Abstract
Introduction
It has been postulated that up to 11 million “silent” strokes occur annually. While these patients are without classic neurologic deficits, they may exhibit cognitive decline. In this study, we examine the cognitive function of patients with carotid stenosis. Additionally, we evaluate a noninvasive measure of strain in pulsating carotid artery plaques to determine its ability to predict cognitive decline.
Methods
We administered the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) to 44 patients with carotid stenosis. All patients had stenosis meeting NASCET or ACAS criteria for endarterectomy, and were classified as symptomatic or asymptomatic as defined by these publications. Age-adjusted scores for each of the 5 RBANS domains (immediate memory, visuospatial ability, language, attention, and delayed memory) were compared between symptomatic and asymptomatic patients. Mean score for each of the 5 domains was then compared to all other domains, regardless of symptom status. From this cohort, 23 patients underwent assessment of carotid plaque strain by tracking displacements in ultrasound radiofrequency data to estimate axial and principal strains over the cardiac cycle.
Results
Thirty symptomatic and 14 asymptomatic patients were studied. Visuospatial scores were significantly lower than any other domain regardless of symptoms (p<0.05 for all pairwise comparisons). No other domain score was significantly different from any other. In the language domain, asymptomatic patients scored significantly higher than symptomatic patients (p<0.05. For all other domains, no difference was found. Asymptomatic patients showed a relationship between plaque strain and immediate memory (r= −.61, p=ns). Left carotid disease was associated with poorer performance across multiple cognitive domains with increasing accumulated strain. This was not seen in right carotid disease.
Conclusion
Patients with large carotid plaques (>70% stenosis) exhibit significant difficulties in mental status whether classically symptomatic or asymptomatic. While language deficits may be a non-specific marker for stroke symptoms, visuospatial deficits are seen before classic symptoms, suggesting that carotid disease may become symptomatic earlier and more subtly than previously suspected. Abnormal strain distribution with pulsation may be related to cognition.
Keywords: Impaired cognitive function, visuospatial cognitive deficit, carotid atherosclerosis, stroke, ultrasound strain measurement, carotid stenosis
Introduction
Stroke is a major cause of morbidity and mortality in the United States, with over 700,000 strokes occurring annually. As large as this number is, recent studies have suggested that for every recognized clinical stroke, there are 5 silent strokes(1). Likewise, imaging studies have shown that as many as 11 million silent strokes may occur in the US annually(2). What are the implications of this multitude of small cerebral infarctions? Are these strokes truly “silent,” or do they manifest with deficits other than the classic definition of stroke: motor, sensory, language, or visual deficit? The purpose of this work is to explore the relationship between carotid stenosis and cognitive decline, with the hypothesis that atherosclerotic disease is a risk factor for cognitive decline, and that non-invasive studies may be able to stratify that risk for individual patients.
Stroke and Cognitive Decline
The relationship between stroke and cognitive impairment is of vital importance. One fourth of cognitively impaired patients have had a stroke, and more than 60% of stroke patients have some form of cognitive impairment(3). In the classic nun study, 93% of subjects who had a clinical stroke also had evidence of cognitive decline(4–6). Furthermore, decline in cognitive function, particularly executive function has been found to predict 10-year stroke risk(7). These findings suggest that some ongoing pathologic process leads to both cognitive decline and stroke, and that intervention directed at this process may be useful in abating the effects of both.
Recent developments suggest that the distinction between Alzheimer’s dementia and vascular cognitive decline is rapidly becoming blurred(8). Furthermore, the processes at work in the carotid plaque: lipid deposition, inflammation, angiogenesis, and microhemorrhages, are the same processes at work in the brain with degenerative dementia. Our previous work, utilizing GeneChip microarray analysis of plaque samples from classically symptomatic and asymptomatic subjects has shown that the symptomatic plaques have altered regulation of 236 genes coding largely for proteins involved in cell proliferation and growth, but also genes implicated in neurodegenerative disorders such as Huntington disease, ALS, and Alzheimer’s(9–11). It is with these findings in mind that we sought to determine the relationship between carotid stenosis, whether or not it is classically symptomatic, and cognitive decline.
Plaque Strain and Cognitive Decline
A series of studies have suggested that cerebral emboli, as measured by transcranial Doppler, may predict cognitive impairment(12–14). Along with the classic work of C. Miller Fisher showing artery-to-artery embolization as the major mechanism of cerebral ischemia with carotid plaque, these findings stress the importance of determining which plaques are at risk for rupture and embolization.
Previous studies have shown that plaque vulnerability to rupture is determined primarily by the mechanical/elastic properties of the vessel wall and plaque(15–17). The location of carotid atherosclerosis at the bifurcation of the common carotid artery in the neck makes it particularly suitable for noninvasive study using ultrasonography. We therefore postulate that there might be parameters of plaque strain, measurable by ultrasound, that would predict stable versus unstable plaques and hence that plaque strain measurement may correlate with cognitive decline. In the second part of the current work, we investigate the relationship of plaque strain to cognition using a subset of the patients studied for cognitive function.
Methods
Under University of Wisconsin Institutional Review Board approval, we studied a cohort of patients undergoing carotid endarterectomy for clinically significant carotid stenosis. Data were analyzed retrospectively. Patients were eligible for study if they had symptomatic carotid stenosis meeting NASCET criteria or asymptomatic stenosis meeting ACAS criteria and were scheduled to undergo carotid endarterectomy by the principal investigator (RJD) (18, 19). Patients were considered to be symptomatic if they had had any transient or permanent neurological deficit in speech, motor, somatic sensation, or vision. All patients underwent testing with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which consists of 12 subtests and yields 5 index scores: Attention, Language, Visuospatial/Constructional, Immediate Memory, and Delayed Memory(20). Each of the 5 index scores is age-adjusted and normalized(21). Scores for each cognitive domain were then compared to all other domains in pairwise fashion with a significant difference defined as p<0.05 using Student’s t-test. The scores of symptomatic patients were compared to asymptomatic patients within each of the 5 domains tested. Again, statistical comparison was performed with Student’s t-test.
Plaque Strain Data Acquisition
A subset of the patients undergoing RBANS testing also underwent ultrasound carotid plaque analysis. Carotid plaque strain was assessed by tracking displacements in ultrasound radiofrequency data to estimate axial and principal strains over cardiac cycle.
Carotid ultrasound scanning for in-vivo data acquisition on patients scheduled for carotid endarterectomy with standard clinical indications was performed at the University of Wisconsin-Madison Hospital and Clinics. Patients were scanned using a Siemens SONOLINE Antares system (Siemens Healthcare, Malvern, PA, USA) equipped with the Axius direct ultrasound research interface (URI), which provides the raw radiofrequency (RF) data.
A standard clinical carotid examination was performed on the patient, by placing a linear array transducer (VFX 13-5) on the skin at the location of the carotid artery. The scanning location was selected based on the surgical site for the carotid endarterectomy procedure. The linear array transducer was pulsed at 11.43 MHz, and RF data was acquired at a 40 MHz sampling rate, with a single transmit focus and data acquired to a depth of 4 cm. Dynamic focusing was used on receive. The longitudinal RF data obtained at the plaque location was processed offline to track the displacement and obtain the accumulated axial-strain distribution.
Displacement Tracking and Strain Estimation
A 2D multi-level cross-correlation method utilizing a coarse-to-fine tracking scheme was previously developed in our laboratory for displacement tracking and strain estimation for discontinuous tissue(22, 23). The current algorithm also uses the strategy described above, however the image sets created at each level are scale-space representations(24). A three-level pyramid was used for displacement tracking, and recursive Bayesian regularization is used to denoise the tracked displacement at each level(25). Eulerian frame-to-frame strains are estimated at the final level utilizing RF data and are estimated using a least squares strain estimator, after utilizing a 3×3 median filter to remove outliers(26).
RF data for carotid strain imaging was obtained at the highest frame-rate, depending on system settings, approximately 27 frames/second. Previously, displacement and strain estimates were computed between two consecutive frames, however the frame strain can vary significantly over the cardiac cycle with high strain-rates during systole and lower strain-rates during diastole(22). As previously described, the ability of an algorithm to obtain a high strain signal-to-noise ratio is dependent on the magnitude of the deformation of strain(27). If the deformation is too low, electronic and quantization noise reduce image quality. If the deformation is too large, signal decorrelation limits the improvements in the signal-to-noise obtained. A dynamic frame-skip algorithm was therefore used to obtain an optimal inter-frame strain within the carotid plaque(26).
Accumulated axial strain values estimated from the accumulated displacement over the cardiac cycle were then computed within specified regions of interest (ROI) that define plaque location on the ultrasound B-mode image that were segmented by a radiologist. Finally, the relationship between the maximum accumulated axial strain indices estimated from the variation of the accumulated strain curve in a region of interest containing plaque (marked by the radiologist on the B-mode image) and each of the RBANS index scores was assessed using Pearson’s r.
Results
Carotid Stenosis and Cognitive Function
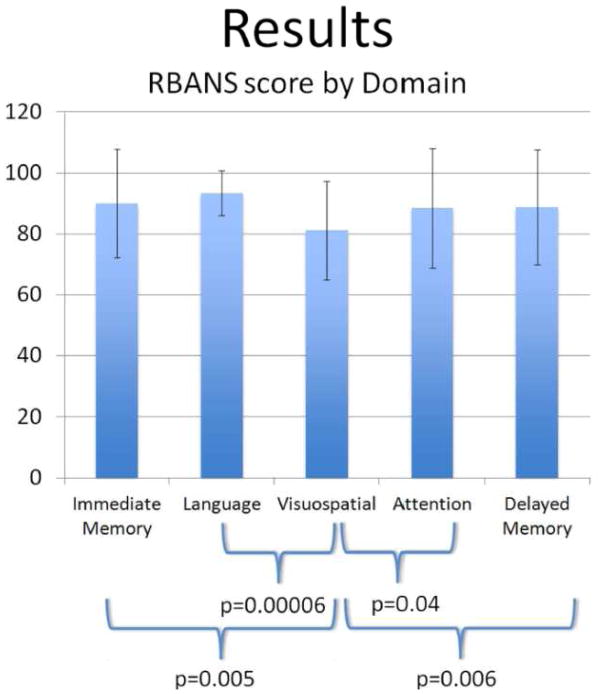
Forty-four patients underwent RBANS testing. Thirty were defined as symptomatic and 14 asymptomatic. Patients had similar baseline demographic characteristics. For all domains tested with the RBANS metric, the average score was 87 with an age-adjusted normal score defined as 100. Comparing each RBANS domain in pairwise fashion to the others, regardless of symptoms, showed significantly lower scores in visuospatial/constructional abilities than in any other domain (Figure 1). These differences were statistically significant for all pairwise comparisons. No other comparison between any two domains showed significant difference.
Figure 1.
Comparison of RBANS scores for each domain.
Visuospatial/constructional score is significantly lower than all others.
Taking each domain individually, symptomatic versus asymptomatic patients showed no difference in visuospatial/constructional ability, attention, immediate or delayed memory. There was a significant difference between symptomatic and asymptomatic patients in the language domain (both picture naming and semantic fluency tasks) (p=0.016).
Plaque Strain and Cognitive Function
Twenty-three patients from the above cohort were additionally tested with ultrasound for plaque strain analysis. Sixteen were symptomatic and seven were asymptomatic. Asymptomatic patients showed a negative relationship between plaque strain and immediate memory (r= −.61, p = ns). Cognitive performance of patients with left carotid disease (n = 14) did not differ from that of patients with right carotid disease (n = 9). However, correlational analyses revealed that poorer performance across multiple cognitive abilities was negatively correlated with increasing accumulated principal strain (r’s ranging from −0.065 [language] to − 0.531 [visuospatial construction]; see Table 1) in patients with left carotid disease. A similar relation was found between cognitive abilities and increasing accumulated axial strain (r’s ranging from .068 [language] to −.394 [visuospatial construction]. In other words, increasing accumulated strain was associated with deficits in cognitive performance. This relationship was not seen in right carotid disease.
Table 1.
Left vs. Right Carotid Disease: Cognitive Performance and Principal/Axial Strain
| Left Carotid Disease | Right Carotid Disease | |||
|---|---|---|---|---|
| Principal Strain | Axial Strain | Principal Strain | Axial Strain | |
| Immediate Memory | r= −.387 p= .171 |
r= −.265 p= .359 |
r= −.165 p= .649 |
r= −.299 p= .401 |
| Delayed Memory | r= −.435 p= .120 |
r= −.174 p= .551 |
r= .225 p= .533 |
r= .162 p= .654 |
| Visuospatial Construction | r= −.531 p= .050 |
r= −.394 p= .164 |
r= .750 p= .012 |
r= .540 p= .107 |
| Language | r= −.065 p= .825 |
r= .068 p= .818 |
r= .058 p= .874 |
r= −.120 p= .741 |
| Attention | r= −.177 p= .545 |
r= −.151 p= .607 |
r= −.047 p= .897 |
r= −.124 p= .733 |
Discussion
We have shown that visuospatial/constructional abilities are significantly impaired in all patients with carotid stenosis regardless of the presence of major symptoms. Underlying this finding is a new understanding of carotid disease, borne out by studies of plaque genetics, cerebral and carotid imaging, and studies of cerebral embolization.
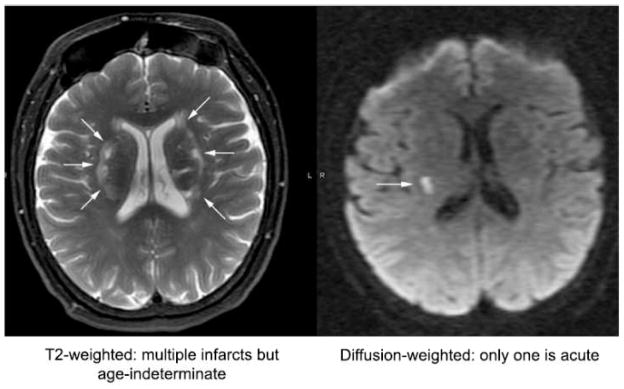
Cerebral imaging investigations based on diffusion weighted and gradient echo MRI techniques have suggested that microinfarcts, both hemorrhagic and non-hemorrhagic, are far more common than clinically recognized. Furthermore, these techniques show that the infarcts are of multiple ages (Figure 2). Studies of patients undergoing cardiac surgery or catheterization have shown remarkably high numbers of cerebral emboli, as measured by transcranial Doppler, and correlated this with cognitive decline following these procedures(28, 29). It follows that the stability of the plaque and its propensity for embolization, more than the degree of luminal narrowing, may be the most important factors in defining the risk to the patient with atherosclerotic carotid stenosis.
Figure 2.
MRI scans showing infarcts of multiple ages.
Our preliminary studies suggest a relationship between strain parameters measurable in carotid plaques and significantly worse cognitive function, which is present in both “symptomatic” and “asymptomatic” patients. Therefore, whether a patient has had a classically defined TIA or stroke with motor, sensory, visual, or language deficit may not be an adequate characterization of this disease process. Cognitive abnormality due to the combined effects of microemboli from structurally unstable carotid plaques may be far more prevalent than classic stroke.
Carotid plaques are not random in their distribution. The plaque is almost always located at the carotid bifurcation in the neck, specifically on the back wall of the internal carotid artery. It is at this location that the jet of flow coming off of the flow divider is most turbulent, leading to the development of plaque. The plaque itself, with varying degrees of calcification, hemorrhage, lipid deposition, hard and soft components must change the physical properties of the vessel. While a normal vessel pulsates radially with its intact elastic and smooth muscle components, the plaque-bearing region cannot. This suggests that in order to pulsate, these plaques may need to crack or flex along stress points that may be measurable. We hypothesize that these may be the source of microemboli and a signature of the instability of the plaque in question.
Using ultrasound elastography measurements we are able to identify areas of greatest strain and areas where strain measurements vary due to different consistencies of adjacent tissues within the plaque. Furthermore, this can be quantified by measuring the strain vector, realizing that the strain displacement may not take place in a radial direction as it would in a normal vessel, but may be longitudinal or tangentially directed. The change in strain vector with pulsation may be an important sign of plaque instability.
In the current study, we have shown preliminary data suggesting that the strain in a pulsating plaque may correlate with cognitive decline. Future studies will examine the relationship between strain measurements and cerebral emboli using transcranial Doppler measurements. By examining strain tensors, we hope to identify areas of high probability of failure of a plaque during pulsation. Overlying strain measurements represented as elliptical glyphs on an anatomic image can give a visual representation of the internal architecture of a plaque (Figure 3). In patients who undergo carotid endarterectomy, this map can be compared to the physical specimen, thus correlating areas of high strain or change in strain to the histologic examination of the specimen. In this way, the ultrasound strain mapping can be validated with potential future use for planning clinical interventions.
Figure 3.
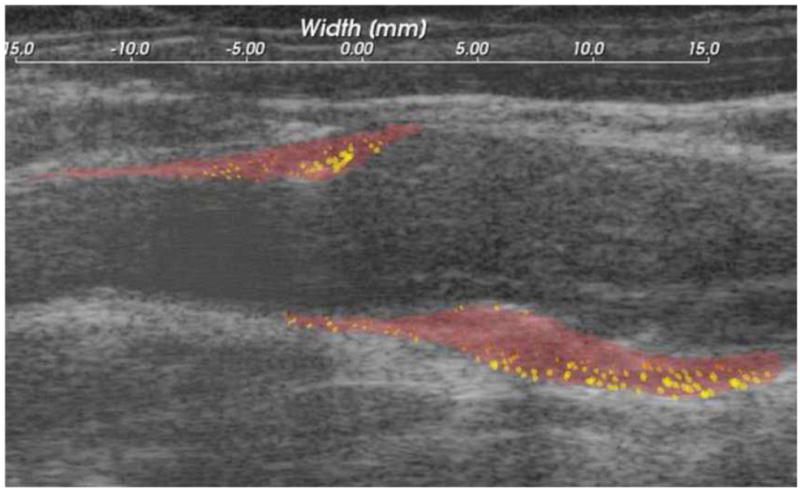
Strain values represented as elliptical glyphs overlaid on anatomic image of carotid plaque.
Limitations and Future Directions
The present study of cognitive function is limited in a few ways. It lacks a control group of age-matched subjects against whom the cognitive function of subjects with carotid disease can be compared. Ultrasound strain measurements were performed on a small sample of patients, thus limiting the power of this study to detect correlation between strain and cognitive function. Further study is ongoing, with a prospective study designed to address the shortcomings of the current work, and to evaluate the role of cerebral emboli, the relationship between ultrasound and histopathologic examination, and the effect of treatment on cognitive function.
Conclusion
The future for these studies is vitally important. Quantitative comparison of various strain parameters with symptomatology needs to be performed. The relationship of cerebral embolization to vessel strain and to cognition as well as the effect of carotid endarterectomy on these emboli -- i.e. whether emboli stop with surgery, whether vessel strain is normalized, and whether cognitive decline is altered – are the issues which will define the utility of these measurements and their effect on patient outcome.
Continued study should involve noninvasive plaque imaging, genetic studies of the factors that make a plaque more susceptible to emboli, and the effects of microemboli on the aging brain. In the future, we may be able to direct medical and surgical treatments for atherosclerotic carotid stenosis at an earlier stage of the disease by noninvasively recognizing at-risk patients with structurally high-risk plaques.
Acknowledgments
This work was supported by the NIH Grant R01NS064034.
Footnotes
Conflict of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003 Mar 27;348(13):1215–22. doi: 10.1056/NEJMoa022066. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 2.Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis. 2003;16(3):280–5. doi: 10.1159/000071128. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 3.Cordoliani-Mackowiak MA, Henon H, Pruvo JP, Pasquier F, Leys D. Poststroke dementia: influence of hippocampal atrophy. Arch Neurol. 2003 Apr;60(4):585–90. doi: 10.1001/archneur.60.4.585. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 4.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006 Feb;37(2):345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 5.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997 Mar 12;277(10):813–7. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- 6.Greiner PA, Snowdon DA, Greiner LH. Self-rated function, self-rated health, and postmortem evidence of brain infarcts: findings from the Nun Study. J Gerontol B Psychol Sci Soc Sci. 1999 Jul;54(4):S219–22. doi: 10.1093/geronb/54b.4.s219. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 7.Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Beiser A, Au R, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004 Feb;35(2):404–9. doi: 10.1161/01.STR.0000103141.82869.77. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 8.Korczyn AD, Vakhapova V. Is vascular cognitive impairment a useful concept? J Neurol Sci. 2010 Dec 15;299(1–2):2–4. doi: 10.1016/j.jns.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey RJ, Vemuganti R, Varghese T, Hermann BP. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery [Research Support, NIH, Extramural Review] 2010 Aug;67(2):484–93. doi: 10.1227/01.NEU.0000371730.11404.36. discussion 93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vemuganti R, Dempsey RJ. Increased expression of genes that control ionic homeostasis, second messenger signaling and metabolism in the carotid plaques from patients with symptomatic stroke. J Neurochem. 2006 Apr;97( Suppl 1):92–6. doi: 10.1111/j.1471-4159.2005.03516.x. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 11.Tureyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery [Comparative Study] 2006 May;58(5):971–7. doi: 10.1227/01.NEU.0000210246.61817.FE. discussion -7. [DOI] [PubMed] [Google Scholar]
- 12.Purandare N, Voshaar RC, Morris J, Byrne JE, Wren J, Heller RF, et al. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007 Aug 15;62(4):339–44. doi: 10.1016/j.biopsych.2006.12.010. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 13.Purandare N, Welsh S, Hutchinson S, Riding G, Burns A, McCollum C. Cerebral emboli and paradoxical embolisation in dementia: a pilot study. Int J Geriatr Psychiatry. 2005 Jan;20(1):12–6. doi: 10.1002/gps.1202. [DOI] [PubMed] [Google Scholar]
- 14.Purandare N, Burns A, Morris J, Perry EP, Wren J, McCollum C. Association of cerebral emboli with accelerated cognitive deterioration in Alzheimer’s disease and vascular dementia. Am J Psychiatry. 2012 Mar 1;169(3):300–8. doi: 10.1176/appi.ajp.2011.11010009. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 15.Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–4. doi: 10.1016/s0140-6736(89)90953-7. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 16.Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation [Review] 1990 Sep;82(3 Suppl):II-47–59. [PubMed] [Google Scholar]
- 17.Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 1999 Jan;19(1):2–13. doi: 10.1161/01.atv.19.1.2. [Review] [DOI] [PubMed] [Google Scholar]
- 18.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991 Aug 15;325(7):445–53. doi: 10.1056/NEJM199108153250701. [Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 19.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Jama. 1995 May 10;273(18):1421–8. [Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- 20.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998 Jun;20(3):310–9. doi: 10.1076/jcen.20.3.310.823. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 21.Duff K, Patton D, Schoenberg MR, Mold J, Scott JG, Adams RL. Age- and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. Clin Neuropsychol. 2003 Aug;17(3):351–66. doi: 10.1076/clin.17.3.351.18082. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 22.Shi H, Varghese T. Two-dimensional multi-level strain estimation for discontinuous tissue. Phys Med Biol. 2007 Jan 21;52(2):389–401. doi: 10.1088/0031-9155/52/2/006. [DOI] [PubMed] [Google Scholar]
- 23.Shi H, Mitchell CC, McCormick M, Kliewer MA, Dempsey RJ, Varghese T. Preliminary in vivo atherosclerotic carotid plaque characterization using the accumulated axial strain and relative lateral shift strain indices. Phys Med Biol. 2008 Nov 21;53(22):6377–94. doi: 10.1088/0031-9155/53/22/008. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindeberg T. Scale-Space Theory in Computer Vision. Springer; 1994. [Google Scholar]
- 25.McCormick M, Rubert N, Varghese T. Bayesian regularization applied to ultrasound strain imaging. IEEE Trans Biomed Eng. 2011 Jun;58(6):1612–20. doi: 10.1109/TBME.2011.2106500. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick M. Carotid plaque characterization with medical ultrasound. Madison, WI: University of Wisconsin; 2011. [Google Scholar]
- 27.Varghese T, Ophir J. A theoretical framework for performance characterization of elastography: the strain filter. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44(1):164–72. doi: 10.1109/58.585212. [DOI] [PubMed] [Google Scholar]
- 28.Lund C, Nes RB, Ugelstad TP, Due-Tonnessen P, Andersen R, Hol PK, et al. Cerebral emboli during left heart catheterization may cause acute brain injury. Eur Heart J. 2005 Jul;26(13):1269–75. doi: 10.1093/eurheartj/ehi148. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 29.Lund C, Sundet K, Tennoe B, Hol PK, Rein KA, Fosse E, et al. Cerebral ischemic injury and cognitive impairment after off-pump and on-pump coronary artery bypass grafting surgery. Ann Thorac Surg. 2005 Dec;80(6):2126–31. doi: 10.1016/j.athoracsur.2005.06.012. [Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]