Abstract
Altered immunity and inflammation are increasingly recognized features of pulmonary arterial hypertension (PAH). This is suggested by infiltration of various inflammatory cells (e.g., macrophages, T and B lymphocytes), increased cytokine and growth factor (e.g., VEGF and PDGF) expression in remodeled pulmonary vessels, and the presence of circulating chemokines and cytokines. In certain diseases associated with PAH, increased expression of growth and transcriptional (e.g., Nuclear Factor of Activated T cells or NFAT) factors, and viral protein components (e.g., HIV-1 Nef), appear to contribute directly to recruitment of inflammatory cells in remodeled vessels, and may potentially serve as specific therapeutic targets. This section provides an overview of inflammatory pathways highlighting their potential role in pulmonary vascular remodeling in PAH and the possibility of future targeted therapy.
A. Introduction
Pulmonary arterial hypertension (PAH) is Group 1 of the pulmonary hypertension (PH)classification and is defined by a mean pulmonary arterial pressure greater than 25 mm Hg with a capillary wedge pressure ≤ 15 mmHg. PAH includes a heterogeneous group of clinical entities sharing similar pathological changes that have been subcategorized as idiopathic PAH, familial PAH, pulmonary hypertension associated with other diseases such as connective tissue diseases (CTD, e.g., systemic sclerosis (SSc), lupus erythematosus, and mixed CTD), portal hypertension and pulmonary hypertension related to HIV infection, drugs and toxins1. PAH is characterized by increased pulmonary vascular resistance due to remodeling and occlusion of the pulmonary arterioles. Left untreated, PAH leads irremediably to right ventricular (RV) hypertrophy and eventually failure from pressure and volume overload load resulting in death within 2–3 years2.
In the past two decades, novel therapies have been developed based on the observation that endothelial cell dysfunction and an imbalance between endothelial-derived vasoactive substances were central to the pathogenesis of severe PAH3, eventually leading to the use of agents targeting specifically three biological pathways, i.e., the endothelin, nitric oxide/cyclic GMP, and prostacyclin pathways4. These therapeutic drugs have had a clear but variable impact depending on the etiology of PAH and thus agents targeting alternate pathways in this disease are needed.
The role of altered immunity and inflammation has been increasingly recognized in various types of PH, such as IPAH and diseases associated with PAH (e.g., CTD and HIV infection) based on the observation of circulating autoantibodies, such as antinuclear antibodies, elevated levels of circulating proinflammatory cytokines interleukin-1 (IL-1) and IL-6 7, as well as association of PAH with inflammatory conditions (e.g., CTD and HIV). Inflammation is also prominent in various experimental animal models of PH which will be reviewed here. Consequently, a better understanding of inflammatory pathways and their role in the pathogenesis of PAH (see Figure 1) may lead to targeted therapeutic approaches similar to those achieved from an improved understanding of endothelial dysfunction.
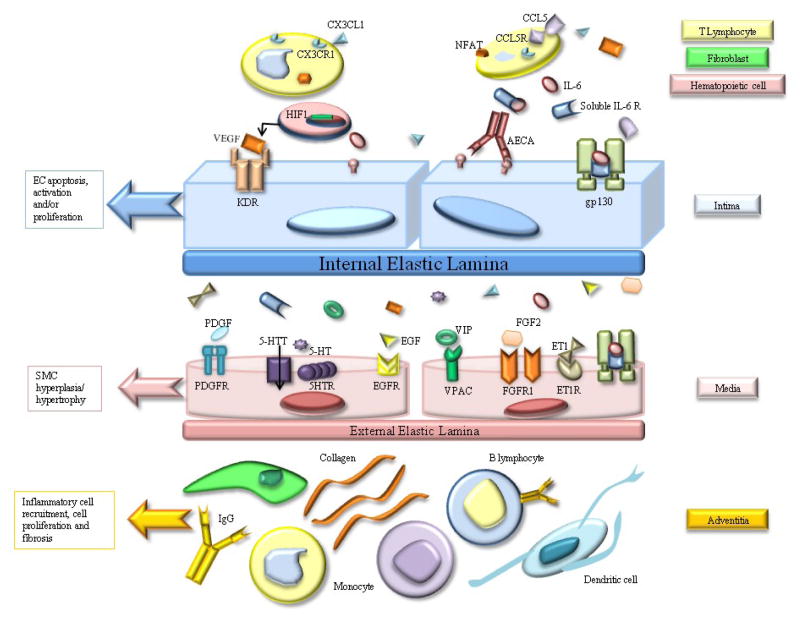
Figure 1.
Main inflammatory mediators in pulmonary vascular remodeling in PAH. CX3CL1 (Fractalkine) causes upregulation of its receptor CX3CR1 on CD4+and CD8+T lymphocytes. CCL5 (RANTES) and Nuclear factor of activated T cells (NFAT) attract T and B lymphocytes. IL-6 (Interleukin-6) binds to its soluble receptor (soluble IL-6 Receptor) and the complex then binds to gp130. Vascular Endothelial Growth Factor (VEGF) acts through its receptor KDR, a receptor tyrosine kinase. Other growth factors involved in vascular remodeling include platelet derived Growth Factor (PDGF), epithelial Growth Factor (EGF), and Fibroblast Growth Factor (FGF-2). The receptor (5-HTR) and transporter (5-HTT) of serotonin (5-HT), and VPAC (the receptors of Vasoactive intestinal peptide (VIP)) are all coupled to G-proteins. Endothelin1 (ET1) is a potent vasoconstrictor and mitogen.
B. The role of inflammation in human PAH
Inflammation appears to be a prominent pathologic feature in PAH as suggested by infiltration of inflammatory cells, including macrophages and T and B lymphocytes, and dendritic cells in pulmonary perivascular spaces and around the plexiform lesions in PAH. In addition, levels of macrophage inflammatory protein-1 alpha, IL-1β and IL-6 and P-selectin11 (expressed on leukocytes and platelets and able to bind to their ligands on endothelial cells (EC)), are increased in severe IPAH.
Recruitment of inflammatory cells such as leukocytes, macrophages, and lymphocytes abound in the complex vascular lesions of IPAH as described by Tuderet al12 and Dorfmuller et al 13 who suggested that the inflammatory response is not confined to plexiform lesions but also observed in other vascular lesions of PAH lungs.
Chemokines in PAH
Interactions between EC and leukocytes are important in the inflammatory process, and involve successive events (e.g., rolling, adhesion, and extravasation of leukocytes) in response to a chemoattractant gradient related to release of chemokines. Thus the presence of such chemokines in PAH further support a role for inflammation in this syndrome.
The chemokine fractalkine or chemokine (C-X3-C motif) ligand 1 (CX3CL1) promotes the receptor, CX3CR1-expressing leukocyte recruitment and mediates the rapid capture, integrin-independent adhesion, and activation of circulating CX3CR1+ leukocytes under high blood flow. CX3CR1 is upregulated in circulating CD4+ and CD8+ T lymphocytes from PAH patients as compared with controls14, which accounts for increased sensitivity of these cells to soluble CX3CL1. This abnormal response of T lymphocytes to CX3CL1 appears specific to PAH since PAH patients have elevated soluble CX3CL1 plasma concentrations as compared with patients with other forms of PH (e.g., chronic thromboembolic PH) and normal controls. Increased CX3CL1 mRNA expression is also found in lung tissue samples from PAH patients as compared with controls, and pulmonary artery (PA) EC obtained from these patients express CX3CL1 protein 14.
Chemokines produced from small PA of PAH patients appear to contribute to inflammatory cell recruitment and PA-SMC proliferation as demonstrated by Perros et al15. Examining the expression of CX3CL1 and CX3CR1 by immunohistochemistry and competitive reverse transcription polymerase chain reaction (RT-PCR) on laser-captured microdissected PA in monocrotaline-induced PH, these authors revealed that CX3CL1 was expressed by inflammatory cells surrounding PA lesions and that PA-SMC had increased CX3CR1 expression. Thus, fractalkine may act as a growth factor for PA-SMC. Further evidence of a chemokine participation in PA remodeling is suggested by significant increases in Chemokine (C-C motif) ligand 2 (CCL2, also known as monocyte chemotactic protein-1 (MCP-1)) protein levels in plasma and lung tissue of IPAH patients compared to controls 16. The cellular sources of CCL2 in IPAH appear to be EC and PA-SMC. Monocyte migration is markedly increased in the presence of pulmonary EC from patients with IPAH, which is significantly prevented by CCL2-blocking antibodies. Finally, CCL2 expression is markedly increased in PA-SMC from IPAH patients, likely explaining stronger migratory and proliferative responses of these cells to CCL2 as compared to cells obtained from controls 16.
RANTES (Regulated upon Activation, Normal T cell expressed and secreted: CCL5), an important chemoattractant for monocytes and T-cells, plays a central role in several vascular inflammatory processes such as glomerulonephritis, Kawasaki disease, and Takayasu’s arteritis. CCL5 may also play an indirect role in PAH through the induction of endothelin-converting enzyme-1 and endothelin-1, a potent endothelium-derived factor with strong vasoconstrictive and mitogenic action. Involvement of this potent mediator in the pathogenesis of PAH is suggested by increased CCL5 mRNA copies detected by RT-PCR in lung samples from PAH patients as compared to controls17, with EC being the major source of CCL5 as demonstrated by in situ hybridization and immunohistochemistry of lung samples 17. The exact relevance of these findings to the pathophysiology of PAH requires further investigation.
Cytokines in PAH
Recent studies suggest that cytokines are key players in the pathogenesis of PAH. IL-6, a pleiotropic cytokine, is significantly elevated in the serum and lungs of patients with PAH 7 and its level is independently associated with mortality in one study 18. Similarly, patients with COPD with PH have elevated circulating IL-6, that correlates with mean pulmonary arterial pressure (mPAP) whereas patients without PH do not. Furthermore, elevations in mPAPare highest in patients with certain polymorphisms of the gene encoding IL-6 (i.e., IL-6 GG genotype as compared to CG or CC)19.
At this time, there is convincing experimental evidence linking IL-6 to excess cellular proliferation and pulmonary vascular remodeling. Injection of recombinant human IL-6 leads to PH and RV hypertrophy in mice 20. Steiner et al reported that lung-specific overexpression of IL-6 in mice mimics the pathobiological lesions observed in advanced PAH (e.g., distal PA muscularization and formation of plexiform-like lesions) and increases RV systolic pressure, changes that were amplified in response to chronic hypoxia exposure 21. The vascular proliferative lesions were composed essentially of EC and T lymphocytes. In addition, IL-6-induced vascular lesions were accompanied by activation of vascular endothelial growth factor (VEGF), proto-oncogene transcription factors c-MYC and MAX, and the anti-apoptotic proteins survivin and Bcl-2 while the pro-apoptotic kinases JNK and p38 were downregulated21.
In contrast, IL-6 deficiency attenuates hypoxia-induced PH (as assessed by increases in RV systolic pressure and pulmonary vascular remodeling) and lung macrophage accumulation in a mouse model of PH although increased expression of adhesion molecules (ICAM-1 and VACM-1) and cytokines (MCP-1) was unchanged 22.
The source of IL-6 in the lung is unclear as several cells can express this cytokine including leukocytes, vascular cells such as EC and SMC, fibroblasts and myocytes. IL-6 induces migration of PA SMC in vitro, and while cultured human PA SMCs constitutively express IL-6, this expression is significantly increased in response to hypoxic exposure 19. Taken together, these findings suggest a preponderant role for this cytokine in SMC growth and vascular remodeling triggered by a hypoxic stimulus.
Regulatory T cells
PAH is associated with a defect in the CD4+T-cell compartment reflected by an absolute deficiency of CD4+cells, a decreased CD4+/CD8+ratio and/or a diminished relative percentage of CD4+CD25+cells, the putative regulatory T-cell (Treg) subset 9. The role of T-cells in the control of self-reactive T-cells and B-cells in the maintenance of self-tolerance is well known23. In fact the depletion of suppressor T-cells, and more specifically the CD4+CD25+Tregs, is a pivotal event in the development of autoimmunity 24 and is enough to break natural self-tolerance leading to chronic autoimmune diseases. Patients with IPAH have diminished circulating CD8+ T cells compared with controls, as well as an elevated proportion of FoxP3+ cells within the CD4+ T cell population, presumably identifying an increase in circulating Tregs with suppressor activity 25. In a recent study, Tamosiuniene et al demonstrated that vascular endothelial growth factor receptor 2 (VEGFR2) blockade induced significant pulmonary endothelial apoptosis in T-cell-deficient rats but not in immune-reconstituted (IR) rats. IR with either CD4+CD25hi or CD4+CD25− T cell subsets prior to vascular injury attenuated the development of PAH26. Taken together, these studies suggest that T regs normally function to limit vascular injury and may play a protective role against the development of PAH.
C. Growth factors in PAH: tyrosine kinase signalling as a potential target in PAH
Several growth factors, including platelet-derived growth factor (PDGF), basic fibroblast growth factor (b-FGF) 29, epidermal growth factor (EGF) 30 and VEGF 31–33, have been implicated in the abnormal proliferation and migration of PA vascular cells. These growth factors form homo- or heterodimers, stimulate cell surface receptors which activate the major signaling transduction pathways like the Ras-mitogen activated protein kinase pathway, and act as potent mitogens and chemoattractants for SMCs, fibroblasts and EC. Responses to these growth factors include inflammation, cellular proliferation, migration and resistance to apoptosis.
Growth factors and inflammation
Cool et al demonstrated exuberant expression of the VEGF receptor KDR, coupled with a reduced expression of p27/kip1 (a cell cycle inhibitory protein) in the EC of plexiformlesions34. However, phenotypically distinct EC lined the plexiform lesions and the sites of neoangiogenesis, suggesting differential angiogenic activity within the plexiform lesion. Other growth factors, such as VEGF and hypoxia inducible factor (HIF-1) subunits α and β are highly expressed in EC of plexiform lesions in severe PAH 35. In addition, decreased expression of c-Src kinase 35, a protein that mediates VEGF-induced production of prostacyclin and nitric oxide in the EC 36, is decreased in PAH. Taken together, these findings suggest a central role in PAH for VEGF, a mediator of angiogenesis but also a factor involved in permeability and inflammatory processes of the vascular endothelium.
Platelet derived growth factor (PDGF)
Active PDGF is composed of polypeptides (A and B chain) that form homo- or heterodimers and stimulate α and β cell surface receptors. Ligand binding induces receptor dimerization and autophosphorylation of tyrosine residues in the PDGF Receptor kinase domain followed by phosphorylation events involving sequential activation of mitogen-activated protein kinase Ras-mitogen activated protein kinase cascades. These events play a role in inflammation, cell proliferation and differentiation.
PDGF, which is synthesized by many different cell types including SMC, EC, and macrophages, induces the proliferation and migration of SMC and fibroblasts, and is a key mediator in the progression of several fibroproliferative disorders such as atherosclerosis, lung fibrosis, and PH 27. The pathogenic role of PDGF in human PAH is also demonstrated by increased expression of PDGF and its receptors by RT-PCR performed on laser capture microdissected PA from explanted lungs of patients with severe IPAH 37. PDGF-A, PDGF-B, PDGF-Rα and PDGF-Rβ mRNA expression is increased in small PA from patients with severe IPAH as compared to controls with more pronounced PDGF-B and PDGF-R β than PDGF-A and PDGF-Rα expression. In small PA, PDGF-B is mainly expressed in EC, SMC, and in some perivascular inflammatory cells, while PDGFR-β is mainly expressed in SMC. Furthermore PDGF-BB-induced proliferation and migration of PA-SMC was found to be inhibited by imatinib treatment 37. Recently, tyrosine kinase inhibitors targeting PDGF have been shown effective in experimental models of PH.
Transforming growth factor-β1 (TGF-β1)
Abnormalities of signaling involving the TFG-β1 superfamily, and specifically one of its members the bone morphogenetic protein (BMP) receptor-2 are closely linked to the pathogenesis of PAH since germline mutations of the BMP receptor-2 gene are found in about 80% of patients with the familial form of PAH and between 10 and 40% of patients with the sporadic form40. There is also evidence for a general imbalance in TGF signaling in PAH; for instance, loss of function mutations of the TGF-β receptor I, Alk1, has been linked with PAH related to the hereditary hemorrhagic telangiectasia 41, and somatic microsatellite instability of the TGF-β receptor II gene is found in the plexiform lesions of IPAH patients 42. Increased TGF-β expression and signaling smad (e.g., smad2) is found predominantly in EC lining remodeled vessels in IAPH patients. Finally, an imbalance in TGF-β signaling has also been demonstrated in some animal models of PH with early enhancement of TGF-β/Alk5 signaling in the rat monocrotaline model of PH. In this model, SD-208, an orally active small-molecule TGF-β receptor I inhibitor, attenuates the development of PH and pulmonary vascular remodeling43. Further work is needed to elucidate the complexities of TGF signaling in PAH and how to target this important pathway.
Fibroblast growth factor (FGF)
FGF-2 (previously known as basic FGF) is ubiquitously expressed and known to be upregulated in response to hypoxia and shear stress in PA-SMC and in lambs with increased pulmonary blood flow and PH 29. However, due to a lack of selective inhibitors of the FGFR system, no functional data are currently available and the role of this growth factor in PAH remains to be further explored.
Epidermal growth factor (EGF)
The EGF receptor (EGFR), has intrinsic tyrosine kinase activity, and is widely expressed on numerous cell types. Upon binding, the intrinsic kinase is activated and EGFR tyrosyl-phosphorylates itself and several intermediary effector molecules. This initiates a multitude of signaling pathways. The integrated biological responses to EGFR signaling include mitogenesis or apoptosis, enhanced cell motility, protein secretion, and differentiation or dedifferentiation. EGF-dependent proliferation and migration of SMC is dependent on the extracellular matrix component Tenascin C 45. In addition, EGF colocalizes with Tenascin C in PAH lesions 46, suggesting a direct role in disease progression. In this context, it is noteworthy that the EGF receptor inhibitor PKI166 reverses established monocrotaline-induced PH in rats 30, which further supports a role for EGF in pulmonary vascular remodeling.
Vasoactive intestinal peptide (VIP) and PAH
VIP is a 28-amino-acid peptide that belongs to the glucagon-secretin family of peptides. The effects of VIP are mediated by G protein-coupled receptors. Two subtypes cloned from human tissues, VPAC1 and VPAC2, are widely distributed in the central nervous system, the heart, blood vessels, and other tissues. VIP has a high affinity for both receptor subtypes. Binding of VIP to its receptors results in activation of adenylyl cyclase and formation of cAMP, which is responsible for vasodilation and many other vascular effects of the peptide. Other actions may be mediated by inositol trisphosphate synthesis and calcium mobilization. Some of the actions of VIP include, but are not limited to, relaxation of vascular SMC 47, inhibition of PA-SMC proliferation in vitro48, reduction of pulmonary vasoconstriction induced by endothelin49, and antithrombotic effects 50.
Genetic deletion of VIP results in overexpression of genes that promote pulmonary vascular SMC proliferation, downregulation of antiproliferative genes, as well as upregulation of pro-inflammatory genes 51 suggesting a preponderant role of VIP in pulmonary vascular remodeling and inflammatory response in the setting of PAH. Said et al demonstrated that VIP knockout mice spontaneously develop PAH (i.e., in the absence of exposure to hypoxia) 52. Histological examination of the lungs of mice lacking the VIP gene demonstrates pulmonary vascular smooth muscle and collagen proliferation, right ventricular (RV) hypertrophy and evidence of lung inflammation, all features relevant to human PAH. Furthermore, both the right ventricular hypertrophy and the vascular remodeling can be significantly decreased after VIP treatment of the mice 52. In piglets with high ouput-induced PAH (achieved by a shunt from the left subclavian artery to the PA trunk), circulating VIP is significantly decreased together with an up-regulation of VPAC1 53, further supporting a role for VIP in the remodeling process.
Preliminary clinical experience suggests that PAH patients treated with the inhaled VIP analog Aviptadil have significant improvements in PA pressure, cardiac output, and peripheral vascular resistance (PVR) 54. This suggests that local VIP administration exerts an immuno-regulatory role in humans, and that VIP inhalation might be an attractive therapeutic option for patients with PAH, although randomized clinical trials are needed to confirm these findings.
Serotonin and the serotonin transporter
Serotonin (5-HT) exerts vasoactive and mitogenic effects on PA-SMCs. In contrast to the constricting action of 5-HT on SMC, which is mainly mediated by 5-HT receptors (5-HT 1B/D, 2A, and 2B) 55, the 5-HT transporter (5-HTT) is required for the mitogenic and co-mitogenic effects of 5-HT. Serotonin is synthesized by EC in the normal lung as a result of tryptophan hydroxylase-1 enzyme activity, and appears to be the main growth factor produced by ECs, acting on PA-SMCs in a paracrine fashion. Such cross-talk between ECs and SMCs mediated by 5-HT appears unique to the pulmonary circulation, since 5-HT is not a potent mitogenic factor for SMCs from systemic vessels 58. Also, the uptake of indoleamine in this process is central. Accordingly, drugs that competitively inhibit 5-HTT also block the mitogenic effects of 5-HT on SMC.
5-HTT is abundantly expressed in the lung, where it is predominantly located in PA-SMCs. 5-HTT is required as a mediator of the mitogenic activity of 5-HT as suggested by studies demonstrating that mice with targeted 5-HTT gene disruption developed less severe hypoxic PH than wild-type controls 61, and that selective 5-HTT inhibitors attenuated hypoxia- and monocrotaline (MCT)-induced PH 62. It is noteworthy that 5-HTT-deficient mice have altered expression of genes responsible for stress response and immune regulation, genes related to muscle structure, vasoreactivity, or actin dynamics, and genes involved in cell cycle and apoptosis, energy metabolism, and matrix formation 63. Conversely, transgenic mice with selective overexpression of 5-HTT in SMC spontaneously develop PH64 which is exacerbated after exposure to hypoxia 65. Even without any change in the bioavailability of 5-HT, PH appears to develop in these mice as a result of the increased expression of the 5-HTT protein in SMC. Taken together, these observations suggest a close correlation between 5-HTT expression and/or activity and the extent of pulmonary vascular remodeling in experimental PH.
There is also abundant evidence that 5-HTT plays an important role in the pathogenesis of human IPAH. 5-HTT expression is increased in circulating platelets and in lungs from patients with IPAH, where it predominates in the media of thickened PA 60. Moreover, PA-SMC obtained from patients with IPAH exhibit faster growth compared to cells obtained from controls when stimulated with serotonin, due to increased 5-HTT expression 60, an effect abrogated in the presence of 5-HTT inhibitors. In conclusion, PA-SMC hyperplasia in IPAH appears to result from the overexpression of tryptophan hydroxylase-1 enzyme and 5-HTT, causing an abnormal 5-HT production by ECs and an enhanced PA-SMC proliferative response 66.
D. Transcriptional factors and their role in PAH
The NFAT in inflammation and vascular remodeling
The nuclear factor of activated T cells (NFAT) increases the transcription of multiple inflammatory mediators, such as interleukins and tumor necrosis factor. NFAT also activates T and B cells 67. An increase in [Ca2+]i, in response to hypoxia or other stimulus, activates calcineurin, which dephosphorylates cytoplasmic NFAT, allowing its entry to the nucleus where it forms complexes with other important transcription factors (e.g., activator protein-1) regulating gene transcription 67.
NFAT activation causes downregulation of Kv1.5 68, a channel with a preponderant role in pulmonary vasoconstriction and development of PAH. ET can activate NFAT, which increases B-cell lymphoma-2 expression, contributing to the antiapoptotic effects of ET in the heart 69. In addition, NFAT regulates the transcription of several genes that affect mitochondrial function (e.g., pyruvate decarboxylase and the electron transport chain enzyme cytochrome C oxidase) 70. Importantly, NFAT is upregulated and activated in circulating inflammatory cells in patients with PAH, including IPAH and scleroderma-associated PAH71. CD3-positive cells with activated NFAT are present in remodeled PAs. However, NFAT is also activated in the PA-SMCs of remodeled arteries.
Directly relevant to the pathogenesis of PAH, PA-SMCs isolated from lungs of PAH patients, but not from normal lungs, have a unique phenotype (downregulated Kv1.5, upregulated B-cell lymphoma-2 bc, hyperpolarized mitochondria), which is associated with activated NFAT and resistance to apoptosis. Experimental inhibition of NFATc2 (which is the predominant NFAT isotype in PAH) by VIVIT (a competitive peptide that inhibits the docking of NFAT to calcineurin) or cyclosporine (an inhibitor of calcineurin), restores Kv1.5 expression and current and decreases [Ca2+]i, [K+]i, bcl-2, and mitochondrial membrane potential, leading to increased apoptosis in vitro 71. In the rat, cyclosporine treatment decreases established MCT-induced PAH 71. A role for NFAT in the pathogenesis of PH is further suggested by the observation that exposure of PA-SMCs to chronic hypoxia leads to NFAT activation, hyperpolarization of mitochondria, and downregulation of Kv1.5, similar to the SMC phenotype observed in PAH. Finally, inhibition with VIVIT or cyclosporine reverses this phenotype, normalizing the mitochondrial membrane potential and level/function of Kv1.5 in these cells.
HIF-1
PASMC mitochondria normally produce reactive O2 species (ROS), which are converted by superoxide dismutase 2 to diffusible H2O2. This serves as a redox-signaling mechanism, regulating pulmonary vascular tone and structure through effects on Kv1.5 and transcription factors. Thus, O2 sensing is mediated by this mitochondria-ROS-HIF-1a-Kv1.5 pathway. In PAH, mitochondrial metabolism and redox signaling are reversibly disordered, creating a pseudohypoxic redox state characterized by normoxic decreases in ROS, a shift from oxidative to glycolytic metabolism and HIF-1a activation, which appears to decrease expression of Kv1.5 74.
Bonnet et al demonstrated that dichloroacetate, the pyruvate dehydrogenase kinase inhibitor, restores oxidative metabolism in fawn-hooded rats (FHR) (which spontaneously develop PAH 75) PASMCs. The metabolic changes induced reverse the hypoxic phenotype of FHR, restoring the relatively depolarized PASMCs and increasing ROS production to normoxic levels. This reverses HIF-1a activation and restores Kv1.5 expression, reducing the severity and mortality of FHR/PAH 74.
The Signal Transducers and Activators of Transcription STAT
STAT lung expression is enhanced in a model of hypoxic pulmonary hypertension suggesting a potential role for this signaling pathway in the pathogenesis of the syndrome 76. STAT3in particular has been recognized to play a critical role in cell growth stimulation and has significant anti-apoptotic effects 77. Normally, bone morphogenetic proteins (BMPs) induce growth arrest through intracellular signaling pathways of the Smad proteins78, which act in part through interaction with, and inactivation of, STAT3. Thus, loss of the inhibitory BMP pathway is hypothesized to lead to amplified activation of STAT3. It is therefore intriguing that PA-EC of IPAH patients have increased arginase II, a STAT1/STAT3-regulated gene, essential for endothelial cell proliferation. Taken together, these data suggest that increased proliferation and survival of EC in IPAH may be dependent on persistent STAT3 activation 81.
E. Human immunodeficiency virus and PAH
PAH associated with HIV infection is classified in WHO Group 1 along with IPAH and CTD-associated PAH. Perhaps even more so than for other syndromes in this group (with the exception of CTD-PAH), there is strong evidence for underlying inflammatory mechanisms in the pathogenesis of the pulmonary vascular remodeling in HIV-PAH.
Although the exact mechanisms of pathogenesis of HIV-PAH are not well understood, several facts have been established, including the lack of evidence of a direct role for the HIV virus in the development of PAH. While the virus is present in macrophages and other inflammatory cells in the lungs, which may provide a potential reservoir for the transmission of the virus to circulating T-cells and a source for localized viral proteins such as Nef, Tat and gp120, it has not been detected in EC of patients with HIV-PAH. Rather, the pathophysiology of HIV-PAH has been linked to indirect mechanisms involving mediators such as cytokines, growth factors, and vasoconstrictors 83.
The vasoconstrictor Endothelin 1 (ET-1) which is significantly elevated in a variety of PAH etiologies, is also elevated in HIV-PAH. The glycoprotein 120, which is crucial for the binding of the virus to CD4+cells, stimulates the secretion of ET-1 as well as cytokines, including TNF from macrophage 84. Ehrenreich et al also demonstrated that circulating monocytes in those infected with HIV show persistent activation of the ET-1 gene. Like in IPAH, PDGF is another key mediator in HIV-PAH. PDGF-A chain mRNA is significantly increased in both IPAH and HIV associated PAH when compared to controls 27.
Marecki et al. have recently demonstrated the pathogenic role of the HIV nef gene in the development of HIV-PAH 85. The Nef protein plays a key role in the pathogenesis of simian immunodeficiency virus infection 86. HIV-1 Nef, an accessory protein made early in HIV infection, down-regulates CD4 87 and blocks major histocompatibility antigen I trafficking to the membrane 88, thus allowing cells with HIV to evade immune surveillance 89. In human monocyte-derived macrophages, Nef activates several inflammatory pathways, such as the STAT1 pathway, and secretion of MIP-1, IL-6, and TNF-α 90. In macaques infected with either the chimeric SHIV-nef virions (simian immunodeficiency virus containing a cloned HIV-1 nef gene) or with parental SIV strains containing the native SIV nef allele, vascular changes in the lungs characteristic of PAH were observed in the first group of animals, but were absent in animals infected with parental SIV strains. These experiments strongly suggest that nef is in part responsible for vascular remodeling in HIV-PAH. In addition, nef expression has been shown by immunohistochemistry in the lungs of HIV PAH patients 85.
F. Autoantibodies in PAH
Several autoantibodies have been reported in PAH, in particular IPAH and SSc-PAH. For example, PAH-SSc patients have been found to have antifibrillarin antibodies 91, and the poorly characterized anti-EC antibodies which correlate with digital infarcts 92. Nicolls et al suggested that anti-EC antibodies can activate EC, induce the expression of adhesion molecules, and trigger apoptosis, thus contributing to the pathogenesis of PAH 9. Other antibodies reported in PAH-SSc include antibodies to fibrin-bound tissue plasminogen activator (in patients with limited cutaneous SSc93 and in IPAH patients with HLA-DQ7 antigen 94), and anti-topoisomerase II-alpha antibodies, particularly in association with HLA-B35 antigen 95. In vitro, autoantibodies from patients with CTD (antifibrillarin antibodies and anti-dsDNA) can upregulate adhesion molecules (e.g., endothelial leukocyte adhesion molecule-1) and histocompatibility complex class II molecules on human pulmonary arterial EC 96, suggesting an inflammatory process can lead to proliferation and remodeling of the pulmonary vasculature.
The detection of anti-fibroblast antibodies in the serum of PAH-SSc and IPAH patients has significant pathogenic importance since fibroblasts are necessary components of the pulmonary vascular wall remodeling in PAH, and can be found in the remodeled neointimal layer in both SSc-PAH and IPAH. These antibodies can activate fibroblasts, in turn inducing collagen synthesis, which contributes potentially directly to the remodeling process. Antibodies from sera of patients with SSc induce a pro-adhesive and pro-inflammatory response in normal fibroblasts 98. Patients with IPAH and PAH-SSc have IgG antifibroblast antibodies present in their sera which have distinct reactivity profiles in these two conditions 99. A two-dimension immunoblotting technique showed that fibroblasts antigens which are recognized by serum IgG from IPAH and SSc-PAH patients included proteins involved in regulation of cytoskeletal function, cell contraction, cell and oxidative stress, cell energy metabolism and other pathways that play key roles in cell biology and maintenance of homeostasis100. Although the specific membrane antigen targeted by these auto-antibodies has not yet been determined, it is likely that the antibodies react to membrane components since they typically bind to unpermeabilized fibroblasts mediating the release of cytokines and growth factors which in turn might contribute to the pathogenesis of vascular remodeling in PAH 99.
G. Conclusion
Inflammatory processes appear to play an important role in the vascular remodeling characteristic of PAH and might be important targets for PAH therapy. While preclinical trials targeting specific inflammatory pathways have shown promising results in animal models, translating these findings to human PAH remains largely unexplored except in the case of the tyrosine kinase pathway where clinical trials are on-going. Recognizing the complexity of specific inflammatory pathways in animal models and human PAH, some of which may be predominant in certain forms of PAH but not in others, is important. However, deciphering their exact mechanisms of action in the process of pulmonary vascular remodeling will provide a wide spectrum of potentially novel targets that are desired considering the currently limited array of modern PAH therapies.
Acknowledgments
This work was supported by a grant from the National Heart, Lung and Blood Institute (NIH/NHLBI HL084946)
Abbreviation List
- BMP
Bone morphogenic protein
- EC
Ecndothelial cells
- CTD
Connective tissue disease
- CX3CL1
chemokine (C-X3-C motif) ligand 1
- CX3CR1
Receptor to CX3CL1
- CCL2
(C-C motif) ligand 2
- FGF
Fibroblast growth factor
- EGF
Epidermal growth factor
- ET-1
Endothelin-1
- HIV
Human immunodeficiency virus
- 5HT
5-hydroxytryptophane (serotonine)
- 5-HTT
serotonine transporter
- MAPK
Mitogen activated protein kinase
- MCT
Monocrotaline
- mPAP
Mean pulmonary arterial pressure
- NFAT
nuclear factor of activated T cells
- PAH
Pulmonary Arterial Hypertension
- PDGF
Platelet derived growth factor
- RV
Right ventricle
- SMC
Smooth muscle cell
- SSc
Scleroderma
- STAT
The Signal Transducers and Activators of Transcription
- TGF
Transforming growth factor
- Tregs
Regulatory T cells
- VEGF
Vascular endothelial growth factor
- VIP
Vasoactive intestinal peptide
Footnotes
COI statement: The authors have no conflicts of interest regarding this manuscript. PMH is supported by a grant from the National Heart, Lung and Blood Institute (NIH/NHLBI HL084946). Otherwise, He serves on scientific advisory boards for Gilead, Pfizer, Novartis, and Merck. He has also received research funding from Actelion/United Therapeutics for the REVEAL registry of PAH patients.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–36. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–9. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Isern RA, Yaneva M, Weiner E, et al. Autoantibodies in patients with primary pulmonary hypertension: association with anti-Ku. Am J Med. 1992;93:307–12. doi: 10.1016/0002-9343(92)90238-7. [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–31. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 8.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114:225S–30S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 9.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005;26:1110–8. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 10.Fartoukh M, Emilie D, Le Gall C, Monti G, Simonneau G, Humbert M. Chemokine macrophage inflammatory protein-1alpha mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest. 1998;114:50S–1S. doi: 10.1378/chest.114.1_supplement.50s. [DOI] [PubMed] [Google Scholar]
- 11.Sakamaki F, Kyotani S, Nagaya N, et al. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation. 2000;102:2720–5. doi: 10.1161/01.cir.102.22.2720. [DOI] [PubMed] [Google Scholar]
- 12.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Balabanian K, Foussat A, Dorfmuller P, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–25. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 15.Perros F, Dorfmuller P, Souza R, et al. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J. 2007;29:937–43. doi: 10.1183/09031936.00104706. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez O, Marcos E, Perros F, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–7. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 17.Dorfmuller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:534–9. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 18.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. 2009;34:662–8. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 19.Chaouat A, Savale L, Chouaid C, et al. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136:678–87. doi: 10.1378/chest.08-2420. [DOI] [PubMed] [Google Scholar]
- 20.Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest. 2005;128:572S–3S. doi: 10.1378/chest.128.6_suppl.572S-a. [DOI] [PubMed] [Google Scholar]
- 21.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–44. doi: 10.1161/CIRCRESAHA.108.182014. 28p following 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–86. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration. 2008;75:272–80. doi: 10.1159/000111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109:867–79. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humbert M, Monti G, Fartoukh M, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11:554–9. [PubMed] [Google Scholar]
- 28.Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–21. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wedgwood S, Devol JM, Grobe A, et al. Fibroblast growth factor-2 expression is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res. 2007;61:32–6. doi: 10.1203/01.pdr.0000250013.77008.28. [DOI] [PubMed] [Google Scholar]
- 30.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–31. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 31.Sakao S, Taraseviciene-Stewart L, Cool CD, et al. VEGF-R blockade causes endothelial cell apoptosis, expansion of surviving CD34+ precursor cells and transdifferentiation to smooth muscle-like and neuronal-like cells. Faseb J. 2007;21:3640–52. doi: 10.1096/fj.07-8432com. [DOI] [PubMed] [Google Scholar]
- 32.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–76. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 33.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1998;18:768–76. doi: 10.1165/ajrcmb.18.6.2980. [DOI] [PubMed] [Google Scholar]
- 34.Cool CD, Stewart JS, Werahera P, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–9. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endothelium Clin Chest Med. 2001;22:405–18. doi: 10.1016/s0272-5231(05)70280-x. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995;95:1798–807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perros F, Montani D, Dorfmuller P, et al. Platelet Derived Growth Factor Expression and Function in Idiopathic Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 38.Klein M, Schermuly RT, Ellinghaus P, et al. Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation. 2008;118:2081–90. doi: 10.1161/CIRCULATIONAHA.108.779751. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramaniam V, Le Cras TD, Ivy DD, Grover TR, Kinsella JP, Abman SH. Role of platelet-derived growth factor in vascular remodeling during pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2003;284:L826–33. doi: 10.1152/ajplung.00199.2002. [DOI] [PubMed] [Google Scholar]
- 40.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3:680–6. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 41.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–34. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 42.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res. 2001;88:E2–E11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 43.Zaiman AL, Podowski M, Medicherla S, et al. Role of the TGF-beta/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:896–905. doi: 10.1164/rccm.200707-1083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L897–903. doi: 10.1152/ajplung.00044.2001. [DOI] [PubMed] [Google Scholar]
- 45.Jones PL, Rabinovitch M. Tenascin-C is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circ Res. 1996;79:1131–42. doi: 10.1161/01.res.79.6.1131. [DOI] [PubMed] [Google Scholar]
- 46.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol. 1997;150:1349–60. [PMC free article] [PubMed] [Google Scholar]
- 47.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 48.Petkov V, Mosgoeller W, Ziesche R, et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2003;111:1339–46. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janosi T, Petak F, Fontao F, Morel DR, Beghetti M, Habre W. Differential roles of endothelin-1 ETA and ETB receptors and vasoactive intestinal polypeptide in regulation of the airways and the pulmonary vasculature in isolated rat lung. Exp Physiol. 2008;93:1210–9. doi: 10.1113/expphysiol.2008.042481. [DOI] [PubMed] [Google Scholar]
- 50.Cox CP, Linden J, Said SI. VIP elevates platelet cyclic AMP (cAMP) levels and inhibits in vitro platelet activation induced by platelet-activating factor (PAF) Peptides. 1984;5:325–8. doi: 10.1016/0196-9781(84)90228-6. [DOI] [PubMed] [Google Scholar]
- 51.Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J. 2008;31:135–9. doi: 10.1183/09031936.00105807. [DOI] [PubMed] [Google Scholar]
- 52.Said SI, Hamidi SA, Dickman KG, et al. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260–8. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- 53.Vuckovic A, Rondelet B, Brion JP, Naeije R. Expression of vasoactive intestinal peptide and related receptors in overcirculation-induced pulmonary hypertension in piglets. Pediatr Res. 2009;66:395–9. doi: 10.1203/PDR.0b013e3181b33804. [DOI] [PubMed] [Google Scholar]
- 54.Leuchte HH, Baezner C, Baumgartner RA, et al. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008;32:1289–94. doi: 10.1183/09031936.00050008. [DOI] [PubMed] [Google Scholar]
- 55.MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161–8. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res. 1991;68:1362–8. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 57.Eddahibi S, Fabre V, Boni C, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res. 1999;84:329–36. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- 58.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:L795–806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 59.Eddahibi S, Humbert M, Fadel E, et al. Hyperplasia of pulmonary artery smooth muscle cells is causally related to overexpression of the serotonin transporter in primary pulmonary hypertension. Chest. 2002;121:97S–8S. [PubMed] [Google Scholar]
- 60.Eddahibi S, Humbert M, Fadel E, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–50. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eddahibi S, Hanoun N, Lanfumey L, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–62. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guignabert C, Raffestin B, Benferhat R, et al. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005;111:2812–9. doi: 10.1161/CIRCULATIONAHA.104.524926. [DOI] [PubMed] [Google Scholar]
- 63.Crona D, Harral J, Adnot S, Eddahibi S, West J. Gene expression in lungs of mice lacking the 5-hydroxytryptamine transporter gene. BMC Pulm Med. 2009;9:19. doi: 10.1186/1471-2466-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLean MR, Deuchar GA, Hicks MN, et al. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–5. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- 65.Guignabert C, Izikki M, Tu LI, et al. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98:1323–30. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- 66.Dempsie Y, Morecroft I, Welsh DJ, et al. Converging Evidence in Support of the Serotonin Hypothesis of Dexfenfluramine-Induced Pulmonary Hypertension With Novel Transgenic Mice. Circulation. 2008;117:2928–37. doi: 10.1161/CIRCULATIONAHA.108.767558. [DOI] [PubMed] [Google Scholar]
- 67.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 68.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res. 2004;94:1340–50. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- 69.Kawamura T, Ono K, Morimoto T, et al. Endothelin-1-dependent nuclear factor of activated T lymphocyte signaling associates with transcriptional coactivator p300 in the activation of the B cell leukemia-2 promoter in cardiac myocytes. Circ Res. 2004;94:1492–9. doi: 10.1161/01.RES.0000129701.14494.52. [DOI] [PubMed] [Google Scholar]
- 70.Bushdid PB, Osinska H, Waclaw RR, Molkentin JD, Yutzey KE. NFATc3 and NFATc4 are required for cardiac development and mitochondrial function. Circ Res. 2003;92:1305–13. doi: 10.1161/01.RES.0000077045.84609.9F. [DOI] [PubMed] [Google Scholar]
- 71.Bonnet S, Rochefort G, Sutendra G, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104:11418–23. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michelakis ED, McMurtry MS, Wu XC, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–50. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 73.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1. 5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 74.Bonnet S, Michelakis ED, Porter CJ, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–41. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 75.Kentera D, Susic D, Veljkovic V, Tucakovic G, Koko V. Pulmonary artery pressure in rats with hereditary platelet function defect. Respiration. 1988;54:110–4. doi: 10.1159/000195509. [DOI] [PubMed] [Google Scholar]
- 76.Wang GS, Qian GS, Bai L, et al. Enhanced expression of signal transducers and activators of transcription in lung tissue of hypoxic pulmonary hypertension rat models. Zhonghua Nei Ke Za Zhi. 2003;42:768–72. [PubMed] [Google Scholar]
- 77.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 78.Kawamura C, Kizaki M, Yamato K, et al. Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood. 2000;96:2005–11. [PubMed] [Google Scholar]
- 79.Xu W, Comhair SA, Zheng S, et al. STAT-1 and c-Fos interaction in nitric oxide synthase-2 gene activation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L137–48. doi: 10.1152/ajplung.00441.2002. [DOI] [PubMed] [Google Scholar]
- 80.Xu W, Kaneko FT, Zheng S, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–8. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 81.Masri FA, Xu W, Comhair SA, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–54. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 82.Mette SA, Palevsky HI, Pietra GG, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis. 1992;145:1196–200. doi: 10.1164/ajrccm/145.5.1196. [DOI] [PubMed] [Google Scholar]
- 83.Humbert M. Mediators involved in HIV-related pulmonary arterial hypertension. AIDS. 2008;22 (Suppl 3):S41–7. doi: 10.1097/01.aids.0000327515.55041.da. [DOI] [PubMed] [Google Scholar]
- 84.Ehrenreich H, Rieckmann P, Sinowatz F, et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol. 1993;150:4601–9. [PubMed] [Google Scholar]
- 85.Marecki JC, Cool CD, Parr JE, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174:437–45. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ali SA, Huang MB, Campbell PE, et al. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. 2010;26:173–92. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lundquist CA, Tobiume M, Zhou J, Unutmaz D, Aiken C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J Virol. 2002;76:4625–33. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology. 2001;282:267–77. doi: 10.1006/viro.2000.0816. [DOI] [PubMed] [Google Scholar]
- 89.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 90.Olivetta E, Percario Z, Fiorucci G, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol. 2003;170:1716–27. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 91.Okano Y, Steen VD, Medsger TA., Jr Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis. Arthritis Rheum. 1992;35:95–100. doi: 10.1002/art.1780350114. [DOI] [PubMed] [Google Scholar]
- 92.Negi VS, Tripathy NK, Misra R, Nityanand S. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol. 1998;25:462–6. [PubMed] [Google Scholar]
- 93.Fritzler MJ, Hart DA, Wilson D, et al. Antibodies to fibrin bound tissue type plasminogen activator in systemic sclerosis. J Rheumatol. 1995;22:1688–93. [PubMed] [Google Scholar]
- 94.Morse JH, Barst RJ, Fotino M, et al. Primary pulmonary hypertension, tissue plasminogen activator antibodies, and HLA-DQ7. Am J Respir Crit Care Med. 1997;155:274–8. doi: 10.1164/ajrccm.155.1.9001324. [DOI] [PubMed] [Google Scholar]
- 95.Grigolo B, Mazzetti I, Meliconi R, et al. Anti-topoisomerase II alpha autoantibodies in systemic sclerosis-association with pulmonary hypertension and HLA-B35. Clin Exp Immunol. 2000;121:539–43. doi: 10.1046/j.1365-2249.2000.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okawa-Takatsuji M, Aotsuka S, Fujinami M, Uwatoko S, Kinoshita M, Sumiya M. Up-regulation of intercellular adhesion molecule-1 (ICAM-1), endothelial leucocyte adhesion molecule-1 (ELAM-1) and class II MHC molecules on pulmonary artery endothelial cells by antibodies against U1-ribonucleoprotein. Clin Exp Immunol. 1999;116:174–80. doi: 10.1046/j.1365-2249.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamby MC, Chanseaud Y, Humbert M, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765–72. doi: 10.1136/thx.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chizzolini C, Raschi E, Rezzonico R, et al. Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 2002;46:1602–13. doi: 10.1002/art.10361. [DOI] [PubMed] [Google Scholar]
- 99.Tamby MC, Humbert M, Guilpain P, et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J. 2006;28:799–807. doi: 10.1183/09031936.06.00152705. [DOI] [PubMed] [Google Scholar]
- 100.Terrier B, Tamby MC, Camoin L, et al. Identification of target antigens of antifibroblast antibodies in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1128–34. doi: 10.1164/rccm.200707-1015OC. [DOI] [PubMed] [Google Scholar]