Synopsis
Myeloproliferative neoplasm (MPN) animal models accurately re-capitulate human disease in mice and have been an important tool for the study of MPN biology and therapy. Transplantation of BCR-ABL transduced bone marrow cells into irradiated syngeneic mice established the field of MPN animal modeling and the retroviral bone marrow transplantation (BMT) assay has been used extensively since. Genetically engineered MPN animal models have enabled detailed characterization of the effects of specific MPN associated genetic abnormalities on the hematopoietic stem and progenitor cell (HSPC) compartment and xenograft models have allowed the study of primary human MPN-propagating cells in vivo. All models have facilitated the pre-clinical development of MPN therapies. JAK2V617F, the most common molecular abnormality in BCR-ABL negative MPN, has been extensively studied using retroviral, transgenic, knock-in and xenograft models. MPN animal models have also been used to investigate additional genetic lesions found in human MPN and to evaluate the bone marrow microenvironment in these diseases. Finally, several genetic lesions, although not common, somatically mutated drivers of MPN in humans induce a MPN phenotype in mice. Future uses for MPN animal models will include modeling compound genetic lesions in MPN and studying myelofibrotic transformation.
Keywords: Myeloproliferative neoplasms, preclinical murine models, BCR-ABL, JAK2V617F, hematopoietic stem cells, bone marrow microenvironment, myelofibrosis, oncogenes
A. Introduction
Animal models have been used extensively in the study of myeloproliferative neoplasms (MPN) and have played a key role in advancing the biological understanding of these diseases. In general, these models have faithfully recapitulated human MPN in mice, enabled detailed characterization of the effects of specific MPN associated genetic abnormalities on the hematopoietic stem and progenitor cell (HSPC) compartment and provided excellent in vivo models for testing novel MPN therapeutic agents. In this review, we focus primarily on murine models of the JAK2V617F mutation and on the insights these have provided, and also briefly outline the central role BCR-ABL models played in establishing and developing the field. We discuss models of additional genetic lesions found in human MPN and outline genetic models that induce a MPN phenotype in mice. We describe the use of JAK2V617F models in the pre-clinical development of JAK2 kinase inhibitors and other MPN therapies. We summarize the studies of the bone marrow microenvironment that have been performed using MPN models and conclude with some thoughts as to how MPN animal models might be used in the future.
B. MPN Murine Models
BCR-ABL
The faithful modeling of human MPN in mice began in 1990 with the demonstration that retroviral transduction of BCR-ABL into murine bone marrow cells, followed by transplantation into irradiated syngeneic mice, recapitulated human chronic myelogenous leukemia (CML) in mice5,6. These groundbreaking experiments established the retroviral bone marrow transplantation (BMT) assay and with it the ability to accurately model human hematologic malignancies in mice.
1) Retroviral
The retroviral BCR-ABL model has provided a number of important insights into the molecular pathophysiology of CML. These include the demonstration that BCR-ABL expression in vivo is sufficient to induce CML5,6, the identification of key BCR-ABL activated downstream signaling pathways in CML stem cells7 and the provision of an accurate pre-clinical model that continues to be used in the evaluation of novel CML therapeutics8.
2) Transgenic
Several BCR-ABL transgenic models have been developed and used primarily to investigate the kinetics and therapeutic susceptibilities of CML-propagating stem cells. Here, we discuss two models that have been particularly informative in this regard. Perez-Caro et al. generated transgenic mice that expressed BCR-ABL under the control of the Sca-1 promoter, thus restricting transgene expression to the Sca-1 positive cell population, which includes HSC9. The mice developed a CML-like disease characterized by neutrophilia and hepatosplenomegaly as a result of extra-medullary hematopoiesis, with the majority of animals progressing to blast crisis. A subset of mice developed additional solid tumors, indicating that Sca-1 driven BCR-ABL expression is not restricted to hematopoietic cells. The course of the CML-like disease was not modified by treatment with imatinib, suggesting that the HSC compartment in CML is insensitive to ABL kinase inhibition.
Koschmieder et al. developed an inducible BCR-ABL transgenic model in which the transactivator protein, tTA, was placed under the control of the murine stem cell leukemia (SCL) gene 3’ enhancer, and double transgenic SCLtTA/BCR-ABL mice were generated10. With tetracycline withdrawal, BCR-ABL expression was induced in the HSPC compartment. These mice developed a CML-like disease that is reversible and re-inducible, suggesting that sustained BCR-ABL expression is required for the development of a CML disease phenotype10. The CML-initiating cell population was found to be present in the lineagelowSca1+Kithigh (LSK) compartment11 and more recently this population has been further refined and shown to be present solely in the most immature LT-HSC compartment12,13. BCR-ABL positive LT-HSCs out-compete normal LT-HSC and aberrantly mobilize to the spleen in this model12,13. Finally, CML-propagating stem cells have been found to be resistant to treatment with imatinib alone11, while the combination of imatinib with histone deacetylase inhibitors (HDACi) appears to mitigate some of this resistance, in this BCR-ABL inducible murine model14.
3) Xenograft
Xenograft models of chronic phase CML have been problematic in that the majority of SCID-repopulating cells are BCR-ABL negative15, a finding thought to relate, at least in part, to the presence of a reservoir of normal HSC in chronic phase CML. These findings suggest that BCR-ABL positive SCID-repopulating cells (SRC) from chronic phase CML do not have a proliferative advantage over wild-type SRC. A major caveat of these experiments is the incompatibilities between cytokine receptors on human cells and cytokines produced by a murine microenvironment. Transplanting BCR-ABL positive cells taken from long-term culture-initiating cell (LT-CIC) assays results in more durable leukemic cell engraftment in NOD/SCID mice16, as does the transplantation of cells from CML patients in accelerated or blast phase17.
JAK2V617F
JAK2V617F is the most common molecular abnormality in BCR-ABL negative MPN and it has been extensively studied in vivo using retroviral, transgenic, knock-in and xenograft murine models. In general, these models reliably recapitulate the clinical features of human MPN in mice (see Figure 1, Figure 2 and Table 1) and they have helped advance the biological understanding of JAK2V617F-mediated MPN in humans.
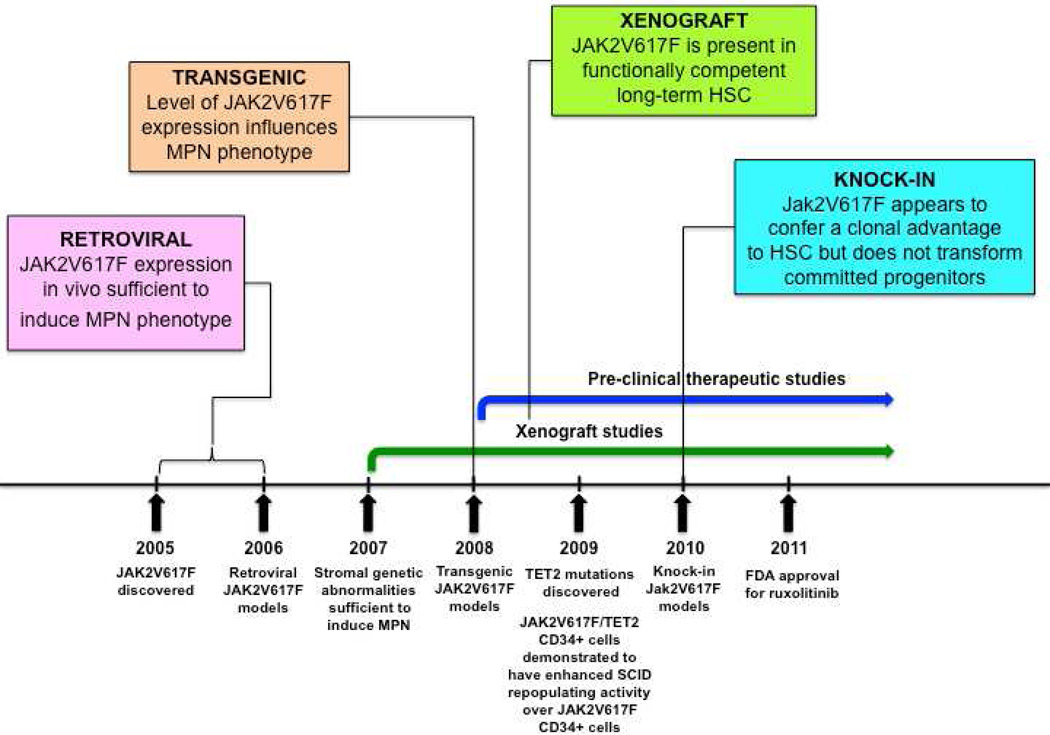
Figure 1. Timeline for development of JAK2V617F murine models.
MPN = myeloproliferative neoplasm; HSC = hematopoietic stem cell; SCID = severe combined immunodeficiency; FDA = Food and Drug Administration
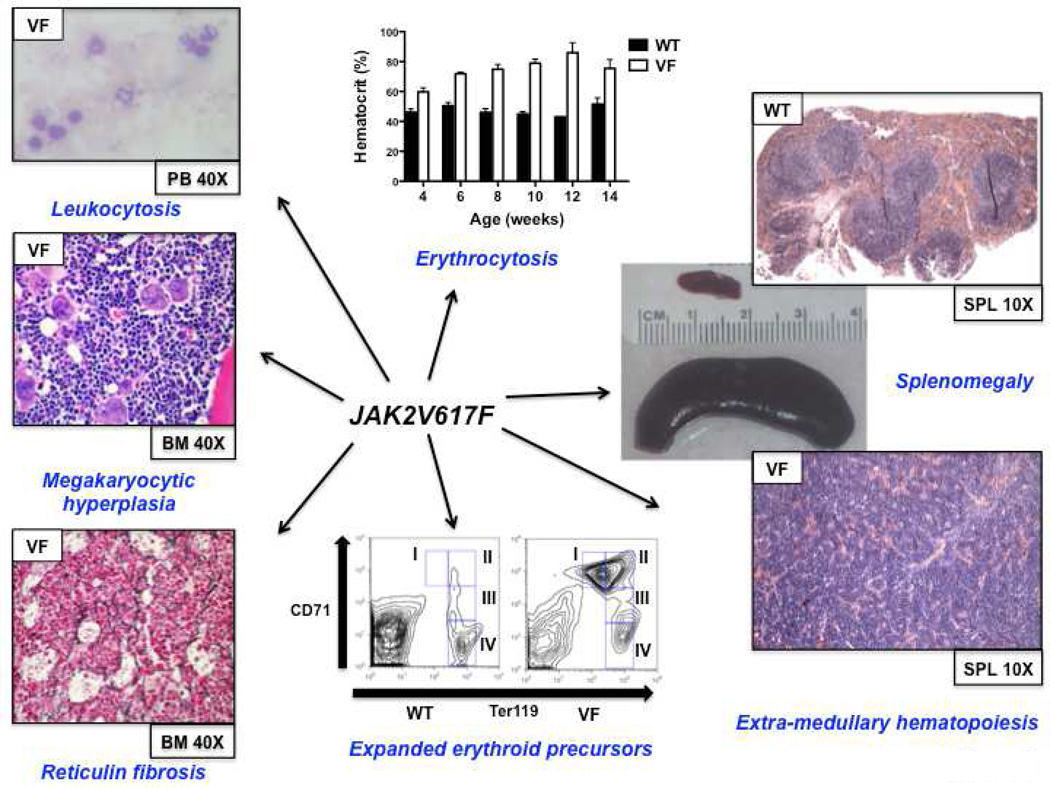
Figure 2. JAK2V617F murine models faithfully recapitulate features of human MPN.
MPN features common to the majority of retroviral and genetic JAK2V617F models (data taken from knock-in model of Mullally et al.28)
WT = wild-type; VF = JAK2V617F; PB = peripheral blood; BM = bone marrow; SPL = spleen
Table 1.
Jak2V617F knock-in murine MPN models
| First Author |
JAK2 Origin |
Strain | Type | VF:WT Expression |
Homozygotes | Phenotype | Myelofibrosis |
|---|---|---|---|---|---|---|---|
| Akada | Mouse | 129Sv/C57Bl/6 | Conditional | < 1:1 | Yes | PV-like | Yes |
| Marty | Mouse | 129Sv/C57Bl/6 | Constitutive | ~ 1:1 | No | PV-like | Yes |
| Mullally | Mouse | 129Sv/C57Bl/6 | Conditional | < 1:1 | No | PV-like | Yes, with BMT |
| Li | Human | 129Sv/C57Bl/6 | Conditional | ~ 1:1 | No | ET-like | No |
PV = polycythemia vera; ET = essential thrombocythemia; BMT = bone marrow transplantaton
1) Retroviral
Immediately following the discovery of the JAK2V617F mutation, retroviral models were generated to assess the phenotypic effects of JAK2V167F in vivo18,19,20,21,22. Mice transplanted with JAK2V617F expressing donor bone marrow developed striking polycythemia after a short latency with a high degree of penetrance. Leukocytosis, splenomegaly due to extramedullary hematopoiesis and the development of myelofibrosis were also seen. All groups observed constitutive activation of Jak2 kinase signaling and Stat5 phosphorylation in addition to cytokine-independent colony formation. These models demonstrated that JAK2V617F expression in vivo was sufficient to induce a MPN phenotype and indicated that genetic modifiers of JAK2V617F-mediated fibrosis exist (more prominent fibrosis was seen in the Balb/c strain as compared to C57Bl/6 strain19).
2) Transgenic
Three groups generated transgenic JAK2V617F models in 2008, with the main goal of correlating disease phenotype with differences in JAK2V617F gene dosage23,24,25. Tiedt et al. conditionally expressed human JAK2V617F from the endogenous human JAK2 promoter and crossed with Vav-Cre or Mx1-Cre transgenic mice to induce hematopoietic specific JAK2V617F expression. The ratio of human JAK2V617F to murine wild-type Jak2 expression was quantified in mRNA23. Xing et al. expressed human JAK2V617F from the Vav promoter24 while Shide et al. expressed murine Jak2V617F from the H-2Kb promoter25.
In the model of Tiedt et al., JAK2V617F Vav-Cre mice had constitutive and sustained Cre expression, low transgene copy number and a JAK2 mutant to wild-type ratio of approximately 0.5. In JAK2V617F MxCre mice, Cre expression was induced following treatment with pI:pC to activate the interferon response, transgene copy number was higher than in JAK2V617F Vav-Cre animals and the JAK2 mutant to wild-type mRNA was approximately 1.0. The lower copy number in Vav-Cre compared to MxCre was attributed to sustained Cre expression in the Vav-Cre model, leading to more efficient deletion of additional transgene copies. The phenotype of JAK2V617F Vav-Cre mice was more consistent with essential thrombocythemia (ET) (high platelets, normal WBC count and hemoglobin), while the JAK2V617F MxCre animals more closely recapitulated polycythemia vera (PV) (elevated hemoglobin with suppressed erythropoietin (Epo), high WBC count and platelets)23. In the model of Xing et al., the level of mutant JAK2 expression was extremely low and the phenotype most consistent with ET24. In the model of Shide et al., the MPN was incompletely penetrant in the founder line in which mutant Jak2 was expressed at a lower level than wild-type Jak2. In a second founder line in which mutant Jak2 expression was higher than wildtype Jak2, a MPN with myelofibrotic transformation developed25.
The major conclusion to be drawn from the transgenic models is that the level of JAK2V617F expression influences the MPN phenotype, with the suggestion that lower JAK2V617F expression results in an ET phenotype and higher JAK2V617F expression give rises to a PV phenotype23,24. However, some of the MPN phenotypic differences observed by Tiedt et al. could be related to tissue specific Cre effects (i.e. Vav-Cre versus Mx1-Cre)23. JAK2V617F expression is driven by exogenous promoters in some of these transgenic models24, 25, which could also influence the MPN phenotype.
3) Knock-in
Four JAK2V617F knock-in models were published in 201026,27,28,29. These were generated with the major goals of: (i) recapitulating human MPN through physiological Jak2V617F expression, (ii) assessing the impact of Jak2V617F gene dosage on disease phenotype and (iii) evaluating the effects of Jak2V617F on the HSPC compartment.
The models of Akada et al., Marty et al. and Mullally et al. all expressed murine Jak2V617F from the endogenous murine Jak2 promoter, but each in a slightly different manner. Akada et al. and Mullally et al. both generated conditional Jak2V617F knock-in models while Jak2V617F expression was constitutive in the model of Marty et al. Human JAK2V617F was conditionally expressed from the endogenous murine Jak2 promoter in the model of Li et al29. In all of the models, the ratio of mutant to wild-type Jak2 expression was 1:1 or less. The model of Akada et al. was the only one in which homozygous Jak2 mutant mice were generated, but since the Jak2 mutant allele expressed at approximately 50% of the wild-type allele the level of Jak2V617F expression in homozygous mice was relatively low26. A summary of the Jak2V617F knock-in models is outlined in Table 1 and a schematic representation of the targeting strategies for each of the models can be found in a recent review by Li et al30.
The phenotype of the three models that expressed murine Jak2V617F was broadly similar26,27,28. In each of these models, mice heterozygous for the Jak2V617F mutation had profound erythrocytosis, leukocytosis, splenomegaly due to extramedullary hematopoiesis and myelofibrosis developed over time (although this was only observed in transplant recipients by Mullally et al.31). Compared with the other models, the hematologic phenotype was relatively mild in the model of Li. et al29. Only a modest increase in platelets and hemoglobin, more reminiscent of ET than PV, was observed (Epo levels were not suppressed), while approximately 10% mice developed marked erythrocytosis with prolonged follow up. In this model, human JAK2V617F was expressed from the endogenous murine Jak2 promoter, and whether human JAK2V617F signals differently in murine cells remains undetermined.
In all of the models, the MPN was transplantable into secondary recipients indicating that the MPN is cell autonomous. The ability to transplant the disease enabled a functional analysis of the stem cell properties of Jak2V617F-expressing HSC. Recent reports on this analysis suggest that Jak2V617F mutant HSC have a clonal advantage over wild-type HSC31,32,33. Mullally et al. found that Jak2V617F mutant HSC have a subtle competitive repopulating advantage over normal HSC28,31, while Marty et al.32 and Lundberg et al.33 recently described a stronger competitive advantage for Jak2V617F mutant HSC in a knock-in model and a transgenic model respectively. The HSC phenotype in the knock-in model of Li. et al. differs from that of the others in that LSK cell numbers were reduced in JAK2V617F mice and demonstrated decreased cell cycle and increased senescence. In competitive repopulation experiments, JAK2V617F mutant HSC were out-competed by wild type cells in primary bone marrow transplant recipients as early as 5 weeks post transplantation, preventing transplantation of the MPN29. It remains unclear whether this finding is related to expression of human JAK2V617F from the endogenous murine Jak2 promoter, or due to another cause such as increased replicative stress or immunological rejection. Finally, by transplanting sorted populations of progenitor cells, Mullally et al. found that expanded Jak2V617F progenitor cell populations such as megakaryocytic erythroid progenitor (MEP) cells are incapable of reconstituting MPN in a transplanted animal28. These results are consistent with previously published reports indicating that oncogenic kinase alleles are incapable of transforming progenitor cells34,35.
The main conclusions from the Jak2V617F knock in models are that: (i) physiologic heterozygote expression of murine Jak2V617F in mice causes a polycythemia phenotype26,27,28, (ii) the Jak2V617F mutation appears to provide a clonal advantage to HSC31,32,33, and (iii) Jak2V617F does not confer self-renewal properties to committed myeloid progenitor cells28. While these models closely recapitulate human MPN, some differences remain. These include the fact that although Jak2V617F expression is driven by the endogenous Jak2 promoter in these knock-in models, the mutant allele is expressed in all hematopoietic cells, rather than in a MPN clone as occurs in the human disease36. It is also important to note that reconstitution in competitive repopulation experiments is polyclonal and occurs in a bone marrow niche that has been perturbed by irradiation.
4) Xenograft
To study BCR-ABL negative MPN cells in vivo, xenograft models have been generated. These models enable a functional assessment of the repopulating capacity of primary human MPN CD34+ cells, which possess the full germline and somatic genotype of human MPN.
Peripheral blood CD34+ cells from patients with myelofibrosis engraft NOD/SCID mice and show clonal hematopoiesis with myeloid skewing1. Two independent groups have found relatively poor engraftment of JAK2V617F mutant CD34+ cells from patients with PV and ET2,3 and the ratio of JAK2V617F to JAK2 wild-type SRCs was found to be higher in myelofibrosis than in PV3. Functionally, JAK2V617F SRCs did not gain a proliferative advantage over wild-type SRCs over time3.
The major conclusions from these studies are that: (i) JAK2V617F is found in a functionally competent LT-HSC population1,2,3, (ii) the functional JAK2V617F LT-HSC compartment is expanded in myelofibrosis as compared with PV3 and (iii) poor JAK2V617F CD34+ engraftment in xenografts may be related to downregulation of CXCR437. Its important to note that incompatibilities between human cytokine receptors and murine cytokines in xenograft models, may be particularly relevant in assessing the in vivo functional effects of the JAK2V617F allele, which activates cytokine receptor signaling. In general, reliable xenotransplantation across a wide spectrum of genetically diverse MPN requires further optimization.
MPLW515L
A somatic activating mutation in MPL (MPLW515L) was originally identified in JAK2V617F negative myelofibrosis patients38 and MPLW515L or MPLW515K mutations were subsequently found in approximately 5% and 1% of myelofibrosis and ET patients respectively39. In a retroviral BMT assay, MPLW515L induced a MPN in mice, characterized by leukocytosis, thrombocytosis, splenomegaly and reticulin fibrosis38. This model has subsequently been used to provide preclinical evidence in support of the use of JAK2 kinase inhibitors in MPN patients who carry the MPLW515L mutation40,41.
TET2
TET2 deletions and loss-of-function mutations occur in up to 12% of MPN patients42,43. Initial studies by Delhommeau et al. indicated that TET2-JAK2V617F co-mutated CD34+ cells from MPN patients show an increased capacity over JAK2V617F-mutated CD34+ cells to repopulate NOD-SCID mice, suggesting that loss of TET2 enhances HSC self-renewal42. These findings have been validated with the publication by several groups of Tet2 conditional knock-out mice, which demonstrate that Tet2 null HSC have a competitive repopulating advantage over wild-type HSC44,45,46,47. Future studies will likely focus on murine modeling of compound mutants (e.g. Jak2V617F/Tet2) to evaluate the impact of loss of Tet2 on the MPN phenotype, on MPN-propagating stem cell function and on the therapeutic susceptibilities of Jak2V617F/Tet2 compound mutant hematopoietic cells.
LNK
Heterozygote loss-of-function mutations in the inhibitory adaptor protein, LNK, were originally reported in JAK2 wild-type MPN patients48. Overall the frequency of LNK mutations in MPN is low and although mutations in LNK have been reported in JAK2V617F positive MPN, it is not known if both mutations co-occur in the same clone49. Knock-out mice for Lnk were generated prior to the identification of LNK mutations in MPN; Lnk null mice exhibit an MPN phenotype (CML-like disease), while heterozygous Lnk mice have an intermediate disease phenotype50,51. Lnk negatively regulates Mpl through its SH2 domain. Consistent with this, Lnk null mice exhibit potentiation of Jak2 signaling in response to thrombopoietin (Thpo), increased HSC quiescence and decelerated HSC cell cycle kinetics52. Lnk retains the ability to bind and inhibit Jak2V617F and consistent with this loss of Lnk exacerbates JAK2V617F-mediated MPN and accelerates the development of JAK2V617F-induced myelofibrosis, through the potentiation of JAK2V617F signaling51. These studies in Lnk null mice have provided important insights into the role of the Thpo/Mpl/Jak2/Lnk pathway in regulating HSC self-renewal and quiescence and informed the understanding of MPN stem cell kinetics.
c-CBL
Mutations in c-CBL were identified in myelofibrosis patients in a region of acquired uniparental disomy (aUPD) on 11q53. Most variants were missense substitutions in the RING or linker domains that abrogated CBL ubiquitin ligase activity53. These mutations were modeled in mice using retroviral over-expression in c-Cbl null cells, and were demonstrated to further augment the enhanced cytokine sensitivity of c-Cbl null HSPC, indicating a gain-of-function activity above that seen in c-Cbl null cells alone54. Subsequent c-Cbl mutant knock-in models, have demonstrated that mutations in the c-Cbl RING finger domain (but not germline deletion of c-Cbl) cause loss of E3 ubiquitin ligase activity and an inability of c-Cbl to interact with target receptor tyrosine kinases, such as Flt3. This in turn, causes activation of downstream signaling pathways and the development of a MPN in vivo, which is dependent on Flt3 signaling for the maintenance of disease55.
Other genetic lesions in MPN
Other genetic lesions have been described in the epigenetic machinery in MPN such as in ASXL156, EZH256,57 and DNMT3A58,59. IDH1 and IDH2 mutations have been identified at low frequency in MPN60. Genetic murine models exist for some of these genetic abnormalities61,62 while others are in development.
Other MPN animal models
Additional murine models have an MPN phenotype, though they are driven by alleles that are not common, somatically mutated drivers of MPN in humans. These include the K-RasG12D knock-in model, in which oncogenic K-Ras was conditionally expressed from its endogenous murine promoter63,64 and the FLT3 internal tandem duplication (ITD) knock-in model, in which a FLT3-ITD mutation was engineered into the Flt3 locus and expressed from the endogenous murine Flt3 promoter65,66. RAS and FLT3 are both recurrently mutated in human AML, but K-RasG12D and Flt3ITD mice both develop MPN, indicating that these genetic lesions alone are insufficient to induce AML. K-RasG12D mice develop a MPN characterized by leukocytosis, extra-medullary hematopoiesis and growth factor hypersensitivity, while Flt3ITD mice develop MPN reminiscent of human chronic myelomonocytic leukemia (CMML), with prominent monocytosis and splenomegaly.
The AP-1 transcription factor, JunB, is a transcriptional regulator of myelopoiesis and its expression is downregulated in AML67. Inactivation of JunB in post-natal mice results in LT-HSC expansion and a MPN resembling early CML68. Murine models of some of the genetic lesions found in human juvenile myelomonocytic leukemia (JMML) have been developed and these also result in a MPN phenotype in mice. Conditional deletion of Nf1 and conditional activation of Ptpn11D61Y both induce a MPN characterized by leukocytosis and hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF)69,70.
Each of these in vivo models has advanced the biological understanding of these genetic abnormalities, particularly with respect to their effects on the HSPC compartment, and they continue to serve as useful preclinical models for testing novel pharmacological agents71.
C. The use of JAK2V617F models in the preclinical development of MPN therapies
JAK2V617F and MPLW515L models have been employed extensively in the pre-clinical development of MPN therapies. These studies have provided the following insights:
Provision of a “proof of concept” that JAK2 inhibitors could be safe and efficacious in the treatment of JAK2V617F72,73 and MPLW515L mediated MPN40,41
Demonstration that MPN-propagating stem cells are not effectively targeted with JAK2 inhibitor monotherapy28
Identification of heat shock protein 90 (HSP90) as a promising therapeutic target in JAK2 driven neoplasms74,75
Demonstration of the efficacy of the HDACi, vorinostat for the treatment of JAK2V617F-mediated MPN76
Indication that pharmacological inhibition of STAT5 (if possible in MPN patients) has the potential to be efficacious in treating JAK2V617F-mediated MPN77
These insights have facilitated the clinical advancement of JAK2 kinase inhibitors, provided a rationale for clinical trials of HSP90 inhibitors in JAK2 driven cancers and offered supportive evidence for the use of HDACi in MPN patients. No doubt, these models will continue to be employed in the evaluation of future MPN therapies.
D. The bone marrow microenvironment in MPN
The bone marrow microenvironment is perturbed in MPN (at least in the late stages of the disease), sometimes to such an extent that myelofibrosis and bone marrow failure develop. Murine models in this area have helped advance understanding in three main areas:
(i) Demonstration that genetic alterations in bone marrow stroma are sufficient to induce MPN
In 2007, Walkley et al. showed in two separate genetic models that MPN could be induced as a result of perturbation of the bone marrow microenvironment. In the first model, germline deletion of the nuclear hormone receptor, retinoic acid receptor γ (RARγ) resulted in an MPN that was TNFα mediated78, while in the second model an MPN developed following conditional inactivation of the negative cell cycle regulator, Rb in hematopoiesis using Mx1-Cre79. In both scenarios, the MPN could not be propagated into secondary recipients and could not be rescued by transplantation with wild-type HSC indicating that the disease phenotypes were cell extrinsic.
(ii) Investigation of the biological mechanisms underlying myelofibrosis development
Prior to the advent of the JAK2V617F murine models, the main genetic models of myelofibrosis were GATA1low80, thrombopoietin (THPO) transgenic81 and Tri21 mice82. These models are all characterized by marked megakaryocytic hyperplasia, underpinning the central role of the megakaryocytic lineage in the development of a fibrosis phenotype. A retroviral THPO over-expression murine model of myelofibrosis83 has facilitated the demonstration that transforming growth factor beta (Tgfβ1) is required84, while thrombospondin (Tsp) is redundant85, for THPO-induced myelofibrosis, and that osteoprotegerin (Opg) is required for osteosclerotic transformation86. While much data exists on the abnormal production of pro-inflammatory cytokines in patients with myelofibrosis it is unclear which cytokines are the real drivers of fibrotic transformation in MPN. Cytokine profiling has been performed in the retroviral JAK2V617F and MPLW515L models73,40 and further investigation of the specific contributions of individual cytokines to fibrotic transformation in JAK2V617F-mediated MPN will likely follow. There has been some heterogeneity in the fibrosis phenotype between the Jak2V617F knock-in models, which may be related to the level of Jak2V617F expression.
(iii) Investigation of cell non-autonomous contributions to clonal expansion in MPN
A positive paracrine loop involving interleukin-6 (IL-6) was recently identified in a genetic model of CML, where IL-6 produced by expanded CML myeloid cells was shown to act on leukemic multi-potent progenitors (MPPs) and drive further myeloid expansion12. Using the same inducible transgenic BCR-ABL model, Zhang et al. also recently demonstrated that altered cytokine expression (including of IL-1, IL-6 and TNFα) in CML bone marrow was associated with selective impairment of normal LT-HSC growth and a growth advantage to CML LT-HSC13. In a retroviral JAK2V617F model, TNFα has been demonstrated to facilitate the preferential expansion of JAK2V617F mutant cells87. In aggregate, these studies indicate that differential microenvironmental regulation of HSPC populations in MPN and normal bone marrow contributes to clonal expansion in MPN.
F. Future Directions
As the genetic landscape of MPN has been defined, corresponding genetically engineered murine models have been developed. Going forward, mutations that co-occur in human patients will be modeled in the mouse to investigate the functional effects and therapeutic susceptibilities of compound genetic lesions in MPN. These models will also be useful in understanding the differential molecular dependencies and niche interactions of MPN-propagating stem cells and in studying fibrotic transformation in MPN. Given the recent identification of a nuclear role for JAK288, evaluating the effects of non-canonical JAK2V617F signaling in vivo will be another important focus. These models will continue to be employed in pre-clinical studies, particularly for the evaluation of combinatorial therapeutic strategies, aimed at targeting MPN-propagating stem cells and/or circumventing JAK2 kinase inhibitor resistance.
Key Points.
Retroviral transduction of BCR-ABL into murine bone marrow cells followed by transplantation into irradiated syngeneic mice established the field of myeloproliferative neoplasm (MPN) animal modeling
The effects of the JAK2V617F mutation in hematopoietic cells has been extensively modeled in vivo using retroviral, transgenic, knock-in and xenograft murine models
The considerable phenotypic differences observed between broadly similar JAK2V617F murine models highlights the inherent variability in murine models that can occur as a result of multiple factors, e.g. promoter, oncogene expression level, murine versus human protein, mouse strain
Mutant oncogenes found in human acute myelogenous leukemia (AML), e.g. RAS, FLT3, induce MPN in mice, indicating that these genetic lesions are insufficient to cause AML, and suggesting that additional co-operating genetic events are required for AML development
As the increasing genetic complexity of MPN has become apparent, additional genetic models have been developed to investigate the functional effects and therapeutic susceptibilities of compound genetic lesions in MPN
Box 1 – Retroviral Bone Marrow Transplant Murine MPN Models.
In the retroviral bone marrow transplantation (BMT) assay, bone marrow is harvested from donor mice that have been stimulated with 5-fluorouracil (5-FU). The 5-FU stimulated bone marrow cells are then transduced ex vivo with a retroviral construct expressing the gene of interest. This results in stable but random integration of the transgene into the host cell genome. The transduced cells are then transplanted into irradiated syngeneic mice, where hematopoietic reconstitution is polyclonal. Transgene expression is generally high (non-physiologic), and differences in the site of retroviral integration may result in variation in transgene expression level. Since retroviruses preferentially transduce mitotically active cells, quiescent long-term hematopoietic stem cells (LT-HSC) are relatively resistant to retroviral integration.
Box 2 – Genetically Engineered Murine MPN Models.
Genetically engineered murine models can be classified as transgenic or endogenous. Transgenic mice express the gene of interest under the control of ectopic promoter and enhancer elements. They are generated by pronuclear injection of the transgene into a single cell of a mouse embryo, where it randomly integrates into the mouse genome. Knock-in mice express the gene of interest from their native promoters and thus represent endogenous genetically engineered mice. They are generated through the modification of embryonic stem (ES) cells using a DNA construct that contains sequences homologous to the target gene. The relevant mutation is thus introduced to the gene of interest under the control of its endogenous promoter via homologous recombination in ES cells. Conditional knock-in mice use site-specific recombinases, such as Cre, to control the timing and tissue-specificity of gene expression. Inducible transgenic and knock-in models use exogenous ligands (e.g. doxycycline or interferon) to reversibly control the timing of target-gene expression. In general, transgenic models result in over-expression of the gene of interest through the use of exogenous promoters, whereas expression is at physiological levels in knock-in models, where the gene of interest is expressed from its endogenous promoter. Knock-out mice are genetically engineered mice, in which the gene of interest is inactivated by replacing it or disrupting it with an artificial piece of DNA. This is achieved in ES cells via homologous recombination. Since germline homozygous gene deletions can be embryonically lethal, conditional knock-out mice are often generated to circumvent this problem.
Box 3 – Xenograft Murine MPN Models.
In xenograft murine MPN models, human MPN CD34+ cells are transplanted and propagated in immunodeficient mice. To achieve engraftment, investigators have used NOD/SCID (non-obese diabetic, severe combined immunodeficiency) mice absolutely deficient in B and T cells and having impaired NK cell function1,2. Further impairment of the murine immune system has been achieved by depletion of NK cells using CD122 antibodies3 or by crossing with a mouse strain deficient for the common gamma chain of the IL-2 receptor4 (NSG mice). While these models allow the study of primary human MPN cells in vivo, some aspects of the bone marrow microenvironment are not recapitulated due to the absence of immune cells and species incompatibility for some cytokines and cytokine receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu M, Bruno E, Chao J, et al. The constitutive mobilization of bone marrow-repopulating cells into the peripheral blood in idiopathic myelofibrosis. Blood. 2005;105(4):1699–1705. doi: 10.1182/blood-2004-06-2485. [DOI] [PubMed] [Google Scholar]
- 2.Ishii T, Zhao Y, Sozer S, et al. Behavior of CD34+ cells isolated from patients with polycythemia vera in NOD/SCID mice. Exp Hematol. 2007;35(11):1633–1640. doi: 10.1016/j.exphem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.James C, Mazurier F, Dupont S, et al. The hematopoietic stem cell compartment of JAK2V617F-positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood. 2008;112(6):2429–2438. doi: 10.1182/blood-2008-02-137877. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106(5):1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 6.Kelliher MA, McLaughlin J, Witte ON, et al. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Swerdlow S, Duffy TM, et al. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103(45):16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidel FH, Bullinger L, Feng Z, et al. Genetic and pharmacologic inhibition of beta-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10(4):412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Caro M, Cobaleda C, Gonzalez-Herrero I, et al. Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO J. 2009;28(1):8–20. doi: 10.1038/emboj.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koschmieder S, Gottgens B, Zhang P, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105(1):324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- 11.Schemionek M, Elling C, Steidl U, et al. BCR-ABL enhances differentiation of long-term repopulating hematopoietic stem cells. Blood. 2010;115(16):3185–3195. doi: 10.1182/blood-2009-04-215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynaud D, Pietras E, Barry-Holson K, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Ho YW, Huang Q, et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21(4):577–592. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Strauss AC, Chu S, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17(5):427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirard C, Lapidot T, Vormoor J, et al. Normal and leukemic SCIDrepopulating cells (SRC) coexist in the bone marrow and peripheral blood from CML patients in chronic phase, whereas leukemic SRC are detected in blast crisis. Blood. 1996;87(4):1539–1548. [PubMed] [Google Scholar]
- 16.Eisterer W, Jiang X, Christ O, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19(3):435–441. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- 17.Dazzi F, Capelli D, Hasserjian R, et al. The kinetics and extent of engraftment of chronic myelogenous leukemia cells in non-obese diabetic/severe combined immunodeficiency mice reflect the phase of the donor's disease: an in vivo model of chronic myelogenous leukemia biology. Blood. 1998;92(4):1390–1396. [PubMed] [Google Scholar]
- 18.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 19.Wernig G, Mercher T, Okabe R, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107(11):4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacout C, Pisani DF, Tulliez M, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108(5):1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 21.Zaleskas VM, Krause DS, Lazarides K, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66(23):11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 23.Tiedt R, Hao-Shen H, Sobas MA, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111(8):3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 24.Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111(10):5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shide K, Shimoda HK, Kumano T, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22(1):87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 26.Akada H, Yan D, Zou H, et al. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115(17):3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marty C, Lacout C, Martin A, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116(5):783–787. doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- 28.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17(6):584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Spensberger D, Ahn JS, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010;116(9):1528–1538. doi: 10.1182/blood-2009-12-259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Kent DG, Chen E, et al. Mouse models of myeloproliferative neoplasms: JAK of all grades. Dis Model Mech. 2011;4(3):311–317. doi: 10.1242/dmm.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullally A, Poveromo L, Schneider R, et al. Distinct roles for long-term hematopoietic stem cells and erythroid precursor cells in a murine model of Jak2V617F-mediated polycythemia vera. Blood. 2012 May 24; doi: 10.1182/blood-2012-01-402396. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marty C, Lacout C, Cuingnet M, et al. JAK2V617F Promotes Stem Cell Amplification Driving MPN Clonal Dominance in Mice and Treatment by IFNa Prevents This Effect. Blood. 2011;118:616. [Google Scholar]
- 33.Lundberg P, Kubovcakova L, Takizawa H, et al. JAK2-V617F Expressing Stem Cells Display a Competitive Advantage At Low Limiting Dilution and Are Capable of Initiating MPN Phenotype. Blood. 2011;118:615. [Google Scholar]
- 34.Jaiswal S, Traver D, Miyamoto T, et al. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proc Natl Acad Sci U S A. 2003;100(17):10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntly BJ, Shigematsu H, Deguchi K, et al. MOZ-TIF2, but not BCRABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6(6):587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Levine RL, Belisle C, Wadleigh M, et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107(10):4139–4141. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SY, Xu M, Roboz J, et al. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70(8):3402–3410. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 38.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 40.Koppikar P, Abdel-Wahab O, Hedvat C, et al. Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood. 2010;115(14):2919–2927. doi: 10.1182/blood-2009-04-218842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernig G, Kharas MG, Mullally A, et al. EXEL-8232, a small-molecule JAK2 inhibitor, effectively treats thrombocytosis and extramedullary hematopoiesis in a murine model of myeloproliferative neoplasm induced by MPLW515L. Leukemia. 2011;26(4):720–727. doi: 10.1038/leu.2011.261. [DOI] [PubMed] [Google Scholar]
- 42.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Wahab O, Mullally A, Hedvat C, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quivoron C, Couronne L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Cai X, Cai CL, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118(17):4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko M, Bandukwala HS, An J, et al. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A. 2011;108(35):14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardanani A, Lasho T, Finke C, et al. LNK mutation studies in blastphase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia. 2010;24(10):1713–1718. doi: 10.1038/leu.2010.163. [DOI] [PubMed] [Google Scholar]
- 50.Velazquez L, Cheng AM, Fleming HE, et al. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195(12):1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bersenev A, Wu C, Balcerek J, et al. Lnk constrains myeloproliferative diseases in mice. J Clin Invest. 2010;120(6):2058–2069. doi: 10.1172/JCI42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bersenev A, Wu C, Balcerek J, et al. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118(8):2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113(24):6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 54.Sanada M, Suzuki T, Shih LY, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460(7257):904–908. doi: 10.1038/nature08240. [DOI] [PubMed] [Google Scholar]
- 55.Rathinam C, Thien CB, Flavell RA, et al. Myeloid leukemia development in c-Cbl RING finger mutant mice is dependent on FLT3 signaling. Cancer Cell. 2010;18(4):341–352. doi: 10.1016/j.ccr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Abdel-Wahab O, Pardanani A, Patel J, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25(7):1200–1202. doi: 10.1038/leu.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guglielmelli P, Biamonte F, Score J, et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood. 2011;118(19):5227–5234. doi: 10.1182/blood-2011-06-363424. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Wahab O, Pardanani A, Rampal R, et al. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. 2011;25(7):1219–1220. doi: 10.1038/leu.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stegelmann F, Bullinger L, Schlenk RF, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia. 2011;25(7):1217–1219. doi: 10.1038/leu.2011.77. [DOI] [PubMed] [Google Scholar]
- 60.Tefferi A, Lasho TL, Abdel-Wahab O, et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia. 2010;24(7):1302–1309. doi: 10.1038/leu.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neff T, Sinha AU, Kluk MJ, et al. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc Natl Acad Sci U S A. 2012;109(13):5028–5033. doi: 10.1073/pnas.1202258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Challen GA, Sun D, Jeong M, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2011;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braun BS, Tuveson DA, Kong N, et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(2):597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan IT, Kutok JL, Williams IR, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113(4):528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee BH, Tothova Z, Levine RL, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12(4):367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Piloto O, Nguyen HB, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111(7):3849–3858. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorsam ST, Ferrell CM, Dorsam GP, et al. The transcriptome of the leukemogenic homeoprotein HOXA9 in human hematopoietic cells. Blood. 2004;103(5):1676–1684. doi: 10.1182/blood-2003-07-2202. [DOI] [PubMed] [Google Scholar]
- 68.Passegue E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119(3):431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Le DT, Kong N, Zhu Y, et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004;103(11):4243–4250. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 70.Chan G, Kalaitzidis D, Usenko T, et al. Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via cell-autonomous effects on multiple stages of hematopoiesis. Blood. 2009;113(18):4414–4424. doi: 10.1182/blood-2008-10-182626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyubynska N, Gorman MF, Lauchle JO, et al. A MEK inhibitor abrogates myeloproliferative disease in Kras mutant mice. Sci Transl Med. 2011;3(76):76ra27. doi: 10.1126/scitranslmed.3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13(4):311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115(25):5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marubayashi S, Koppikar P, Taldone T, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120(10):3578–3593. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weigert O, Lane AA, Bird L, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209(2):259–273. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akada H, Akada S, Gajra A, et al. Efficacy of vorinostat in a murine model of polycythemia vera. Blood. 2012;119(16):3779–3789. doi: 10.1182/blood-2011-02-336743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119(15):3539–3549. doi: 10.1182/blood-2011-03-345215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice) Blood. 2002;100(4):1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- 81.Kakumitsu H, Kamezaki K, Shimoda K, et al. Transgenic mice overexpressing murine thrombopoietin develop myelofibrosis and osteosclerosis. Leuk Res. 2005;29(7):761–769. doi: 10.1016/j.leukres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Kirsammer G, Jilani S, Liu H, et al. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111(2):767–775. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villeval JL, Cohen-Solal K, Tulliez M, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90(11):4369–4383. [PubMed] [Google Scholar]
- 84.Chagraoui H, Komura E, Tulliez M, et al. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100(10):3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 85.Evrard S, Bluteau O, Tulliez M, et al. Thrombospondin-1 is not the major activator of TGF-beta1 in thrombopoietin-induced myelofibrosis. Blood. 2011;117(1):246–249. doi: 10.1182/blood-2010-07-294447. [DOI] [PubMed] [Google Scholar]
- 86.Chagraoui H, Tulliez M, Smayra T, et al. Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood. 2003;101(8):2983–2989. doi: 10.1182/blood-2002-09-2839. [DOI] [PubMed] [Google Scholar]
- 87.Fleischman AG, Aichberger KJ, Luty SB, et al. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392–6398. doi: 10.1182/blood-2011-04-348144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dawson MA, Bannister AJ, Gottgens B, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461(7265) doi: 10.1038/nature08448. 819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]