Abstract
Objective
In postmenopausal women, a relationship between luteinizing hormone (LH) and cortisol levels has been suggested. Furthermore, LH receptors on the adrenal gland have been shown to mediate ACTH-independent Cushing's Syndrome. In contrast, follicle stimulating hormone (FSH) receptors have not been found on the adrenal gland. Our objective was to explore the relationship of LH with adrenal function in postmenopausal women, as assessed by 24-hour urinary free cortisol (UFC) and aldosterone excretion rate (AER).
Methods
Participants were studied at single time point in the fasting state in the Clinical Research Center of Brigham and Women's Hospital. We studied 36 postmenopausal women in sodium balance to control for variation in endogenous levels of plasma renin activity and angiotensin II. Serum cortisol, aldosterone, LH and FSH levels were measured, as were 24-hour UFC and AER. Correlations were performed by calculation of Pearson's correlation coefficient.
Results
Serum LH correlated significantly with log-transformed UFC (r = 0.43, p=0.01) and inversely with log AER (r = −0.50, p= 0.002). We found no correlation of serum LH with serum cortisol or aldosterone, nor did we find correlation of FSH with these parameters.
Conclusions
In postmenopausal women, serum LH levels correlate significantly with UFC (positively) and AER (negatively). LH stimulation may induce subtle shifts in adrenal function towards cortisol secretion.
Keywords: Luteinizing hormone, cortisol, aldosterone, menopause, adrenal gland
INTRODUCTION
Menopause heralds change in women's health, with increased incidence of metabolic syndrome1 and cardiovascular disease (CVD)2. Loss of estrogen has been postulated to result in increased CV risk. However the rise of gonadotropins is characteristic of menopause and may play a role in CV risk, independent of loss of estrogen.
Luteinizing hormone (LH) receptors have been identified in the adrenal gland, and case reports have described LH-mediated, adrenocorticotropic hormone (ACTH)-independent Cushing's Syndrome in postmenopausal women.3–5 Serum cortisol increases with age in both men and women, and levels are higher in postmenopausal women than in age-matched men.6 A few case reports3–5 and one prior study7 have also suggested a relationship between LH and serum cortisol in postmenopausal women.
Literature indicates that measurements of 24-hour urinary free cortisol (UFC) and aldosterone excretion rates (AER) are superior to isolated serum measurements of cortisol and aldosterone in providing integrated assessment of cortisol and aldosterone secretion over a 24-hour period.8,9 With this study, we explore the relationship of LH with UFC and AER in postmenopausal women.
METHODS
Study Population
Thirty-eight normotensive and hypertensive postmenopausal women studied by the International Hypertensive Pathotype group were selected for this analysis.10 Women were selected for this analysis if they had confirmed postmenopausal status, defined as amenorrhea for at least 12 consecutive months and FSH more than 30 IU/L. Normotensive women had systolic blood pressure <140 mmHg and diastolic DBP<90 mmHg at both screening and study visits. For hypertensive women, diagnosis was confirmed by review of medical records or by elevated blood pressure at screening visit. No participants were taking prescription medications, including anti-hypertensive medications, or hormone therapy at the time of study. For women who had previously taken hormone therapy, at least 3 months had elapsed between use of hormone therapy and study visit. Study participants did not have any active medical issues, including diabetes mellitus. As a previous study suggested a bimodal distribution of association between LH and cortisol, with lack of association above LH>41,7 we restricted analyses to women who had LH<41. This restriction removed only 2 women from our dataset.
Study Protocol
Participants were admitted to the Clinical Research Center of Brigham and Women's Hospital (BWH). After fasting and remaining supine overnight, blood was drawn between 7 AM and 8AM for measurement of LH, FSH, cortisol, aldosterone, plasma renin activity (PRA), and angiotensin II (Ang II). To decrease variability in endogenous PRA and Ang II, all participants ingested a standardized isocaloric high salt diet containing 250 mEq of sodium for one week prior to study. Sodium balance was confirmed by 24-hour urinary sodium excretion ≥ 200 mmol. Twenty-four hour urine collection was performed for determination of UFC and urinary AER. Urinary creatinine confirmed complete urine collection. All participants provided written, informed consent, and the Institutional Review Board at BWH approved the study protocol.
Laboratory Assays
Blood samples were collected on ice and centrifuged in a refrigerated centrifuge. Serum LH, FSH, cortisol, estradiol and progesterone were measured using paramagnetic-particle chemiluminescence immunoassays (Access Chemiluminscent Immunoassay, Beckman Coulter, Fullerton, CA). Serum aldosterone, UFC and AER were measured using Coat-A-Count Extraction radioimmunoassay (Siemens, Los Angeles, CA). The reference range for UFC is 21 – 85 ug/24 hours. In high sodium balance, the reference range for AER is up to 6 ug/ 24hour and for an ad lib sodium diet is up to 25 ug/24 hours. PRA was measured using radioimmunoassay (Diosorin, Inc., Stillwater, MN), and Ang II levels were measured using double-antibody radioimmunoassay (ALPCO Diagnostics, Salem, NH). Urinary sodium and creatinine were measured using autoanalyzers. The analytical sensitivity for LH was 0.2 mIU/mL, with precision 4.3–6.4%. The analytical sensitivity and precision for FSH were 0.2 mIU/mL and 3.1–4.3%. The analytical sensitivity and precision for cortisol were 0.4 ug/dL and 4.4%. The analytical sensitivity and precision for aldosterone were 2.5 ng/dL and 2.5%. The analytical sensitivity and precision for estradiol were 20 pg/mL and 12%. The analytical sensitivity and precision for progesterone were 0.08 ng/mL and 6.1%. The analytical sensitivity for AER and UFC was 0.1 ng/dL and 0.2 ug/dL. The intra-assay variation for AER and UFC were 2.3–5.4% and 3.0–4.8%, respectively. The analytical sensitivity and precision for PRA were 0.1 ng/mL/hr and 4.6%. The analytical sensitivity and precision for Ang II were 0.6 pg/mL and 7.1%.
Statistical Analysis
The Shapiro-Wilk Test was used to test for normal distribution. Non-normally distributed data were log-transformed for correlation. Comparisons of LH and FSH levels to levels of cortisol, aldosterone, UFC and AER were performed individually by calculation of Pearson's correlation coefficient. Multivariate linear regression models were used to examine the effect of LH and covariates on outcome variable. Best-fit lines were obtained using the least-squares method. For normally distributed data, the independent T test was used for two-group comparisons of demographic characteristics and hormone levels between normotensive and hypertensive subgroups. For non-normally distributed data, the Wilcoxon Rank Sum test was used. Data are expressed as mean ± standard deviation (SD). A p-value of less than 0.05 was significant. Analyses were performed using JMP version 9.0.0 (SAS Institute, Inc. Cary, NC).
RESULTS
Eighteen women were normotensive at time of study and 18 women were hypertensive (Table 1). A majority of women were White and overweight. All women completed menopause at least 12 months prior to study, and serum levels of LH and FSH measured in the menopausal range. Estradiol and progesterone levels also measured in the menopausal range, with all subjects having serum estradiol less than 25 pg/mL and serum progesterone less than 1.4 ng/mL. Urinary sodium excretion confirmed sodium balance (Table 1). In sodium balance (250 mEq sodium/day), serum aldosterone and urinary AER were appropriately low. Serum cortisol and UFC were in the normal range.
TABLE 1.
Characteristics of study population
| Characteristic | Entire Group (n=36) | Normotensive Women (n=18) | Hypertensive Women (n=18) |
|---|---|---|---|
| Age (years) | 61 (6) | 63 (5) | 59 (6)a |
| Race | |||
| Whites (%) | 36 (95) | 19 (100) | 17 (89) |
| Blacks (%) | 2 (5) | 0 (0) | 2 (11) |
| Years from menopause | 12 (8) | 14 (8) | 9 (8) |
| BMI (kg/m2) | 27 (4) | 25.8 (3.8) | 28.0 (3.9) |
| LH (mIU/mL) | 23.5 (8.1) | 23.7 (9.4) | 23.3 (6.8) |
| FSH (mIU/mL) | 70.8 (28.7) | 64.8 (27.0) | 77.5 (29.9) |
| Cortisol (ug/dL) | 11.4 (4.7) | 11.1 (3.0) | 11.7 (6.2)c |
| Aldosterone (ng/dL) | 3.5 (2.6) | 2.6 (0.3) | 4.6 (3.6)a,c |
| UFC (ug/24h) | 54.4 (26.3) | 56.9 (18.2) | 45.9 (20.3)c |
| AER (ug/24h) | 5.8 (3.8) | 4.0 (2.4) | 7.8 (4.4)b,c |
| Urinary Sodium (mmol/24 h) | 235 (52) | 236 (49)c | 234 (56)c |
Data are displayed as mean (SD) or number (%).
p<0.05
p<0.005 vs. normotensive subjects
n=17
There was significant direct correlation of serum LH with log UFC (Figure 1). In addition, there was significant inverse correlation between serum LH and log AER, Figure 1). No correlation was found between LH and log serum cortisol or log serum aldosterone, nor were there correlations of FSH with log UFC, log AER, log cortisol or log aldosterone. Multivariate linear regression models showed that LH independently predicted levels of log UFC (β=0.01, standard error 0.005, 95% confidence intervals [0.002, 0.02], p=−.017), after inclusion of age, years from menopause and body mass index (BMI). In addition, LH independently predicted log AER (β= −0.02, SE 0.007, 95% CI [−0.04, −0.009], p=−.003).
Figure 1.
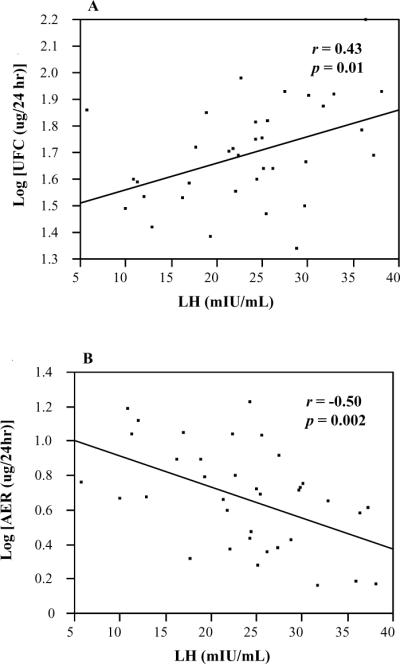
Correlation of LH with UFC and AER
(A) Correlation of LH with UFC
(B) Correlation of LH with AER
r, Pearson's correlation coefficient
LH, leuteinizing hormone; UFC, urinary free cortisol; AER, aldosterone excretion rate.
When comparing the normotensive to the hypertensive subgroup, there were no significant differences in age, racial distribution, years from menopause, LH, FSH, UFC or urinary sodium excretion. Although both subgroups of women were overweight by BMI, women with hypertension did have slightly higher BMI (Table 1). In addition, hypertensive women had significantly higher urinary AER. LH was positively correlated with log UFC in normotensive women (r= 0.64, p= 0.004) and negatively correlated with log AER (r= −0.59, p=0.008). In addition, LH was negatively correlated with log AER in hypertensive women (r= −0.54, p= 0.02) but was not significantly correlated with log UFC (r= 0.17).
There were no significant correlations of age, BMI, years from menopause, estradiol, or progesterone with cortisol, aldosterone, UFC or AER, in the whole study population or in the subgroups.
DISCUSSION
We found that serum LH correlates positively with UFC and a negatively with AER in postmenopausal women, independent of age, BMI, and time from menopause. In contrast, no correlations were observed with FSH and UFC or AER. These data provide evidence that postmenopausal elevations in LH may affect adrenal function and favor cortisol secretion over aldosterone secretion.
Correlation of LH with adrenocortical function in our study population was present for UFC and AER, but not for serum cortisol and aldosterone. Measurements of UFC and AER provide integrated assessment of cortisol and aldosterone secretion over a 24-hour period and are superior to single serum measurements of cortisol and aldosterone in diagnosing cortisol or aldosterone excess.8,9 Our study was able to detect a relationship of LH with cortisol and aldosterone excretion that was not captured by single serum cortisol or aldosterone measurements. It is possible that a parallel relationship would be seen with serum levels with larger sample size.
LH may modulate adrenal function directly by binding to adrenal LH receptors or indirectly, by increasing ACTH levels. Adrenal cortex and gonadal cells have similar origin, originating from urogenital ridge cells.11 Ambiguity of adrenal and gonadal gene expression occurs during the fetal period12 and may return in the presence of high gonadotropin levels.13–15 The fetal adrenal zone is located interior to zones that develop into adrenal cortex, and atrophies after birth.16 A post-mortem study examined adrenal glands from men and women and discovered that the deeper layer of the zona fasciculata and all of the reticularis contained LH receptor protein and transcripts, identified by in situ hybridization and immunocytochemistry.17 LH receptor proteins have also been identified in adrenal glands in cases of ACTH-independent Cushing's Syndrome.4,5,18 Although LH receptors have been identified in aldosterone producing adenomas (APAs),19,20 they have not been found in the zona glomerulosa of patients without adrenal pathology.17 This evidence suggests that LH may act directly by binding to adrenal LH receptors to favor cortisol secretion in patients without overt adrenal pathology.
However, LH may increase ACTH levels and increase cortisol levels indirectly, via ACTH action on adrenal cortex. In a clinical study involving normal women receiving LH injections, parallel increases in ACTH and cortisol were observed.3 Suppression of ACTH by dexamethasone prevented the increase in cortisol observed after LH injection,3 supporting that LH's effects on the adrenal gland were mediated by rise in ACTH. Furthermore, another study showed that with ACTH stimulation, LH releasing hormone (LHRH) does not augment glucocorticoid secretion, suggesting a common mechanism of stimulation.18
Our analyses show that LH is directly correlated with UFC but inversely correlated with AER. The relationship of LH with UFC and AER levels is similar to the observed relationship of ACTH with cortisol and aldosterone secretion in clinical studies.21,22 In these studies, elevated levels of ACTH, achieved by infusion of ACTH, resulted in increased serum cortisol levels but decreased serum aldosterone levels.22 Similarly, in vitro studies have demonstrated that continued daily pulsations of ACTH result in rise of cortisol secretion but decreased aldosterone secretion.23 Based on these findings, it is possible that LH has similar effect to ACTH on adrenal function, or that LH action on the adrenal is mediated via ACTH.
Our study has several strengths. Women were healthy and studied in a research setting without interfering medications, including anti-hypertensive medications. In addition, participants were placed on a standardized salt diet, removing possible confounding due to variable endogenous PRA and Ang II levels. In addition, the urinary assays provided integrated measures of cortisol and aldosterone secretion, and assisted in detecting low levels of aldosterone in high sodium balance. The relationship of LH with AER is further strengthened, as it was observed separately in normotensive and hypertensive subgroups.
There were several limitations to our study. Findings would have been bolstered by measurement of ACTH levels, which were not collected at the time of study. Our sample size was smaller than a previously published study,7 which may have limited our ability to detect correlations of LH with serum cortisol or aldosterone levels, or to determine if the relationship differs between normotensives and hypertensive populations. However, our analyses indicate that the relationship of LH with urinary markers of adrenal function is strong and significant. Further studies need to be done to fully characterize this association and its possible implications for postmenopausal women.
CONCLUSION
In summary, we demonstrate that in a population of postmenopausal women, both normotensive and hypertensive, serum LH correlates positively with UFC and negatively with AER, independent of age, BMI and time from menopause. No relationship was detected with serum FSH. Our findings suggest that elevated levels of LH effect subtle changes in adrenal function in postmenopausal women to favor cortisol secretion over aldosterone secretion. Furthermore, these changes in adrenal function may contribute to the increased incidence of metabolic syndrome and CVD observed in women after menopause.
Acknowledgments
SOURCES OF FUNDING: This work was supported by National Institutes of Health GCRC's Grants M01-RR02635. In addition, EWS received support from K24HL096141 and R01 HL67332, and ARS received support from the National Institutes of Health Training Grant T32HL007609-23 and the Scholars in Clinical Sciences Program, K30RR022292-07.
Footnotes
CONFLICTS OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111(2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone-dependent Cushing's syndrome. N Engl J Med. 1999;341(21):1577–1581. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 4.Feelders RA, Lamberts SW, Hofland LJ, et al. Luteinizing hormone (LH)-responsive Cushing's syndrome: the demonstration of LH receptor messenger ribonucleic acid in hyperplastic adrenal cells, which respond to chorionic gonadotropin and serotonin agonists in vitro. J Clin Endocrinol Metab. 2003;88(1):230–237. doi: 10.1210/jc.2002-020621. [DOI] [PubMed] [Google Scholar]
- 5.Bovenberg SA, Pieters GF, Hofland LJ, Hermus AR. Leuprolide acetate therapy in LH-dependent Cushing's syndrome: in vivo and in vitro observations. Neth J Med. 2004;62(11):456–458. [PubMed] [Google Scholar]
- 6.Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(10):3561–3568. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- 7.Alevizaki M, Saltiki K, Mantzou E, Anastasiou E, Huhtaniemi I. The adrenal gland may be a target of LH action in postmenopausal women. Eur J Endocrinol. 2006;154(6):875–881. doi: 10.1530/eje.1.02165. [DOI] [PubMed] [Google Scholar]
- 8.Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 10.Litchfield WR, Hunt SC, Jeunemaitre X, et al. Increased urinary free cortisol: a potential intermediate phenotype of essential hypertension. Hypertension. 1998;31(2):569–574. doi: 10.1161/01.hyp.31.2.569. [DOI] [PubMed] [Google Scholar]
- 11.Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997;2(2):95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Shaughnessy PJ, Fleming LM, Jackson G, Hochgeschwender U, Reed P, Baker PJ. Adrenocorticotropic hormone directly stimulates testosterone production by the fetal and neonatal mouse testis. Endocrinology. 2003;144(8):3279–3284. doi: 10.1210/en.2003-0277. [DOI] [PubMed] [Google Scholar]
- 13.Fidler WJ. Ovarian thecal metaplasia in adrenal glands. Am J Clin Pathol. 1977;67(4):318–323. doi: 10.1093/ajcp/67.4.318. [DOI] [PubMed] [Google Scholar]
- 14.Romberger CF, Wong TW. Thecal metaplasia in the adrenal gland of a man with acquired bilateral testicular atrophy. Arch Pathol Lab Med. 1989;113(9):1071–1075. [PubMed] [Google Scholar]
- 15.Bachelot A, Meduri G, Massin N, Misrahi M, Kuttenn F, Touraine P. Ovarian steroidogenesis and serum androgen levels in patients with premature ovarian failure. J Clin Endocrinol Metab. 2005;90(4):2391–2396. doi: 10.1210/jc.2004-1734. [DOI] [PubMed] [Google Scholar]
- 16.Rainey WE, Rehman KS, Carr BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin North Am. 2004;31(4):817–35. doi: 10.1016/j.ogc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Pabon JE, Li X, Lei ZM, Sanfilippo JS, Yussman MA, Rao CV. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996;81(6):2397–2400. doi: 10.1210/jcem.81.6.8964884. [DOI] [PubMed] [Google Scholar]
- 18.Andreis PG, Neri G, Nussdorfer GG. Effect of luteinizing hormone-releasing hormone on rat adrenocortical cells. J Steroid Biochem Mol Biol. 1997;63(1–3):17–19. doi: 10.1016/s0960-0760(97)00066-6. [DOI] [PubMed] [Google Scholar]
- 19.Saner-Amigh K, Mayhew BA, Mantero F, et al. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91(3):1136–1142. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- 20.Albiger NM, Sartorato P, Mariniello B, et al. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol. 2011;164(3):405–412. doi: 10.1530/EJE-10-0879. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard RC, Riondel AM, Favrod-Coune CA, Vallotton MB, Muller AF. Aldosterone escape to chronic ACTH administration in man. Acta Endocrinol (Copenh) 1983;103(1):116–124. doi: 10.1530/acta.0.1030116. [DOI] [PubMed] [Google Scholar]
- 22.Seely EW, Conlin PR, Brent GA, Dluhy RG. Adrenocorticotropin stimulation of aldosterone: prolonged continuous versus pulsatile infusion. J Clin Endocrinol Metab. 1989;69(5):1028–1032. doi: 10.1210/jcem-69-5-1028. [DOI] [PubMed] [Google Scholar]
- 23.Braley LM, Adler GK, Mortensen RM, et al. Dose effect of adrenocorticotropin on aldosterone and cortisol biosynthesis in cultured bovine adrenal glomerulosa cells: in vitro correlate of hyperreninemic hypoaldosteronism. Endocrinology. 1992;131(1):187–194. doi: 10.1210/endo.131.1.1319318. [DOI] [PubMed] [Google Scholar]


