we also hypothesize that any condition (drugs, disease, heart rate, etc), which shifts the balance of sarcolemal currents away from INCX preponderance, will also change the electrogenic currents that govern alternans, and hence the magnitude and phase of cellular alternans.
The balance of INCX and ICa determines the phase and gain of Ca-to-Vm coupling
The advantage of manipulating INCX and ICa predominances in the present study stems from the ability to demonstrate a causal relationship between a single current and the development of cellular alternans and Ca-to-Vm coupling. As shown in figure 1, INCX predominance produces positive Ca-to-Vm coupling whereas ICa predominance produces negative Ca-to-Vm coupling. Selective enhancement or reduction of INCX increased or decreased Ca-to-Vm gain, respectively as demonstrated in Figure 2. This finding supports the hypothesis that INCX is the principle electrogenic current for Vm-ALT. Conversely, when INCX was inhibited, ICa becomes the predominant electrogenic carrier transducing Ca-ALT to Vm-ALT. In this case, negative Ca-to-Vm coupling is observed to produce electromechanical discordance, where Ca transient and APD alternate in opposite phase (figure 1).
To check the importance of Ca-dependence of IKs, we repeated the simulations of Figure 4 after removing the Ca-dependence of IKs by replacing Cai by a constant value of 0.1 µM in the formula for gKs given in the supplementary material. The results were unchanged, showing that the Ca-dependence of IKs has a negligible role in determining the sign of Ca-to-Vm coupling.
The balance of INCX and ICa determining the gain of Ca-to-Vm coupling may also explain the differences of Ca-to-Vm gain between canine and guinea pig. It has been reported that the larger Ca-to-Vm gain in the canine compared with guinea pig is due to differences in ion channel expression levels and kinetic properties 21. On the background of smaller IKr and IKs in the canine22, in conjunction with a much smaller ICa during the late AP plateau 23, Ca transient-induced changes in INCX have a much greater modulatory effect on AP repolarization, identifying INCX as the predominant Ca-to-Vm coupler during alternans. The other Ca-dependent currents, ICa and Ito, play a role in shaping the AP during its initial plateau phase, causing crossover between consecutive APs during alternans, but have a minimal effect on APD. The situation can be different, with ICa playing a role in Vm-ALT, in species, where ICa persists into the late phase of the AP (e.g., guinea pig) and the Ca-dependent IKs repolarizing current has a large conductance24, 25. Under such conditions, a large Ca transient can lead to APD shortening during alternans, due to increased Ca-dependent inactivation of ICa. Therefore, the Ca-to-Vm coupling gain in the guinea pig is smaller compared with canine21 and the balance of INCX and ICa determines the gain of Ca-to-Vm coupling.
Our data also suggest that small shifts in the balance of activity between INCX and ICa can dynamically influence Ca-to-Vm coupling. Under conditions of ICa preponderance, negative coupling between Ca-ALT and Vm-ALT was transformed to positive coupling at increased heart rates (Figure 4). To explain the rate dependent shift we determined the rate dependence of ICa and INCX. ICa at different pacing rate were measured and shown in Figure 7. As pacing rate increased ICa density decreased. Since we used same square waveform to initiate ICa faster pacing rate means shorter diastolic interval for ICa to recover from inactivation and less channel available for the activation. Faster pacing also increases diastolic calcium. The rise in calcium at high rates depends primarily on the corresponding accumulation of sodium, due to the activity of INCX 26. Another mechanism that promotes calcium loading at high rates is that the action potential waveform spends more time per period at plateau potentials, and so the time spent in the calcium efflux mode is reduced. Therefore, with increasing pacing rate, INCX increases and ICa decreases in normal myocytes. Pharmacological inhibition of INCX affects the rate dependences of both ICa decrease and INCX increase as shown in Figure 5&6, thereby shifting the balance of INCX and ICa in a rate-dependent fashion. For intermediate pacing rate (240 bpm), ICa dominates due to INCX inhibition, thereby giving rise to negative coupling. In contrast, for even faster pacing rate (333 bpm), the ICa decrease and INCX increase are sufficient to produce a positive coupling despite pharmacological INCX inhibition.
Supplementary Material
ACKNOWLEDGMENTS
The virus (Ad.NCX) was the gift from Dr Hajjar’s lab in Mount Sinai School of Medicine, USA.
Sources of Funding
This study was supported by a grant from the NIH (#RO1-HL54807, DSR). Zhen Song also acknowledges support of an AHA predoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Reference List
- 1.Ikeda T, Saito H, Tanno K, et al. T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol. 2002;89:79–82. doi: 10.1016/s0002-9149(01)02171-3. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 3.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 4.Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: The saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 6.Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL, O'Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res. 2003;93:46–53. doi: 10.1161/01.RES.0000080932.98903.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiferaw Y, Sato D, Karma A. Coupled dynamics of voltage and calcium in paced cardiac cells. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:021903. doi: 10.1103/PhysRevE.71.021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restrepo JG, Karma A. Spatiotemporal intracellular calcium dynamics during cardiac alternans. Chaos. 2009;19:037115. doi: 10.1063/1.3207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Monte F, Lebeche D, Guerrero JL, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranu HK, Terracciano CM, Davia K, et al. Effects of Na(+)/Ca(2+)-exchanger overexpression on excitation-contraction coupling in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2002;34:389–400. doi: 10.1006/jmcc.2001.1521. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda T, Arakawa N, Takuma K, et al. Sea0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- 12.Tanaka H, Nishimaru K, Aikawa T, Hirayama W, Tanaka Y, Shigenobu K. Effect of sea0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br J Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuryshev YA, Wang L, Wible BA, Wan X, Ficker E. Antimony-based antileishmanial compounds prolong the cardiac action potential by an increase in cardiac calcium currents. Mol Pharmacol. 2006;69:1216–1225. doi: 10.1124/mol.105.019281. [DOI] [PubMed] [Google Scholar]
- 14.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan A, Shiferaw Y, Sato D, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kihara Y, Morgan JP. Abnormal Cai2+ handling is the primary cause of mechanical alternans: Study in ferret ventricular muscles. Am J Physiol. 1991;261:H1746–H1755. doi: 10.1152/ajpheart.1991.261.6.H1746. [DOI] [PubMed] [Google Scholar]
- 17.Murphy CF, Horner SM, Dick DJ, Coen B, Lab MJ. Electrical alternans and the onset of rate-induced pulsus alternans during acute regional ischaemia in the anaesthetised pig heart. Cardiovasc Res. 1996;32:138–147. [PubMed] [Google Scholar]
- 18.Orchard CH, McCall E, Kirby MS, Boyett MR. Mechanical alternans during acidosis in ferret heart muscle. Circ Res. 1991;68:69–76. doi: 10.1161/01.res.68.1.69. [DOI] [PubMed] [Google Scholar]
- 19.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 20.Walker ML, Wan X, Kirsch GE, Rosenbaum DS. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation. 2003;108:2704–2709. doi: 10.1161/01.CIR.0000093276.10885.5B. [DOI] [PubMed] [Google Scholar]
- 21.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: Role of camkii and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007;292:H2854–H2866. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hund TJ, Otani NF, Rudy Y. Dynamics of action potential head-tail interaction during reentry in cardiac tissue: Ionic mechanisms. Am J Physiol Heart Circ Physiol. 2000;279:H1869–H1879. doi: 10.1152/ajpheart.2000.279.4.H1869. [DOI] [PubMed] [Google Scholar]
- 23.Banyasz T, Fulop L, Magyar J, Szentandrassy N, Varro A, Nanasi PP. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc Res. 2003;58:66–75. doi: 10.1016/s0008-6363(02)00853-2. [DOI] [PubMed] [Google Scholar]
- 24.Linz KW, Meyer R. Profile and kinetics of L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflugers Arch. 2000;439:588–599. doi: 10.1007/s004249900212. [DOI] [PubMed] [Google Scholar]
- 25.Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca2+ channel and its role in myocyte electrophysiology: A theoretical investigation. Biophys J. 2007;92:1522–1543. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison SM, Boyett MR. The role of the Na(+)-Ca2+ exchanger in the rate-dependent increase in contraction in guinea-pig ventricular myocytes. J Physiol. 1995;482(Pt 3):555–566. doi: 10.1113/jphysiol.1995.sp020539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.