Abstract
Background
It is important to monitor alcohol use in the care of liver disease patients, but patient self-report can be unreliable. We therefore evaluated the performance of urine ethyl glucuronide (EtG) and ethyl sulfate (EtS) in detecting alcohol use in the days preceding a clinical encounter.
Methods
Subjects (n=120) were recruited at a university-based Hepatology clinic or during hospitalization. Alcohol consumption was ascertained by validated self-report measures. Urine EtG (cutoff 100 ng/mL) and EtS (cutoff 25 ng/mL) concentrations were assayed by a contracted laboratory using tandem mass spectrometry. The sensitivity and specificity of each biomarker in the detection of drinking during the 3 and 7 days preceding the clinic visit were determined, as well as the influence of liver disease severity on these results.
Results
Urine EtG (sensitivity 76%, specificity 93%) and urine EtS (sensitivity 82%, specificity 86%) performed well in identifying recent drinking, and liver disease severity does not affect biomarker performance. After elimination of one false negative self-report, urine EtG > 100 ng/mL was 100% specific for drinking within the past week, whereas 9% of the subjects without evidence of alcohol drinking for at least one week had EtS > 25 ng/mL.
Conclusions
Urine EtG and EtS can objectively supplement the detection of recent alcohol use in patients with liver disease. Additional research may determine optimal methods for integrating these tests into clinical care.
Keywords: alcohol drinking, biological markers, liver diseases, ethyl glucuronide, ethyl sulfate
INTRODUCTION
In patients with liver disease the detection and monitoring of alcohol consumption is important, particularly given the high prevalence of alcohol-associated liver disease with or without concurrent viral hepatitis, and the deleterious effect of excessive alcohol use on viral hepatitis (Reuben, 2008). Alcohol use in clinical settings has mainly been assessed by self-report. Screening instruments such as the CAGE (Cut down, Annoyance, Guilt, Eye opener) and AUDIT (Alcohol Use Disorders Identification Test) have been shown to be useful as screening tools, but are vulnerable to underreporting and variable sensitivity across heterogeneous populations (Berner et al., 2007; Fiellin et al., 2000). Direct ethanol detection in breath or body fluids can supplement self-report, but the information it provides, on even heavy drinking, extends back no further than the evening prior to a clinical visit (Swift, 2003). Some laboratory tests such as carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume can identify chronic, heavy alcohol use over the preceding weeks (Salaspuro, 1999). However, limited sensitivity and specificity have precluded their use as stand-alone screening tools for alcohol consumption, and all have diminished specificity in liver disease patients (Hock et al., 2005; Neumann and Spies, 2003).
The need for alcohol consumption biomarkers that supply information on recent drinking, extend the detection window relative to direct ethanol measurement, and also detect moderate drinking in patients who should either drink very little or abstain, has raised interest in urinary ethyl glucuronide (EtG) and ethyl sulfate (EtS) measurement. In humans, 0.5–1.5% of ingested alcohol is eliminated in the urine after undergoing glucuronidation to form EtG (Dahl et al., 2002; Goll et al., 2002). EtG can be detected in the urine for as long as 5 or more days after the consumption of alcohol, but more typically becomes undetectable within 48 to 72 hours (Wurst et al., 2002). EtS is another non-oxidative direct metabolite of ethanol that results from sulfate conjugation. This pathway is responsible for the elimination of less than 0.1% of ethanol consumed and has a similar elimination profile to EtG (Helander and Beck, 2005). Both EtG and EtS have been shown to be sensitive and specific in detecting alcohol consumption in alcohol-dependent patients (Junghanns et al., 2009), although a cutoff concentration above the detection limit is generally favored to minimize false positive results arising from non-beverage ethanol ingestion (e.g., in foods, mouthwashes, and other over-the-counter products). While the use of these ethanol metabolites has promise to improve alcohol assessments in clinical care, little is known about the optimal interpretation of urine EtG and EtS in the setting of liver disease. Erim et al demonstrated that urine EtG could identify otherwise undetected drinking in liver disease patients (Erim et al., 2007), and this was recently confirmed in patients with alcoholic liver disease (Staufer et al., 2011). As a complement to this prior work, this study was designed to estimate the performance of urine EtG as well as EtS in detecting drinking within the time frame these biomarkers may remain positive, utilizing a consistent reference standard that is common in studies involving alcohol epidemiology. As a result, it was anticipated that the results of this study would inform the optimal interpretation of urine EtG and EtS in the clinical care of patients with liver problems (e.g., true sensitivity and insights into recent alcohol use that may be missed by urine EtG and EtS testing).
METHODS
Subjects
This was a cross-sectional study that included the first 120 study subjects participating in an ongoing clinical epidemiologic study of alcohol use at a university-based medical center. Subjects were recruited during their visits to the Hepatology clinics or during hospitalization for liver disease, and all study data for a given subject were collected on the same day. Exclusion criteria were minimal and included encephalopathy sufficient to impair provision of informed consent or accurate recall, and post-liver transplant status. In order to obtain as accurate an estimate of specificity as possible, efforts were also made to exclude subjects who were clinically suspected to be drinking by the hepatologist but who were reporting abstinence. Subjects consented to provide a confidential report on their alcohol use that would be accessible only to research personnel not involved in their healthcare, provided blood/serum, urine, and hair samples, and received $40 to compensate them for their time and effort. The study was approved by the Institutional Review Board of the Medical University of South Carolina.
Determining alcohol consumption
Alcohol use was assessed by trained research staff using alcohol timeline follow back methods (Sobell and Sobell, 1992). The type, brand, and serving size of alcoholic beverages were obtained, and then converted into grams of pure ethanol based on serving size, alcohol content, and the density of ethanol. Alcohol use is reported in “standard drinks”, with one standard drink containing 14 grams of ethanol (e.g., the amount of ethanol in a 12 oz beer containing 5% ethanol, 5 oz of wine containing 12% ethanol, or 1.5 oz of 80-proof liquor). Since EtG and EtS are eliminated from the urine within several days of abstinence, past week drinking was the focus of this report. We also measured several other biomarkers that were useful for estimating the veracity of self-report. These included the ethanol metabolite blood phosphatidylethanol (Aradottir et al., 2006; Stewart et al., 2009) measured by liquid chromatography-tandem mass spectrometry with a limit of quantitation of 8 ng/mL (Gunnarsson, 1998), percent disialotransferrin in the serum measured by high performance liquid chromatography (Helander et al., 2003), where results ≥ 1.7% suggest chronic heavy drinking, and hair ethyl glucuronide (Pragst and Yegles, 2008) measured by liquid chromatography-tandem mass spectrometry with a limit of detection of 2 pg/mg (Morini, 2006). These latter results will be presented in separate reports. Disialotransferrin assays were performed at the Clinical Neurobiology Laboratory at the Medical University of South Carolina, and phosphatidylethanol and hair ethyl glucuronide assays were performed by US Drug Testing Laboratories (Des Plaines, IL).
Laboratory measures of urine biomarkers
Urinary EtG and EtS measurements were completed by US Drug Testing Laboratories using a liquid chromatography-tandem mass spectrometry assay (Dresen et al., 2004). The lower reported limit of EtG was 100 ng/mL, and the lower limit of EtS was 25 ng/mL. These cutoffs were chosen by the contracted laboratory to prevent “false positive” results (positive biomarkers related to incidental, non-beverage alcohol exposure). Continuous EtG and EtS values were reported up to a maximum of 10,000 ng/mL. Urine samples were sent by express courier to the laboratory, usually on the same day the sample was obtained.
Chart review
After confirming the provision of signed informed consent and Health Insurance Portability and Accountability Act authorization, a physician reviewed each subject’s electronic medical record to abstract data on liver disease etiology and severity. This included Hepatology clinical notes, endoscopy results, laboratory values, reports of imaging studies, and liver biopsy results.
Data analysis
The dependent variables of interest were urine EtG and EtS. Since the main clinical use of these markers is to detect any recent drinking, we primarily modeled each dichotomously (i.e., positive or negative, with “positive” indicating levels above the clinical cutoffs of 100 and 25 ng/mL respectively). Based on the typical clearance of EtG and EtS from the urine, the main independent variable was any alcohol consumption during the 3 days preceding the clinic visit (i.e., the day of the clinic visit and the 3 days preceding that visit), and contingency tables were analyzed to estimate the sensitivity, specificity, and predictive values of each biomarker in detecting past-3-day drinking. Because EtG and EtS remain detectable in some individuals beyond 72 hours, we repeated this process for past-7-day drinking as a secondary study outcome. Using non-parametric methods (Wilcoxon test), alcohol consumption was then compared between subjects with discordant results (e.g., true positives vs. false negatives). Logistic regression was used to explore the impact of age, gender, ethnicity, and liver disease severity on the association between biomarker positivity and past-3-day alcohol use. Liver disease severity was estimated by the presence of cirrhosis (limited to subjects with biopsy proven cirrhosis, ascites from chronic liver disease, or known esophageal varices). Among those with cirrhosis, severity was further classified by the Child-Turcotte-Pugh (CTP) score (Child and Turcotte, 1964; Pugh et al., 1973) and MELD score (Kamath et al., 2001) (without the United Network for Organ Sharing [UNOS] modification).
RESULTS
Study subjects (n=120) were 66 men and 54 women, 90 of whom were non-Hispanic whites, 29 non-Hispanic blacks, and one Hispanic white. The mean age was 52 years (SD=11). Cirrhosis was present in 65 subjects (55%), with a median CTP score of 7 (interquartile range 6 to 8), and a median MELD score of 10 (interquartile range 7 to 14). The most common clinical diagnoses were chronic Hepatitis C without alcoholism (n=41), alcoholic liver disease without Hepatitis C (n=25), non-alcoholic fatty liver disease (n=16), and chronic Hepatitis C plus alcoholic liver disease (n=13). As shown in Table 1, those reporting any drinking in the past week were more likely to be male, less likely to be cirrhotic, and more likely to have elevated alcohol consumption biomarkers. Among those with positive urine EtG (including 31 reporting past week drinking and 1 reporting past week abstinence) and positive EtS (including 32 reporting past week drinking and 8 reporting past week abstinence), median EtG was 1,918 ng/mL (interquartile range 556 to >10,000), and median EtS was 459 ng/mL, interquartile range 90 to 2,981). Of the 44 reporting past-week drinking, thirty-four subjects reported some amount of alcohol consumption in the past 3 days (median number of drinks 9.5, interquartile range 3.2 to 18.1, range 0.4 to 80.0).
Table 1.
Sample Characteristics
| Reported Alcohol Use in Past Week (n=44) | No Reported Alcohol Use in Past Week (n=76) | |
|---|---|---|
| Mean age (standard deviation) | 50 (12) | 53 (11) |
| % male | 63% | 50% |
| % non-Hispanic white | 75% | 75% |
| % cirrhotic | 25% | 72% |
| % positive urine EtG | 71% | 1% |
| % positive urine EtS | 73% | 11% |
| Median PEth (interquartile range) | 114 ng/mL (29 to 339) | < 20 ng/mL (<20 to < 20) |
| Median %disialotransferrin (interquartile range) | 1.5% (1.3 to 1.8) | 1.4% (1.2 to 1.6) |
| Median hair EtG (interquartile range) | 26 pg/mg (<2 to 65) | <2 pg/mg (<2 to <2) |
| Median number of drinks in past week (interquartile range) | 16 (6 to 33) | — |
EtG and EtS agreement and overall performance in detecting alcohol use
Among subjects reporting some past 3-day-drinking (n=34) the concentrations of urine EtG and EtS were highly correlated (r=0.94, P<0.001), and EtG and EtS were modestly correlated with total number of drinks in the past-3-days (r=0.60 and 0.61 respectively, both P<0.001). Adjustment for urine creatinine (allowing for differences in urine dilution among subjects) did not alter this correlation. EtG and EtS results (i.e., positive or negative) were in agreement in 110 subjects (92%).
EtG and EtS results for subjects who reported drinking within the previous 3 days (n=34) or 4 to 7 days (n=10) prior to urine collection are summarized in Figures 1 and 2 [Urine biomarker concentrations in past week drinkers; Ends of boxes indicate 25th and 75th percentiles; horizontal line indicates median; square represents mean; vertical lines extend to 95th percentile]. Although this demonstrates prolonged detection in some subjects, the high levels were largely confined to the more recent drinkers, consistent with previously reported reductions in sensitivity beginning after one day of abstinence (Wurst et al., 2002). The sensitivity, specificity, and positive and negative predictive values for EtG and EtS in detection of past-3-day and past-7-day drinking are listed in Table 1. Although there were only an additional 10 subjects whose last reported drink was 4 to 7 days prior to urine sampling (5 with EtG>100ng/mL and 4 with EtS>25ng/mL), there was a trend toward diminished sensitivity (EtG 70 vs. 76% and EtS 73 vs. 82%) and increased specificity (EtG 99 vs. 93% and EtS 89 vs. 82%) when comparing the past-3-day and past-7-day time periods. At the recommended cutoff concentrations there was a non-significant trend towards greater sensitivity for EtS compared to EtG, but EtG was more specific than EtS for self-reported alcohol consumption in this study sample (93 vs. 86% for past-3-day drinking and 99 vs. 89% for past-7-day drinking) and thus had greater positive predictive values (81 vs. 70% and 97 vs. 80% respectively).
Figure 1.
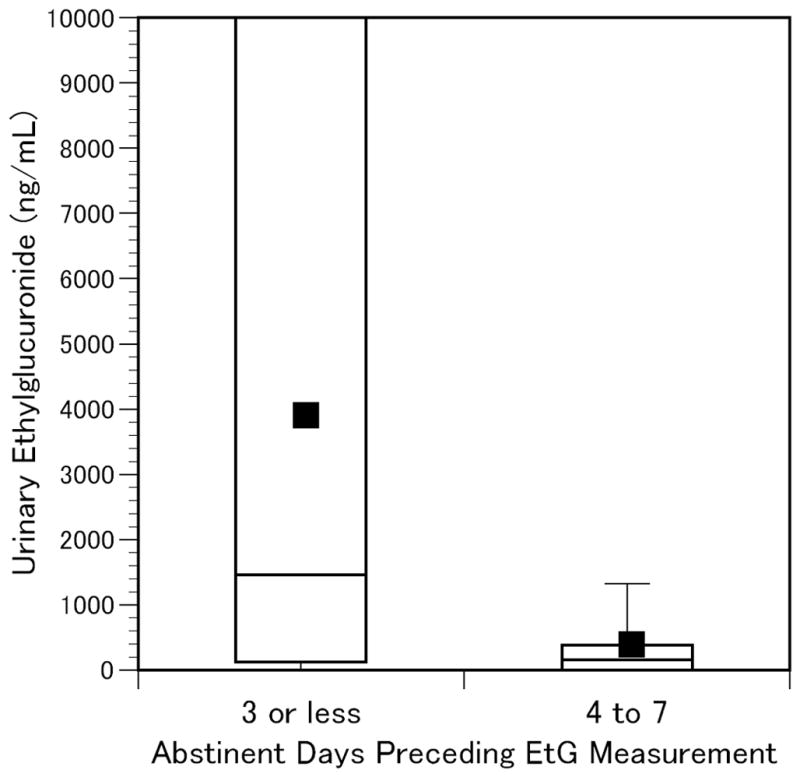
Urine Ethylglucuronide in Self-Reported Past-Week Drinkers
Figure 2.
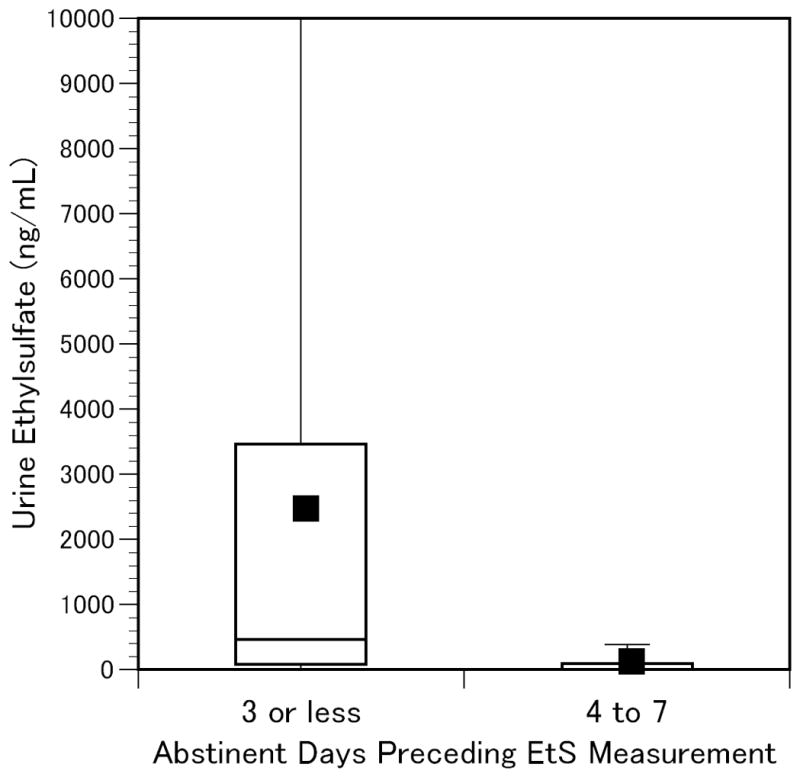
Urine Ethylsulfate in Self-Reported Past-Week Drinkers
Drinking in discordant groups
Subjects with positive EtG who reported past-3-day drinking (n=26) had greater alcohol consumption (a median of 12.1 drinks in the past 3 days, range 1.0 to 80.0) than the subjects (n=8) who reported past-3-day drinking but had a negative EtG (a median of 2.4 drinks in the past 3 days, range 0.4 to 15.1, p=0.008). Subjects with positive EtG but no past-3-day drinking (n=6) reported greater drinking in the past week (a median of 6.0 drinks in the past week, range 0 to 52.3) than the subjects (n=79) who had a negative EtG and did not drink in the past 3 days (a median of 0 drinks in the past week, range 0 to 58.6, p<0.001). Of note, the only EtG positive subject (EtG=184 ng/mL) reporting past week abstinence also reported abstinence for at least 90 days, but was positive for four other alcohol consumption biomarkers (urine EtS was 119 ng/mL, serum % disialo-carbohydrate-deficient transferrin was 2.3%, blood phosphatidylethanol was 435 ng/mL, and hair ethyl glucuronide was 429 pg/mg). Thus this subject was probably drinking in the week preceding urine collection. If so, then the specificity of urine EtG > 100 ng/mL for drinking during the past week was 100%.
Subjects reporting past-3-day drinking with positive EtS results (n=28) reported greater alcohol use (a median of 12.1 drinks in the past 3 days, range 1.0 to 80.0) than the 6 subjects with past-3-day drinking but negative EtS (a median of 1.7 drinks in the past 3 days, range 0.4 to 5.1, p=0.003). Subjects with positive EtS who did not report past-3-day drinking (n=12) had a median of 0 drinks in the past 7 days (interquartile range 0 to 3.7, range 0 to 52.3). The 73 subjects without past-3-day drinking and negative EtS also reported a median of 0 drinks in the past 7-days (interquartile range 0 to 0, range 0 to 58.6). There was however a higher proportion of past-week drinkers in those with positive EtS (4/12) relative to those with negative EtS (6/73).
Of importance, 8 EtS positive subjects (median 67 ng/mL, range 34 to 1099) reported past 7 day abstinence (4 men and 4 women). Five of these subjects had low level urine EtS (range 34 to 72 ng/mL), and it is possible that this represented non-beverage ethanol exposure, a problem that has been well described with low-level urine EtG. Each of these 5 reported greater than 90-day abstinence. We were missing hair and blood from one, and the remaining 4 had negative assays for hair EtG and blood phosphatidylethanol. One had a low positive % disialo-carbohydrate-deficient transferrin of 1.7, which was likely a rare false positive given the absence of detectable phosphatidylethanol. The three remaining subjects had EtS concentrations well above the cutoff of 25 ng/mL (EtS exceeded 100 ng/mL in these cases). One of these (EtS = 119 ng/mL) was the previously mentioned subject with positive urine EtG, % disialo-carbohydrate-deficient transferrin, phosphatidylethanol, and hair EtG, who reported greater than 90 days of abstinence. Assuming that this subject consumed alcohol within a week of study participation, the specificity of urine EtS > 25 ng/mL for past-7-day drinking was 91%. The second allegedly abstinent subject with urinary EtS well above the cutoff (EtS = 556 ng/mL) reported 6 drinks 9 days prior to urine sampling, a total of 20 drinks in the past month, and a total of 52 drinks in the past 3 months. This subject had detectable phosphatidylethanol of 46 ng/mL, and hair EtG of 76 pg/mg, which are more consistent with higher amounts of drinking in the past weeks and months. The third subject (EtS = 1099 ng/mL) reported greater than 90 days of abstinence, there was no known history of heavy drinking or clinical suspicion of drinking, and all other alcohol consumption biomarkers were negative.
Effects of age, gender, ethnicity, and liver disease severity
In logistic regression modeling, age, gender, ethnicity, and liver disease severity (represented by the presence of cirrhosis and CTP score and MELD score for subjects with cirrhosis) did not modify the association of any past-3-day drinking with EtG or EtS positivity (all interaction P-values > 0.250), demonstrating that recent drinking had similar effects on EtG and EtS positivity in all of these groups.
DISCUSSION
The results of this study indicate that, among liver disease patients, urine EtG and EtS are both sensitive (76% and 82% respectively) and specific (93% and 86% respectively) in detecting alcohol consumption within the last three days, regardless of liver disease severity. Urine EtG > 100 ng/mL was particularly specific in detecting past week drinking, but urine EtS > 25 ng/mL was found in several past-week abstainers.
While these biomarkers cannot entirely confirm recent abstinence, our results generally support the use of urine biomarkers as screening and monitoring tools among liver disease patients, as have prior studies. Our estimated specificity for EtG matches that reported by Staufer et al. (2011), but, in our study, sensitivity is somewhat lower. This may be due to a higher proportion of light drinkers that were identified in this current study, as the urine biomarkers are more likely to be positive as recent drinking intensifies. In addition, unlike subjects with positive urine EtG, subjects with negative urine EtG results may have been drinking but were not re-interviewed by Staufer et al, and this may have contributed to an elevated sensitivity estimate (Bossuyt et al., 2003; Sackett and Haynes, 2002). Considering the potential deleterious effect of alcohol ingestion in this patient population, positive tests provide clinically useful information that could be used to engage individuals in conversations regarding alcohol’s impact on disease process, with the goal of improving the clinical outcome, as well as assessing suitability for liver transplantation (Erim et al., 2007; Staufer et al., 2011). Because discussing positive biomarkers with a patient reporting abstinence has the potential to damage rapport, clinical use should be informed by future research that identifies their optimal integration into clinical care, or by referencing existing literature on urine drug testing as a similar treatment paradigm (Moeller et al., 2008).
Positive screens for urine EtG and to a lesser extent EtS provide valuable information, but neither test was perfectly sensitive. We found that in some individuals who reported light or even moderate drinking within three days of sample collection, EtG and EtS were not detected at the recommended cutoff values. Nonetheless the negative predictive values for each test exceeded 90%. Regarding specificity for alcohol consumption, some individuals had EtS levels exceeding 25 ng/mL despite reporting abstinence for greater than one week. In some cases this may be due to non-beverage, minimal ethanol exposure, and specificity for alcohol drinking would be improved with a higher cutoff concentration (e.g., for EtS, 100 ng/mL rather than 25 ng/mL). In general however, determining the optimal use of EtS in detecting alcohol drinking in liver disease patients requires further study. At this time, we see the utility of biomarkers as an additional indicator of alcohol use to be used in conjunction with other clinical information. Conceptually, urine biomarkers could serve a function similar to that of urine drug screens in other settings: while a positive screen is highly suggestive of recent drug use, negative values do support drug abstinence, but may also arise from factors such as dilute urine, intensity of use, ability to abstain from drug use for several days prior to a scheduled vs. random drug screen, or individual differences in drug elimination or the synthesis of drug metabolites.
This study had several limitations. We utilized tandem mass spectrometry for EtG and EtS measurement, and alternative testing methods, such as immunoassays (Böttcher et al., 2008), should be individually validated in the relevant clinical population. The use of alcohol timeline followback methods has been validated for determining alcohol consumption, but remains subject to the limitations of imprecise recall and deliberate misrepresentation. Inaccuracies with this method could have contributed to the estimated imperfect specificity of EtS, although the availability of other alcohol consumption biomarkers measured on our subjects makes this less likely. We did not obtain blood ethanol testing or test for cystitis in this study, and it has been reported that some bacteria can synthesize EtG (but not EtS) in the presence of ethanol (Helander et al., 2007). This should be kept in mind clinically, but false positive EtG results were not a problem in this study. Some bacteria associated with cystitis can also degrade EtG, but not EtS (Helander and Dahl, 2005), and it is possible that this effect could have caused a small underestimation of EtG sensitivity. Similarly, two subjects reporting past-7-day abstinence had EtS concentrations well above the cutoff (i.e., 556 and 1099 ng/mL), but were EtG negative. Asymptomatic bacteruria and undetected alcohol consumption could have played a role in these cases. Finally, one participant in the study continued to screen positive for urine EtG after 6 days of confirmed abstinence (subject was in an intensive care unit for alcoholic hepatitis associated with acute kidney insufficiency prior to study recruitment). One possible explanation for the prolonged presence of urine EtG in this subject was reduced clearance secondary to impaired kidney function, which warrants further study.
In conclusion, our results indicate that urine EtG and EtS are generally reliable indicators of alcohol consumption in liver disease patients. In particular, urine EtG > 100 ng/mL was found to have a specificity approaching 100% for drinking within the past week, but a positive EtS assay in isolation did not entirely confirm recent drinking in this study. Negative EtG and EtS assays are very useful in ruling out moderate to heavy drinking in the prior 3 days, but do not unequivocally confirm recent abstinence. In general, these biomarkers can be used in a qualitative manner, with similar detection windows and interpretation relative to urine drug testing.
Table 2.
Urine Ethyl Glucuronide and Ethyl Sulfate in the Detection of Recent Alcohol Use*
| Ethyl Glucuronide | Ethyl Sulfate | |
|---|---|---|
| Past-3-Day Drinking | ||
| Sensitivity | 76% (62%–91%) | 82% (70%–95%) |
| Specificity | 93% (88%–98%) | 86% (78%–93%) |
| Positive Predictive Value | 81% (68%–95%) | 70% (56%–84%) |
| Negative Predictive Value | 91% (85%–97%) | 93% (87%–98%) |
| Past-7-Day Drinking | ||
| Sensitivity | 70% (57%–84%) | 73% (60%–86%) |
| Specificity | 99% (96%–100%)† | 89% (83%–96%) |
| Positive Predictive Value | 97% (91%–100%)† | 80% (67%–92%) |
| Negative Predictive Value | 85% (78%–93%) | 85% (77%–93%) |
95% confidence intervals shown in parentheses.
See text regarding the likelihood that the specificity and positive predictive value of ethyl glucuronide for past-week drinking were 100%.
Acknowledgments
Grant support: National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (R01AA017911). Dr. Koch is also supported by a Junior Faculty Development Award from the American College of Gastroenterology.
References
- Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- Berner MM, Kriston L, Bentele M, Harter M. The alcohol use disorders identification test for detecting at-risk drinking: a systematic review and meta-analysis. J Stud Alcohol Drugs. 2007;68:461–473. doi: 10.15288/jsad.2007.68.461. [DOI] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol Alcohol. 2008;43:46–48. doi: 10.1093/alcalc/agm153. [DOI] [PubMed] [Google Scholar]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- Dahl H, Stephanson N, Beck O, Helander A. Comparison of urinary excretion characteristics of ethanol and ethyl glucuronide. J Anal Toxicol. 2002;26:201–204. doi: 10.1093/jat/26.4.201. [DOI] [PubMed] [Google Scholar]
- Dresen S, Weinmann W, Wurst FM. Forensic confirmatory analysis of ethyl sulfate-A new marker for alcohol consumption-by liquid-chromatography/electrospray ionization/tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1644–1648. doi: 10.1016/j.jasms.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Erim Y, Bottcher M, Dahmen U, Beck O, Broelsch CE, Helander A. Urinary ethyl glucuronide testing detects alcohol consumption in alcoholic liver disease patients awaiting liver transplantation. Liver Transpl. 2007;13:757–761. doi: 10.1002/lt.21163. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- Goll M, Schmitt G, Ganssmann B, Aderjan RE. Excretion profiles of ethyl glucuronide in human urine after internal dilution. J Anal Toxicol. 2002;26:262–266. doi: 10.1093/jat/26.5.262. [DOI] [PubMed] [Google Scholar]
- Gunnarsson T, Karlsson A, Hansson P, Johnson G, Alling C, Odham G. Determination of phosphatidylethanol in blood from alcoholic males using high-performance liquid chromatography and evaporative light scattering or electrospray mass spectrometric detection. J Chromatogr B, Biomed Sci Appl. 1998;705:243–249. doi: 10.1016/s0378-4347(97)00541-0. [DOI] [PubMed] [Google Scholar]
- Helander A, Beck O. Ethyl sulfate: a metabolite of ethanol in humans and a potential biomarker of acute alcohol intake. J Anal Toxicol. 2005;29:270–274. doi: 10.1093/jat/29.5.270. [DOI] [PubMed] [Google Scholar]
- Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728–1730. doi: 10.1373/clinchem.2005.051565. [DOI] [PubMed] [Google Scholar]
- Helander A, Husa A, Jeppsson JO. Improved HPLC method for carbohydrate- deficient transferrin in serum. Clin Chem. 2003;49:1881–1890. doi: 10.1373/clinchem.2003.023341. [DOI] [PubMed] [Google Scholar]
- Helander A, Olsson I, Dahl H. Postcollection synthesis of ethyl glucuronide by bacteria in urine may cause false identification of alcohol consumption. Clin Chem. 2007;53:1855–1857. doi: 10.1373/clinchem.2007.089482. [DOI] [PubMed] [Google Scholar]
- Hock B, Schwarz M, Domke I, Grunert VP, Wuertemberger M, Schiemann U, Horster S, Limmer C, Stecker G, Soyka M. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100:1477–1486. doi: 10.1111/j.1360-0443.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Graf I, Pfluger J, Wetterling G, Ziems C, Ehrenthal D, Zollner M, Dibbelt L, Backhaus J, Weinmann W, Wurst FM. Urinary ethyl glucuronide (EtG) and ethyl sulphate (EtS) assessment: valuable tools to improve verification of abstention in alcohol-dependent patients during in-patient treatment and at follow-ups. Addiction. 2009;104:921–926. doi: 10.1111/j.1360-0443.2009.02566.x. [DOI] [PubMed] [Google Scholar]
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- Morini L, Politi L, Groppi A, Stramesi C, Polettini A. Determination of ethyl glucuronide in hair samples by liquid chromatography/electrospray tandem mass spectrometry. J Mass Spectrom. 2006;41:34–42. doi: 10.1002/jms.943. [DOI] [PubMed] [Google Scholar]
- Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98 (Suppl 2):81–91. doi: 10.1046/j.1359-6357.2003.00587.x. [DOI] [PubMed] [Google Scholar]
- Pragst F, Yegles M. Determination of fatty acid ethyl esters (FAEE) and ethyl glucuronide (EtG) in hair: a promising way for retrospective detection of alcohol abuse during pregnancy? Ther Drug Monit. 2008;30:255–263. doi: 10.1097/FTD.0b013e318167d602. [DOI] [PubMed] [Google Scholar]
- Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328–338. doi: 10.1097/MOG.0b013e3282fbceca. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ. 2002;324:539–541. doi: 10.1136/bmj.324.7336.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol. 1999;19:261–271. doi: 10.1016/s0741-8329(99)00044-0. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported ethanol comsumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychological and biological methods. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Staufer K, Andresen H, Vettorazzi E, Tobias N, Nashan B, Sterneck M. Urinary ethyl glucuronide as a novel screening tool in patients pre and post liver transplantation improves detection of alcohol consumption. Hepatology. 2011;54:1640–1649. doi: 10.1002/hep.24596. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Reuben A, Brzezinski WA, Koch DG, Basile J, Randall PK, Miller PM. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464–7. doi: 10.1093/alcalc/agp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIFT R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98 (Suppl 2):73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Seidl S, Ladewig D, Muller-Spahn F, Alt A. Ethyl glucuronide: on the time course of excretion in urine during detoxification. Addict Biol. 2002;7:427–434. doi: 10.1080/1355621021000006035. [DOI] [PubMed] [Google Scholar]


