Abstract
Purpose
To identify predictors of ocular surface squamous neoplasm (OSSN) recurrence after surgical resection.
Design
Retrospective case series.
Participants
Three hundred and eighty nine consecutive patients who underwent excisional biopsy for OSSN lesions at the Bascom Palmer Eye Institute from January 1, 2001, to September 20, 2010
Methods
Review of pathology records and patient charts.
Main Outcome Measures
Identification of factors predictive of OSSN recurrence.
Results
Of 389 excised OSSN lesions, forty-four recurred during follow up. The 1 year recurrence rate was 10% and the 5 year recurrence rate was 21% with a mean time to recurrence in those with a recurrence of 2.5 years (standard deviation (SD) 3.4). Using the American Joint Committee on Cancer (AJCC) clinical staging system, T3 and T2 lesions portended a higher risk of recurrence compared to T1 (T2/T1: hazard ratio (HR) = 2.05, p=0.04; T3/T1: HR= 2.31, p=0.07). In addition, a location characteristic that increased the risk of tumor recurrence was tarsal involvement (AJCC T3 stage lesion) (HR=4.12, p = 0.007). Nasal location was associated with a decreased risk of tumor recurrence (HR=0.41, p=0.008). Pathologic characteristics significantly associated with tumor recurrence were the presence of positive margins (HR=2.73, p= 0.008) and higher grade lesions (carcinoma in situ and squamous cell carcinoma versus dysplasia) (HR=2.55, p=0.02). Treatment with adjuvant cryotherapy significantly decreased the risk of tumor recurrence (HR=0.51, p=0.03). In those patients with positive margins, the use of post-operative topical interferon therapy lowered the recurrence rate to a level similar to that of patients with negative margins.
Conclusions
Certain patient and tumor factors are associated with a higher risk of OSSN recurrence after surgical excision, such as tarsal tumor location and positive surgical margins. Post-operative adjuvant therapy should be considered in patients with high risk OSSN characteristics.
Introduction
Ocular surface squamous neoplasia (OSSN) represents a spectrum of disease ranging from mild dysplasia to invasive squamous cell carcinoma (SCC).1, 2 Reported risk factors for disease include ultraviolet light exposure, fair skin, human papilloma virus (HPV), human immunodeficiency virus, dysfunctional DNA repair, and cigarette smoking.3–10 Surgical excision is the predominant treatment modality for OSSN and its approach has evolved with time. Initially, tumors were removed by simple excision11–13 with the later implementation of Shields’“no touch” technique and increased surgical margins.14 The theoretical value of the wide margin “no touch” technique is to avoid the potential risk of seeding and to increase the likelihood clear margins. Some groups have also reported using a modified Mohs technique to further ensure tumor free margins.15, 16
Intraoperative adjuvant agents including cryotherapy14, 16–19, absolute alcohol14, 19, and mitomycin C (MMC)20, 21 are now routinely used and the ocular surface is frequently reconstructed with amniotic membrane instead of primary closure.19 Cryotherapy is applied to the conjunctival and limbal margins in a “double freeze slow thaw” technique and causes cellular death through the rupture of cell membranes and alterations in blood vessel architecture.22 Mitomycin C is an alkylating agent with cytotoxic and antiproliferative effects that targets rapidly diving cells.23 While it is difficult to compare recurrence rates between series as most report recurrences as a percentage in patients with variable follow up times, there is a suggestion that these newer techniques have resulted in decreased tumor recurrences.14, 16, 19, 24
It is important to understand which factors are associated with OSSN recurrence as this information can help to tailor therapy based on risk. For example, physicians may choose to employ intraoperative and postoperative adjuvant agents, such as interferon and MMC, in patients at higher risk of recurrence. However, limited data exists on which patient and tumor factors predict a more severe disease course. Regarding patient factors, Lee et al found that those who presented with ocular irritation had a 2.4 fold risk of recurrence after treatment (95% confidence interval (CI) 1.2–4.8) compared to those without this complaint.11 Regarding tumor factors, the same group found that higher pathologic grade (carcinoma in-situ (CIS) versus dysplasia) increased the risk of recurrence by 3.2 fold (95% CI 1.4–7.3).11 Data from other groups, however, did not support an association between grade and recurrence.12, 13, 18 Positive pathologic margins, on the other hand, have consistently been shown to increase the risk of recurrence.12, 13
Regarding treatment approaches, few papers have directly compared OSSN approaches to determine whether certain techniques lead to superior results. This is important as the physician must consider both the potential benefits of adjuvant agents, such as cryotherapy and MMC, and their potential side effects. Two case series compared outcomes after cryotherapy and surgery versus surgical excision alone and while both found a lower risk of recurrence in patients treated with the combined modality, neither had sufficient sample size to show a statistically significant difference.17, 25 Birkholz et al compared the use of intra- (n=4) or postoperative MMC (n=13) to no MMC (n=15) and found that patients treated with any MMC had significantly fewer recurrences.20 Over the past 10 years, physicians at the Bascom Palmer Eye Institute have treated a large number of patients with OSSN and each has employed his/her own surgical approach to tumor removal. Technical variations in OSSN treatment have included utilizing a variable surgical margin size, some employing a “no touch” technique, and variable use of adjuvant agents such as cryotherapy, absolute alcohol, and interferon. The aim of this study was to evaluate which patient, tumor, and treatment factors affected tumor recurrences with the goal of developing an evidence-based, tailored approach for the treatment of OSSN.
Materials and Methods
Study population
Approval was obtained from the University of Miami Institutional Review Board and the methods adhered to the tenets of the Declaration of Helsinki and were compliant with the Health Insurance Portability and Accountability Act (HIPAA). The pathology records of the Florida Lions Ocular Pathology Laboratory at the Bascom Palmer Eye Institute (BPEI) were searched for diagnoses containing the words “conjunctival” or “corneal” and “dysplasia,” “intraepithelial neoplasia,” or “squamous cell carcinoma.” A total of 703 lesions from 612 subjects were identified in our database from January 1, 2001, to September 20, 2010. Review of patient records revealed that 396 subjects were treated with excisional biopsy at BPEI. The remaining subjects were either treated at an outside institution (n=179) or underwent incisional biopsy only (n=37).
Data collection
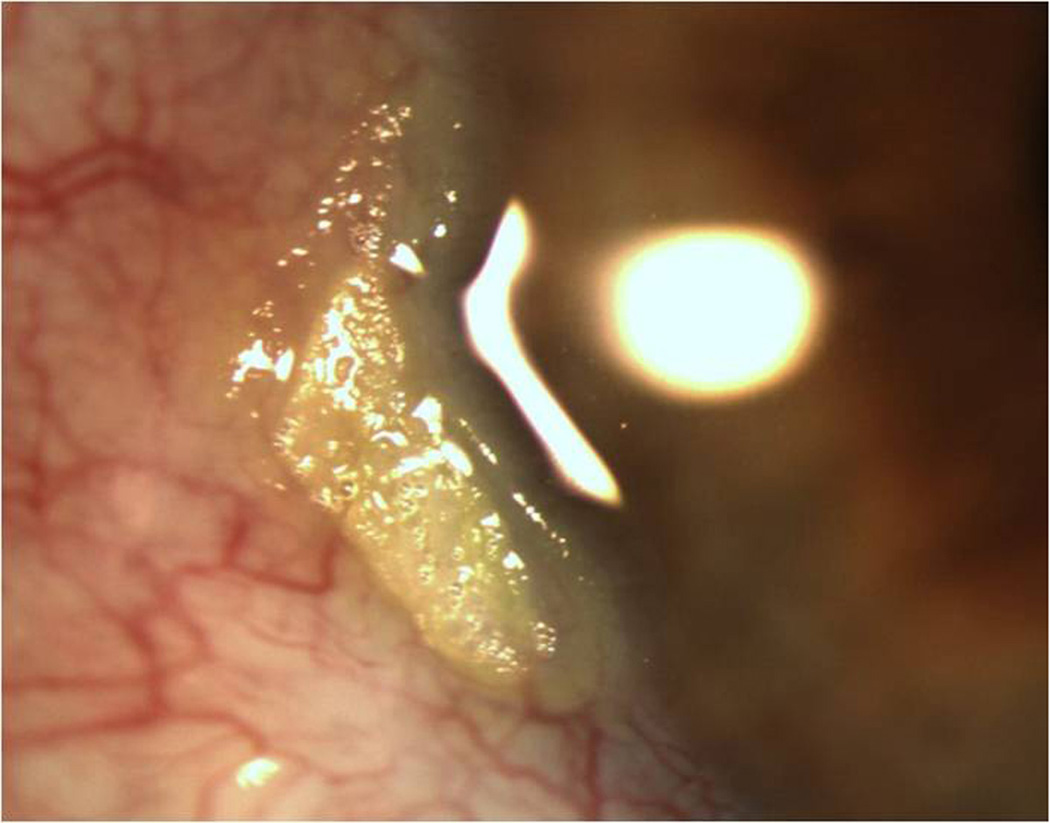
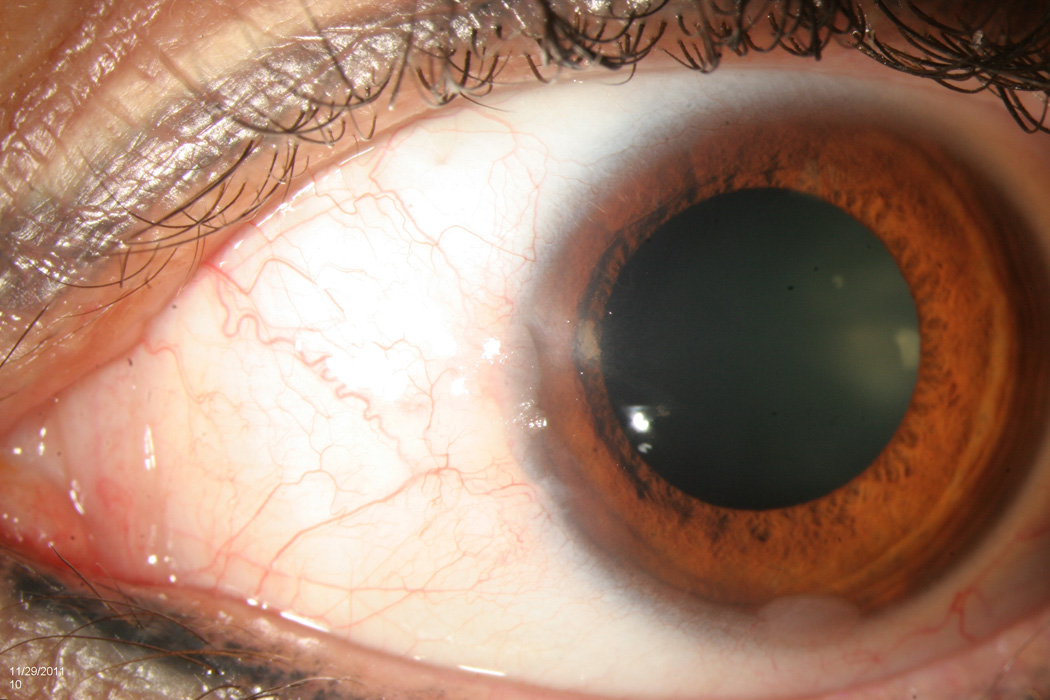
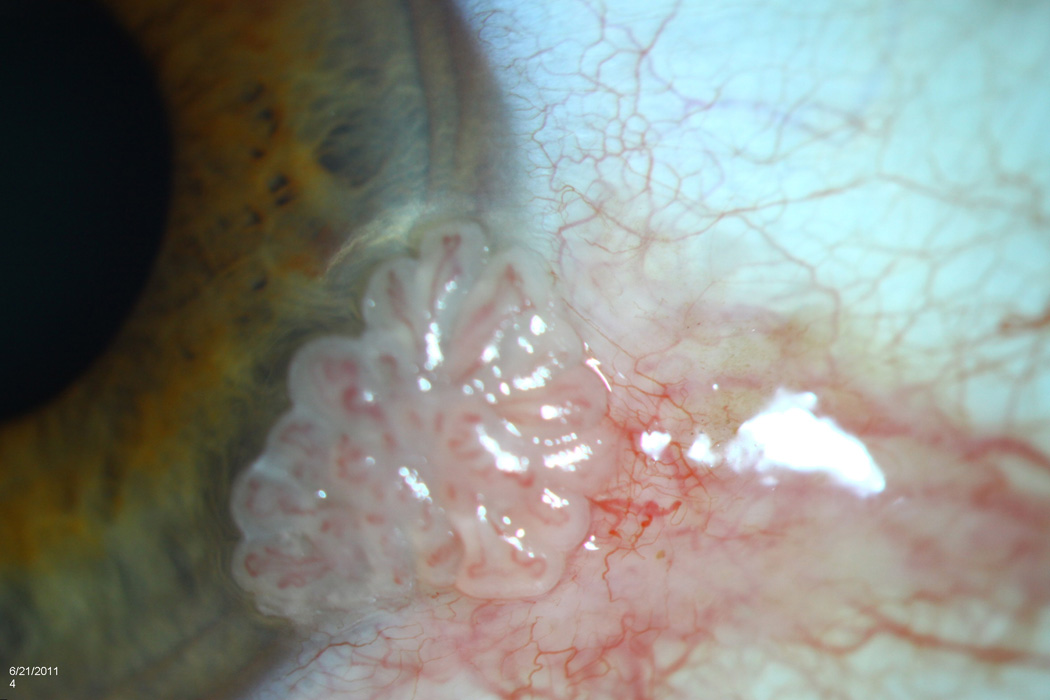
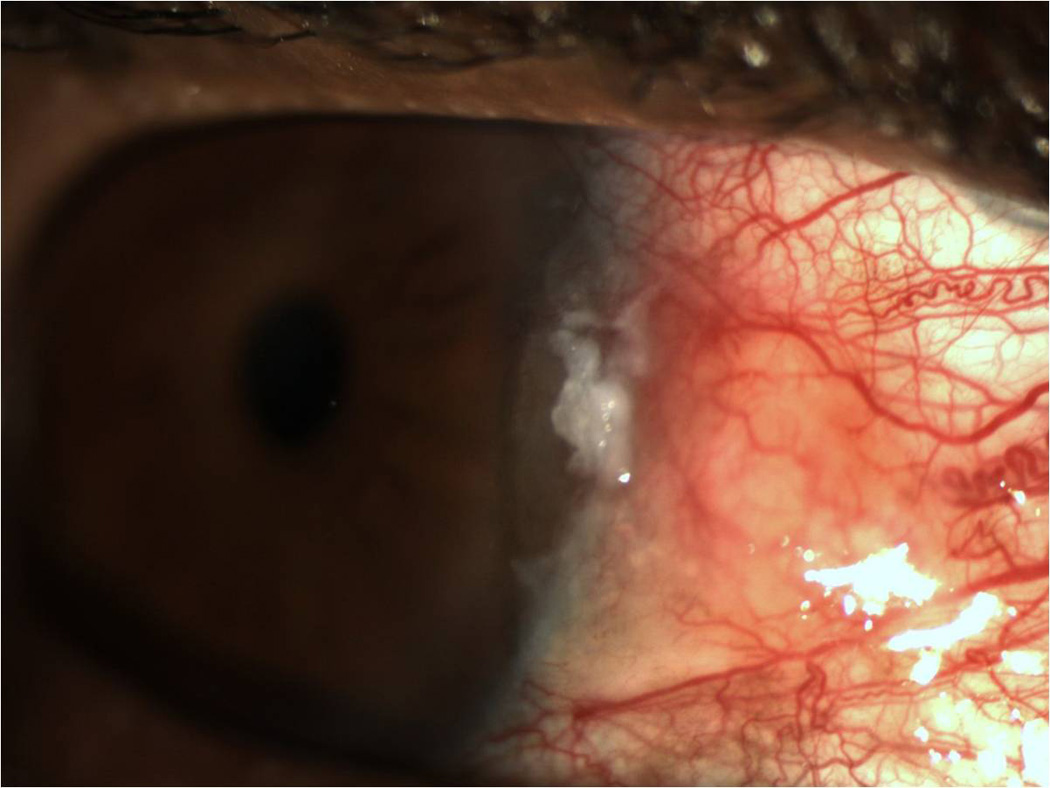
All lesions had been previously examined by a single experienced ocular pathologist (SRD). Pathology reports were reviewed for tumor grade, location, and presence at surgical margins. Corresponding patient records were reviewed for age; gender; self-identified race (Caucasian, African-American, Asian, mixed, other) and self- identified ethnicity (Hispanic, non-Hispanic); involved eye; previous history of OSSN lesions; visible lesion location (tarsal, limbal (with or without corneal involvement), non-limbal conjunctivae, corneal) and affected quadrant(s) of bulbar conjunctivae (superior, inferior, nasal, temporal); clinical appearance of the lesion (papillomatous/not, leukoplakic/not, gelatinous/not, flat/nodular); and size (determined by assuming a corneal dimension of 12 mm × 12 mm and estimating tumor area as it related to cornea/conjunctivae covered). Furthermore, tumors were staged based on the American Joint Committee on Cancer (AJCC) clinical staging system.26 A multifocal lesion was defined as a tumor that had two or more clinically non-connected foci of disease (Figure 1). A papillomatous lesion was defined as a tumor that had multiple abnormal blood vessels with fleshy tissue surrounding the blood vessels (Figure 2). A leukoplakic lesion was defined as one that had a white, keritanized plaque overlying the tumor (Figure 3). A gelatinous lesion was defined as one that had a smooth, gelatin like appearance (Figure 4). A nodular lesion was defined as one that had any appreciable elevation on clinical examination (Figure 5). Charts were reviewed for those descriptive terms, and if clinical photographs were present, these descriptions were verified.
Figure 1.
Slit lamp photograph of a multifocal ocular surface squamous neoplasia demonstrating two foci of tumor, one at 6 and one at 9’oclock.
Figure 2.
Slit lamp photograph of a papillomatous ocular surface squamous neoplasia demonstrating multiple abnormal blood vessels surrounded by fleshy tissue.
Figure 3.
Slit lamp photograph of a leukoplakic ocular surface squamous neoplasia demonstrating a white, keritanized plaque overlying the tumor.
Figure 4.
Slit lamp photograph of a gelatinous ocular surface squamous neoplasia demonstrating a smooth, gelatin like appearance.
Figure 5.
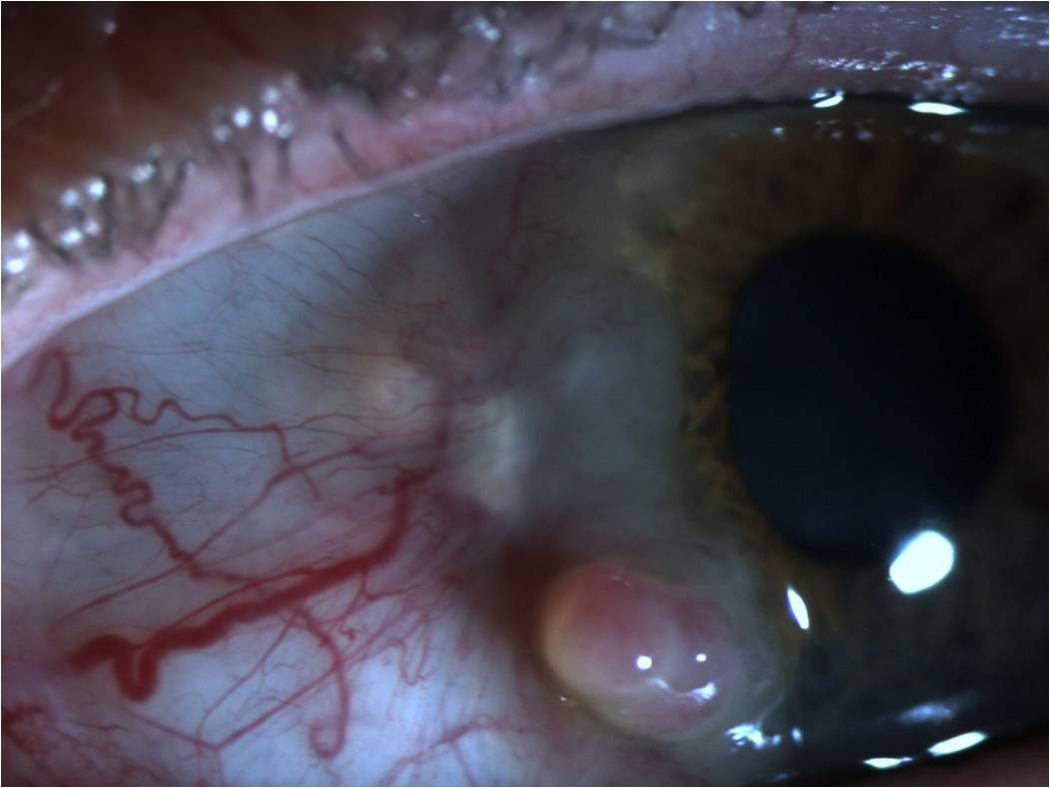
Slit lamp photograph of a nodular ocular surface squamous neoplasia demonstrating significant elevation.
The use of any pre-, intra-, or post-operative adjuvant treatments, including the use of cryotherapy, interferon, 5-Fluorouracil (5FU), and MMC, was recorded. Although we were not always able to determine the exact excisional technique employed by the surgeon, the use of cryotherapy to the limbal and conjunctival margins in a double freeze slow thaw cycle was recorded. In patients with multiple tumor recurrences, only first treatment information was recorded. The main outcome measure was the determination of which patient, tumor, and treatment approaches influenced the risk of disease recurrence. Disease recurrence was defined as the appearance of a clinically identifiable tumor in an eye where there was no identifiable tumor after surgery. This definition likely included patients with a re-growth of incompletely excised disease and along with patients that had new tumor formation.
Statistical analysis
All statistical analyses were performed using the SPSS 18.0 (SPSS Inc, Chicago, IL) statistical package. Frequencies of demographic and clinical variables were calculated for the group. Uni- and multi-variable Cox proportional hazards analyses were employed to evaluate for factors associated with disease recurrence. A forward step-wise approach was used for the multi-variable analysis. Time-to-event curves were generated using the Kaplan-Meier method.
Results
Demographic and clinical information is listed in Table 1. Patient age ranged from 21 to 99 years (mean 61 years), and males (n=282, 71%) outnumbered females (n=108, 29%). Two-hundred ninety-seven subjects were white (79%), and 177 self-identified themselves as Hispanic (47%). Fifty-two had a previous history of OSSN (16%). The right eye was affected in 196 cases (50%).
Table 1.
Demographic and clinical information in patients with ocular surface squamous neoplasia treated by excisional biopsy at Bascom Palmer Eye Institute
| Number of eyes/patients | 396 |
| Age (years), mean [SD] | 61 [16] |
| Gender, male n [%] | 282 [71%] |
| Race, white n [%] black n [%] |
297[79%] 47[12%] |
| Ethnicity, Hispanic n [%] | 177 [47%] |
| Involved eye, right n [%] | 196 [50%] |
| History of OSSN n [%] | 52 [16%] |
n=number of individuals in group; OSSN= ocular surface squamous neoplasia; SD=standard deviation
Regarding treatment, an excisional surgical approach was undertaken in all cases. Two-hundred seventy four patients had concomitant cryotherapy to the surgical margins at the time of tumor removal (69%). Pre- or post-operative adjuvant therapy with interferon, 5FU, or MMC was employed in 110 patients (28%). Table 2 details the treatment information of patients who received adjuvant medical therapy before or after surgery. Of 396 OSSN lesions, 7 lesions were inexcisable, leaving 389 lesions available for the outcome analysis. Of those, forty-four recurred during follow up. Using Kaplan Meier survival analysis, the 1 year recurrence rate was 10% and the 5 year recurrence rate was 21% (Figure 6). The mean time to recurrence in those with a recurrence was 2.5 years (standard deviation (SD) 3.4). Mean follow up was 23.4 months (SD 28.0, range 0.1–166.1 months).
Table 2.
Treatment information in patients with ocular surface squamous neoplasia treated by excisional biopsy who received medical therapy either before or after surgery.
| Medical therapy after excisional surgery |
Medical therapy before excisional surgery |
|
|---|---|---|
| Medical therapy given, n [%] | 85 [77%] | 25 [23%] |
| Interferon drops | 67 [81%] | 16 [19%] |
| Interferon injections | 7 [50%] | 7 [50%] |
| Mitomycin C | 7 [50%] | 7 [50%] |
| 5-FU | 1 [33%] | 2 [67%] |
n=number of individuals in group; 5-FU= 5-fluorouracil
Figure 6.
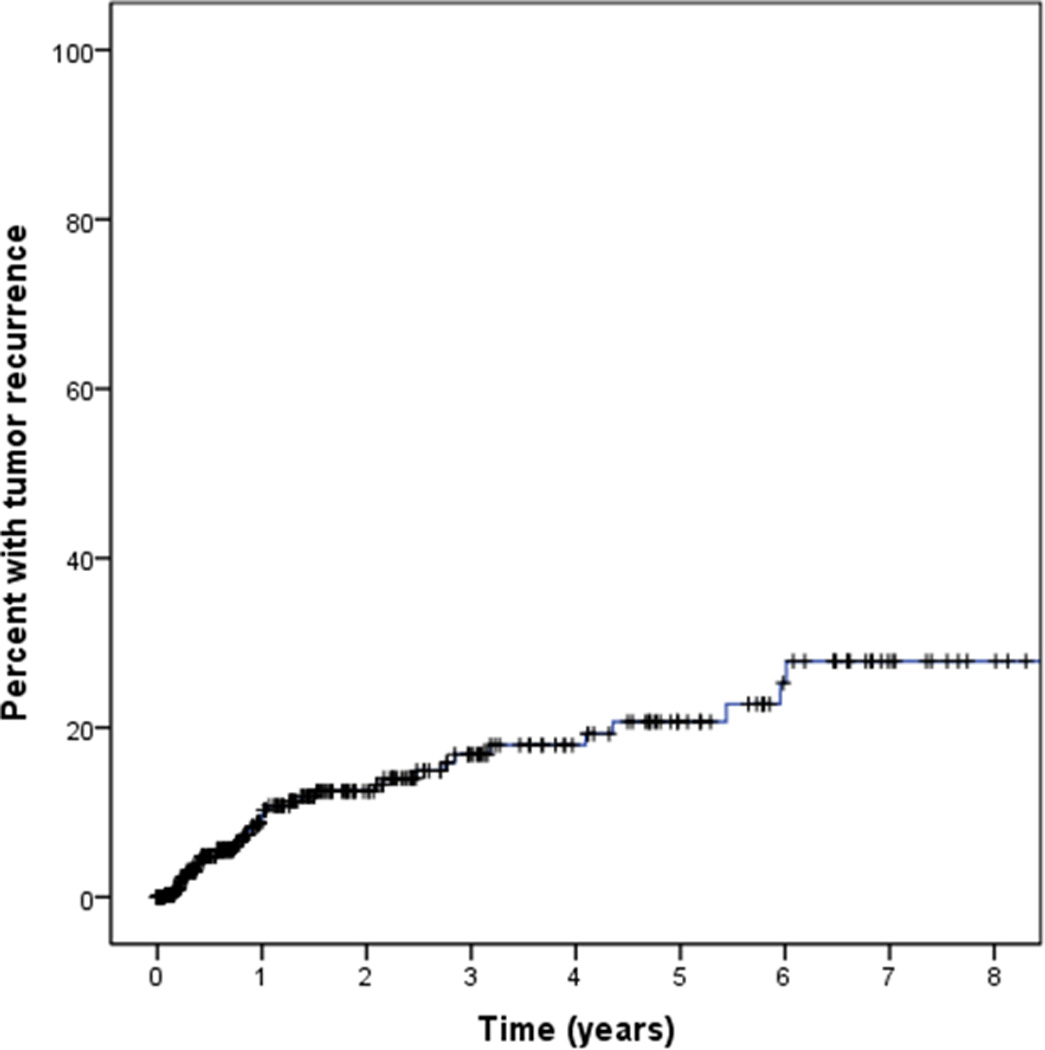
Kaplan Meier survival curve demonstrating the recurrence rate of ocular surface squamous neoplasia (OSSN) over time.
Predictive factors of tumor recurrence on univariable analysis are listed in Table 3. With regards to AJCC classification, 201 lesions were classified as T1 (tumor ≤5 mm in greatest dimension), 140 were classified as T2 (tumor >5 mm in greatest dimension, without invasion of adjacent structures), 36 were classified as T3 (tumor invades adjacent structures, excluding the orbit) in light of tarsal or corneal involvement, and 4 were classified as T4 (tumor invades orbit with or without further extension). No tumors had nodal or metastatic involvement at presentation (i.e. all were N0M0). Larger size (T2) and involvement of adjacent structures (T3) had a higher risk of recurrence compared to smaller size lesions (T1) (p-value =0.04) (Figure 7).
Table 3.
Univariable Cox Proportional Hazards analysis of factors predictive of ocular surface squamous neoplasia recurrence
| Univariable | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Patient factors | |||
| Age | 1.15 | 0.93–1.43 | 0.19 |
| Gender: Male (n=279) /Female (n=110) | 2.13 | 0.95–4.81 | 0.07 |
| Race: Black (n=46) /White (n=292) | 1.16 | 0.48–2.78 | 0.74 |
| Ethnicity: Hispanic (H) (n=173) /NH (n=201) | 0.77 | 0.41–1.42 | 0.40 |
| Recurrent OSSN (n=51) /primary tumor (n=263) | 2.32 | 1.08–4.99 | 0.03 |
| Size of OSSN | 1.00 | 1.00–1.00 | 0.39 |
|
AJCC Classification T2(n=140)/T1 (n=201) T3(n=36) /T1 (n=201) |
2.05 2.31 |
1.04–4.04 0.93–5.77 |
0.04 0.07 |
| Location of OSSN* | |||
| Temporal (n=159) /Not temporal (n=227) | 1.82 | 0.99–3.36 | 0.06 |
| Inferior (n=84) /Not inferior (n=302) | 1.29 | 0.66–2.53 | 0.45 |
| Superior (n=28) /Not superior (n=358) | 3.33 | 1.51–7.37 | 0.003 |
| Nasal (n=210)/Not nasal (n=176) | 0.41 | 0.21–0.79 | 0.008 |
| Tarsal (n=10) /Not tarsal (n=370) | 4.12 | 1.46–11.6 | 0.007 |
| Corneal only†; (n=27)/Not corneal only (n=337) | 1.02 | 0.98–1.02 | 0.98 |
| Multifocal (n=17)/Not multifocal (n=370) | 2.04 | 0.73–5.73 | 0.18 |
| Appearance of OSSN | |||
| Papillomatous (n=124)/Not papillomatous (n=255) | 1.95 | 1.03–3.67 | 0.04 |
| Nodular (n=283)/Not nodular (n=100) | 1.09 | 0.55–2.14 | 0.81 |
| Leukoplakia (n=215)/No leukoplakia (n=162) | 0.88 | 0.47–1.65 | 0.70 |
| Gelatinous (n-240)/Not gelatinous (n=139) | 0.90 | 0.47–1.71 | 0.75 |
| In pterygium (PTG) (n=16) /not in PTG (n=368) | 0.38 | 0.00–46.3 | 0.047 |
| Pathological findings | |||
| Grade: Moderate CIN/mild CIN Severe CIN/mild CIN CIS/mildCIN SCC/mild CIN |
0.58 1.24 1.95 2.93 |
0.10–3.45 0.21–7.44 0.46–8.24 0.62–13.8 |
0.55 0.81 0.36 0.18 |
| Multifocal (n=31)/unifocal (n=356) | 1.21 | 0.43–3.41 | 0.71 |
| Margins: Positive (n=146)/Negative (n=175) | 2.73 | 1.30–5.74 | 0.008 |
| Treatment | |||
| Initial surgery (n=280) /surgery after medical therapy (n=24) | 0.34 | 0.15–0.77 | 0.009 |
| Adjuvant therapy (n=109) /none (n=280) | 1.81 | 0.99–3.30 | 0.05 |
| Cryotherapy (n=271) /none (n=118) | 0.51 | 0.28–0.93 | 0.03 |
Tumors could involve more than one quadrant, e.g. a tumor that involved the temporal and superior bulbar conjunctivae would be represented both in the temporal and the superior location category.
No conjunctival involvement appreciated on clinical examination.
HR = hazard ratio; HR greater than 1 signifies increased risk of tumor recurrence; n=number in each group; CI=confidence interval; CIN=conjunctival intraepithelial neoplasia; CIS= carcinoma
Figure 7.
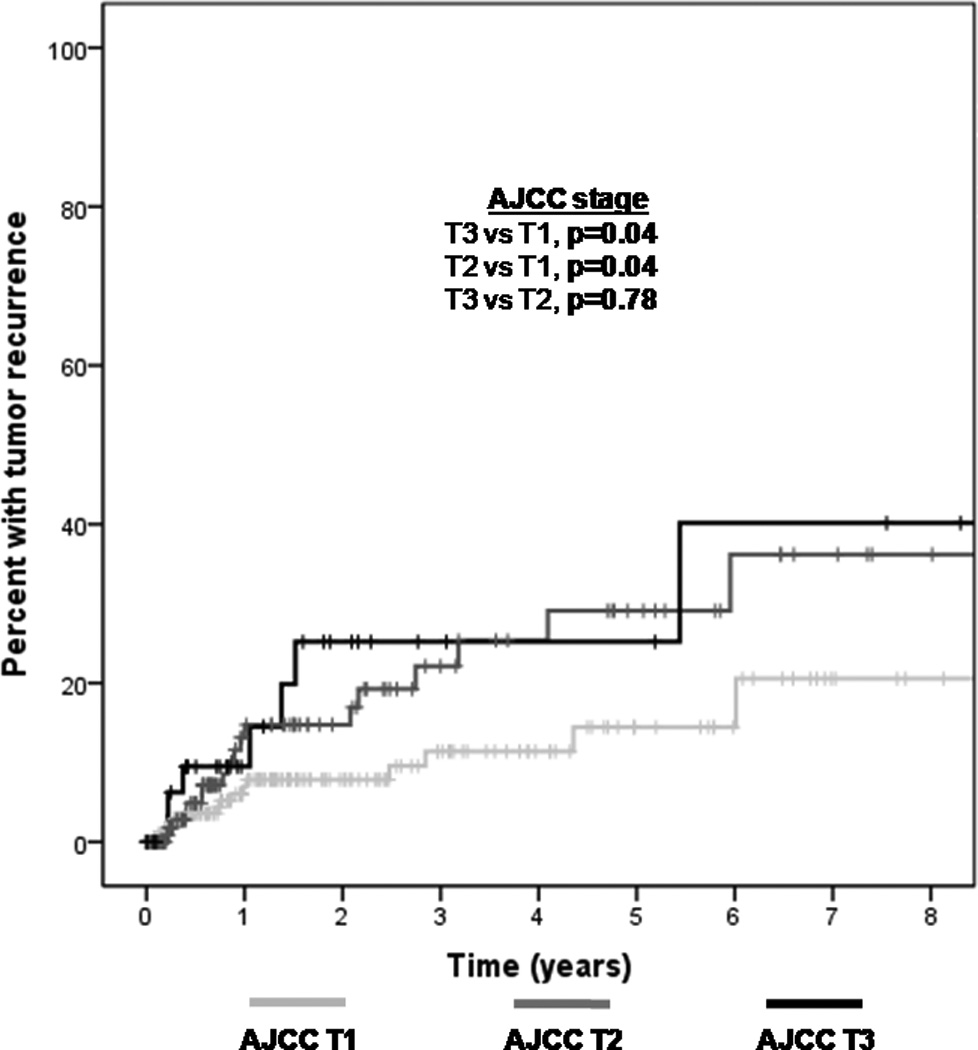
Kaplan Meier survival curve demonstrating an increased recurrence rate of ocular surface squamous neoplasia (OSSN) in patients with higher clinical stage American Joint Committee on Cancer (AJCC) tumors (T3 and T2 compared to T1).
The only patient factor associated with an increased risk of tumor recurrence was a previous history of OSSN (hazard ratio (HR) 2.32, p = 0.03). Location characteristics that increased the risk of tumor recurrence were superior location (HR=3.33, p = 0.003) and tarsal involvement (HR=4.12, p = 0.007). Nasal location was associated with a decreased risk of tumor recurrence (HR=0.41, p=0.008). A tumor characteristic predictive of increased recurrence was papillomatous appearance (HR=1.95, p=0.04). A foci of OSSN within a clinically diagnosed ptergyium was associated with a decreased risk of tumor recurrence (HR=0.38, p=0.047). A pathologic characteristic significantly associated with tumor recurrence was the presence of positive surgical margins (HR=2.73, p= 0.008). No association was noted between tarsal involvement and positive pathologic margins (p-value=0.23), as there was no statistically significant difference in the percent of specimens with positive pathologic margins in eyes with (63% of 11) and without (45% of 307) tarsal disease.
When considering histological grade as a 5 stage categorical variable (SCC, CIS, severe dysplasia, moderate dysplasia, and mild dysplasia), there was a suggestion of increasing risk with increasing grade. When analyzing pathologic grade using two categories: severe (CIS and SCC) versus less severe (mild, moderate, and severe dysplasia), higher pathologic grade was found to be a significant predictor of recurrence with a 2.6 increased risk of tumor reappearance (95% CI 1.18–5.53, p=0.02).
Treatment with adjuvant cryotherapy significantly decreased the risk of tumor recurrence (HR=0.51, p=0.03) (Figure 8). At one year, the recurrence rate was 13% in the no cryotherapy group as opposed to 8% in those who received cryotherapy. At 5 years, the recurrence rate was 31% in the no cryotherapy group as opposed to 16% in those who received cryotherapy. In those patients with positive margins, the use of post-operative topical interferon therapy lowered the recurrence rate to a level similar to that of patients with negative margins (Figure 9). Patients who received post-operative interferon eye drops used the medication for a median of 62.5 days (range 2–385 days).
Figure 8.
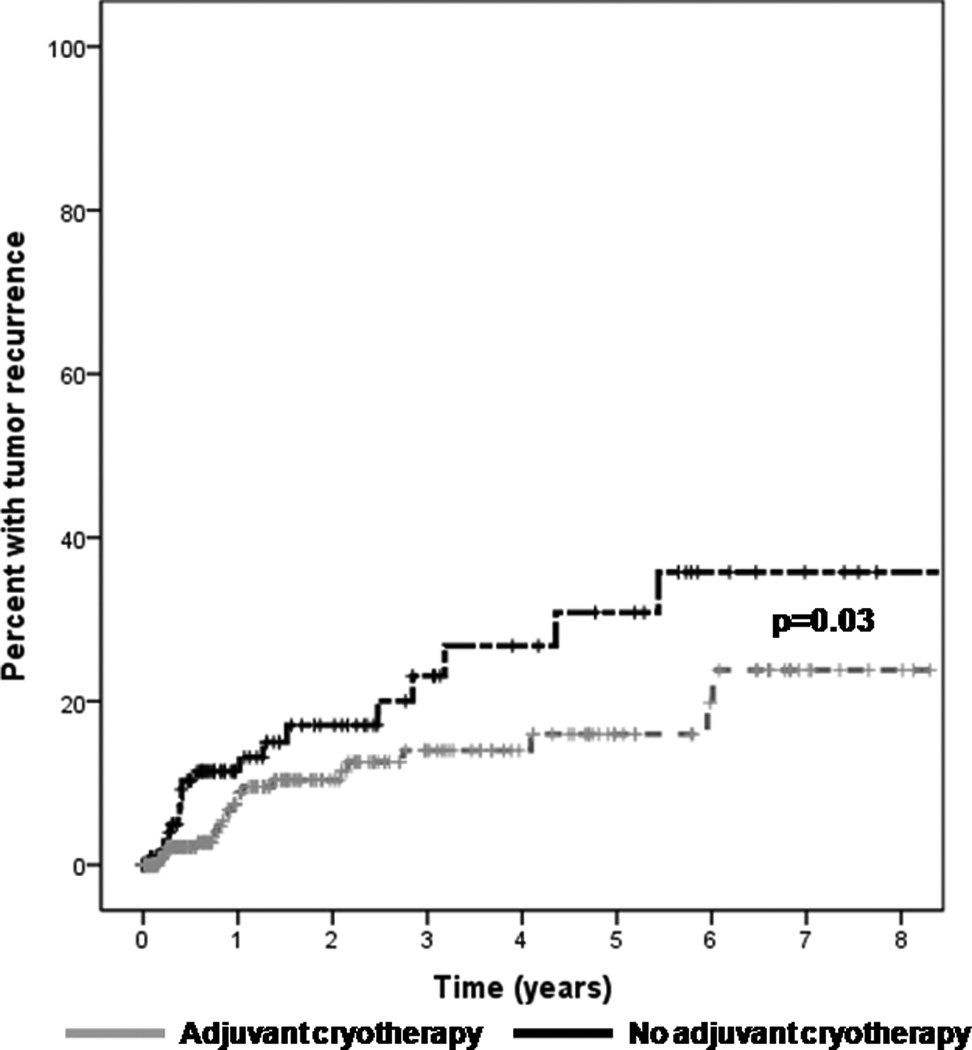
Kaplan Meier survival curve demonstrating a decreased recurrence rate of ocular surface squamous neoplasia (OSSN) in patients treated with adjuvant cryotherapy.
Figure 9.
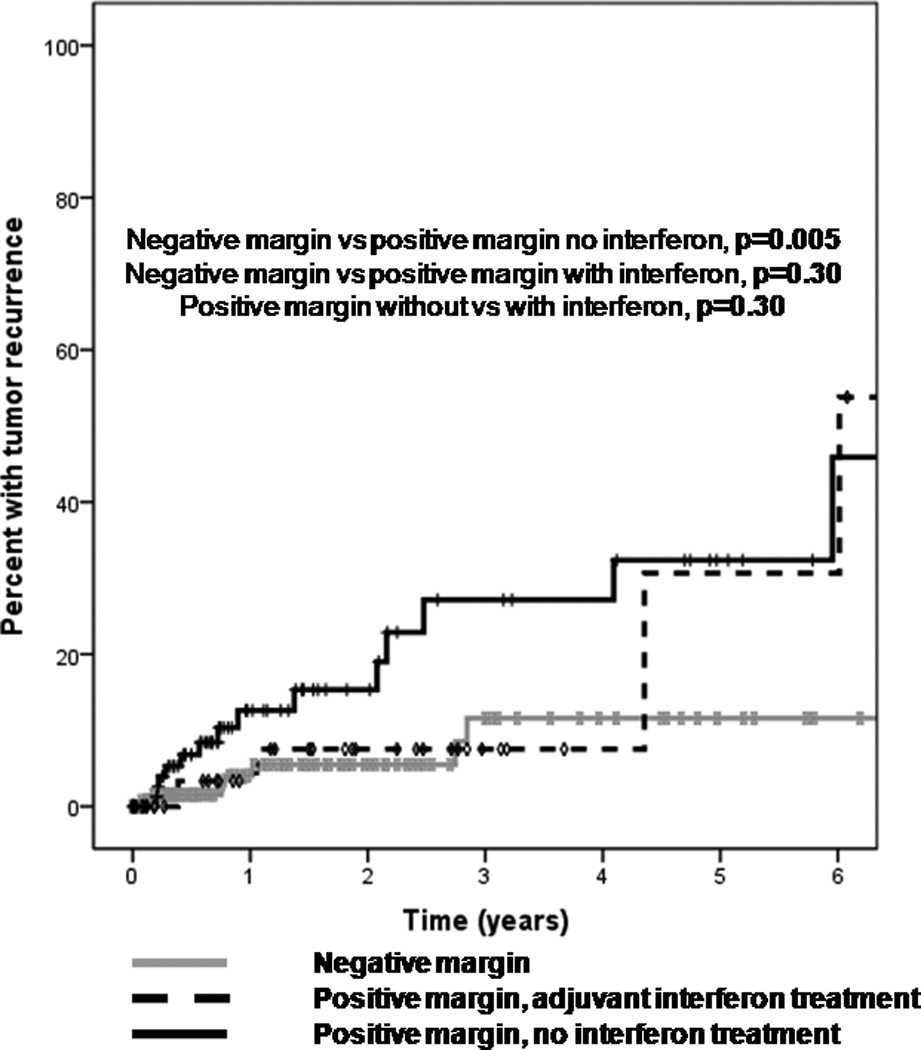
Kaplan Meier survival curve demonstrating a trend towards a decreased recurrence rate of ocular surface squamous neoplasia (OSSN) in patients with positive pathologic margins who were treated with topical interferon therapy after surgery.
In a multivariable Cox proportional hazards analysis evaluating all factors significant in the univariable analysis, tarsal involvement was the strongest predictor of recurrence (HR 5.7 95% CI 1.8–17.8, p=0.003) followed by positive pathologic margins (HR 3.8, 95% CI 1.5–9.9, p=0.006). Nasal location portended a decreased risk of tumor recurrence (HR 0.36, 95% CI 0.14–0.93, p=0.04) as did the use of adjuvant cryotherapy at the time of surgery (HR 0.44, 95% CI 0.20–0.97, =0.04). In a multivariable model including the condensed classification of grade (CIS/SCC versus dysplasia), higher grade remained a significant predictor of recurrence while positive pathologic margins did not remain significant. In our series of patients with excisable tumors, none developed intra-ocular extension, nodal spread, metastasis, or cancer related mortality during the follow up period.
Discussion
Our series is the largest to date to evaluate the patient, tumor, and treatment factors that predict OSSN recurrence after excisional removal. We found that tarsal involvement (i.e. AJCC T3 stage) and positive pathologic margins were the strongest predictors of clinical recurrence. Furthermore, higher grade lesions (CIS and SCC) portended an increased recurrence risk over dysplasia grade lesions. The importance of adequate surgical margins has been demonstrated in previous studies with increased recurrence rates in those with incompletely excised tumors.12, 13 This data also confirms earlier suggestions that cryotherapy reduces the risk of recurrence. Our data compares favorably to that of Yousef and Finger, who similarly demonstrated that higher AJCC stage and higher pathologic grade were associated with an increased risk of tumor recurrence.27
Our current approach to OSSN removal includes taking out the tumor using a “no-touch” technique with 3–4 mm margins, applying absolute alcohol to the limbus for 1 minute, and using a double-freeze thaw cycle of cryotherapy at the limbus and conjunctival margins. Our data suggest that post-operative interferon use may decrease recurrence rates in patients with positive pathologic margins. As an extension of this finding, we advocate considering adjuvant interferon therapy in patients at high risk for recurrence after surgery, namely in those with positive margins, tarsal involvement and recurrent disease. Our preferred approach is to prescribe two months of topical interferon (1 million units/ml) four times daily in such patients. The advantage of topical interferon is that it is a very benign treatment with minimal side effects.28 There are economic implications to this recommendation, however, as the average cost of treatment is approximately $225/month and many insurance companies do not cover this off-label medication. Furthermore, the medication is not commercially available and must be obtained from select compounding pharmacies. As MMC was not routinely used intra- or post-operatively by our physicians, we were not able to evaluate its utility as an adjuvant agent in the management of OSSN. While Birkholz et al found that its use resulted in significantly fewer recurrences20, we favor the use of topical interferon as an adjuvant agent in high risk cases due to its more favorable side effect profile.28–30
Interestingly, other tumor factors, including papillomatous appearance and superior location were univariably associated with increased recurrence risk, although these factors did not remain significant in the multivariable analysis. One may postulate that these two findings are linked to HPV infection, the role of which is still being elucidated in OSSN. Scott and Karp found HPV DNA and mRNA (subtypes 16 and 18) in 10 OSSN specimens (100%) but not in 5 controls or in clinically uninvolved conjunctiva (P < 0.001).31 Other studies, however, did not replicate these findings with some reporting no HPV in affected tissue32–34 and others reporting lower rates of HPV detection.35, 36 As superiorly located lesions have less ultraviolet-B light (UVB) exposure, a known factor in OSSN development6, other factors, like viral presence, may drive the development and aggressiveness of tumors in this location. Similarly, HPV lesions are thought to manifest themselves in papillomatous-like patterns. A limitation of this study, however, is that data on HPV presence was not available for the majority of patients.
Our findings must be interpreted bearing in mind the limitations of this study. This includes its retrospective nature and non-randomized treatment algorithms, including variability in the post operative application of interferon. Furthermore, our main outcome measure of a clinical recurrence likely represents both re-growth of remaining cancer cells and the development of new cancers. Finally, due to its retrospective review, the effect of certain surgical variations, such as “no-touch” technique and the size of surgical margin, could not be assessed as this information was not easily discernable from the medical records. Our study strength, however, lies in the large number of patients with information available for review. In conclusion, we found that several clinical and pathologic factors were associated with OSSN recurrence. Furthermore, our data supports using cryotherapy at the time of surgical removal and employing post-operative topical interferon in patients at higher risk for tumor recurrence. As such, this is the first study to show that topical interferon can be used as a post-operative adjuvant and may decrease the risk of recurrence in those with positive margins to the level of patient with negative margins on pathology. These findings can help clinicians stratify patients with OSSN in terms of recurrence risk, and provides an evidence-based treatment approach to OSSN.
Acknowledgments
Funding: Supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD- Grant#W81XWH-09-1-0675). Florida Lions Eye Bank, Max Kade Foundation, New York, and the Lepke Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Propriety Interests: None
References
- 1.Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22:687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults: a clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Karp CL, Scott IU, Chang TS, Pflugfelder SC. Conjunctival intraepithelial neoplasia: a possible marker for human immunodeficiency virus infection? Arch Ophthalmol. 1996;114:257–261. doi: 10.1001/archopht.1996.01100130253003. [DOI] [PubMed] [Google Scholar]
- 4.Babar TF, Khan MN, Hussain M, et al. Spectrum of ocular surface squamous neoplasia. J Coll Physicians Surg Pak. 2007;17:344–346. [PubMed] [Google Scholar]
- 5.Furahini G, Lewallen S. Epidemiology and management of ocular surface squamous neoplasia in Tanzania. Ophthalmic Epidemiol. 2010;17:171–176. doi: 10.3109/09286581003731544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 7.Nagaiah G, Stotler C, Orem J, et al. Ocular surface squamous neoplasia in patients with HIV infection in sub-Saharan Africa. Curr Opin Oncol. 2010;22:437–442. doi: 10.1097/CCO.0b013e32833cfcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napora C, Cohen EJ, Genvert GI, et al. Factors associated with conjunctival intraepithelial neoplasia: a case control study. Ophthalmic Surg. 1990;21:27–30. [PubMed] [Google Scholar]
- 9.Waddell K, Kwehangana J, Johnston WT, et al. A case-control study of ocular surface squamous neoplasia (OSSN) in Uganda. Int J Cancer. 2010;127:427–432. doi: 10.1002/ijc.25040. [DOI] [PubMed] [Google Scholar]
- 10.Hamam R, Bhat P, Foster CS. Conjunctival/corneal intraepithelial neoplasia. Int Ophthalmol Clin. 2009 Winter;49(1):63–70. doi: 10.1097/IIO.0b013e3181924ec3. [DOI] [PubMed] [Google Scholar]
- 11.Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Aust N Z J Ophthalmol. 1997;25:269–276. doi: 10.1111/j.1442-9071.1997.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 12.Tabin G, Levin S, Snibson G, et al. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–492. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 13.Erie JC, Campbell RJ, Liesegang TJ. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 1986;93:176–183. doi: 10.1016/s0161-6420(86)33764-3. [DOI] [PubMed] [Google Scholar]
- 14.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors: the 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997;115:808–815. doi: 10.1001/archopht.1997.01100150810025. [DOI] [PubMed] [Google Scholar]
- 15.Buus DR, Tse DT, Folberg R, Buuns DR. Microscopically controlled excision of conjunctival squamous cell carcinoma. Am J Ophthalmol. 1994;117:97–102. doi: 10.1016/s0002-9394(14)73021-1. [DOI] [PubMed] [Google Scholar]
- 16.Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol. 1999;83:98–103. doi: 10.1136/bjo.83.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraunfelder FT, Wingfield D. Therapy of intraepithelial epitheliomas and squamous cell carcinoma of the limbus. Trans Am Ophthalmol Soc. 1980;78:290–300. [PMC free article] [PubMed] [Google Scholar]
- 18.Peksayar G, Altan-Yaycioglu R, Onal S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye (Lond) 2003;17:228–232. doi: 10.1038/sj.eye.6700331. [DOI] [PubMed] [Google Scholar]
- 19.Sturges A, Butt AL, Lai JE, Chodosh J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology. 2008;115:1297–1302. doi: 10.1016/j.ophtha.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Birkholz ES, Goins KM, Sutphin JE, et al. Treatment of ocular surface squamous cell intraepithelial neoplasia with and without mitomycin C. Cornea. 2011;30:37–41. doi: 10.1097/ICO.0b013e3181dee560. [DOI] [PubMed] [Google Scholar]
- 21.Siganos CS, Kozobolis VP, Christodoulakis EV. The intraoperative use of mitomycin-C in excision of ocular surface neoplasia with or without limbal autograft transplantation. Cornea. 2002;21:12–16. doi: 10.1097/00003226-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Peksayar G, Soyturk MK, Demiryont M. Long-term results of cryotherapy on malignant epithelial tumors of the conjunctiva. Am J Ophthalmol. 1989;107:337–340. doi: 10.1016/0002-9394(89)90655-7. [DOI] [PubMed] [Google Scholar]
- 23.Santhiago MR, Netto MV, Wilson SE. Mitomycin C: biological effects and use in refractive surgery. Cornea. 2012;31:311–321. doi: 10.1097/ICO.0b013e31821e429d. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Louis D, Dodd T, Muecke J. Mitomycin C as an adjunct in the treatment of localised ocular surface squamous neoplasia. Br J Ophthalmol. 2004;88:17–18. doi: 10.1136/bjo.88.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudesh S, Rapuano CJ, Cohen EJ, et al. Surgical management of ocular surface squamous neoplasms: the experience from a cornea center. Cornea. 2000;19:278–283. doi: 10.1097/00003226-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 26.AJCC Ophthalmic Oncology Task Force. Carcinoma of the conjunctiva. In: Edge SB, Byrd DR, Carducci M, Compton CC, et al., editors. AJC Cancer Staging Manual. 7th ed. New York: Springer; 2009. pp. 531–538. [Google Scholar]
- 27.Yousef YA, Finger PT. Squamous carcinoma and dysplasia of the conjunctiva and cornea an analysis of 101 cases. Ophthalmology. 2012;119:233–240. doi: 10.1016/j.ophtha.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Galor A, Karp CL, Chhabra S, et al. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94:551–554. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 29.Ballalai PL, Erwenne CM, Martins MC, et al. Long-term results of topical mitomycin C 0.02% for primary and recurrent conjunctival-corneal intraepithelial neoplasia. Ophthal Plast Reconstr Surg. 2009;25:296–299. doi: 10.1097/IOP.0b013e3181ac4c39. [DOI] [PubMed] [Google Scholar]
- 30.Frucht-Pery J, Sugar J, Baum J, et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology. 1997;104:2085–2093. doi: 10.1016/s0161-6420(97)30055-4. [DOI] [PubMed] [Google Scholar]
- 31.Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–547. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 32.Eng HL, Lin TM, Chen SY, et al. Failure to detect human papillomavirus DNA in malignant epithelial neoplasms of conjunctiva by polymerase chain reaction. Am J Clin Pathol. 2002;117:429–436. doi: 10.1309/RVUP-QMU3-5X6W-3CQ1. [DOI] [PubMed] [Google Scholar]
- 33.Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–671. doi: 10.1080/02713680903007162. [DOI] [PubMed] [Google Scholar]
- 34.Tulvatana W, Bhattarakosol P, Sansopha L, et al. Risk factors for conjunctival squamous cell neoplasia: a matched case-control study. Br J Ophthalmol. 2003;87:396–398. doi: 10.1136/bjo.87.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura Y, Mashima Y, Kameyama K, et al. Detection of human papillomavirus infection in squamous tumours of the conjunctiva and lacrimal sac by immunohistochemistry, in situ hybridisation, and polymerase chain reaction. Br J Ophthalmol. 1997;81:308–313. doi: 10.1136/bjo.81.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu JJ, Fu P, Pink JJ, et al. HPV infection and EGFR activation/alteration in HIV-infected East African patients with conjunctival carcinoma. [Accessed April 3, 2012];PLoS One [serial online] 2010 5:e10477. doi: 10.1371/journal.pone.0010477. Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.po.ne0010477. [DOI] [PMC free article] [PubMed] [Google Scholar]