Abstract
Purpose:
Mechanistic and observational studies support an independent increase in risk of hypertension and abnormal glucose metabolism associated with obstructive sleep apnea (OSA). However, the specific populations and outcomes that improve with treatment of OSA in clinical practice are not established. We examined the effectiveness of OSA treatment on clinical blood pressure and diabetes control measures in men with preexisting systemic hypertension or type 2 diabetes.
Methods:
A retrospective cohort of veterans (n = 221) with a new diagnosis of OSA and initiation of positive airway pressure treatment was identified using administrative databases and clinical records.
Measurements and Results:
Outcomes were changes in blood pressure (BP; mean of 3 highest recordings; systolic and diastolic) and glycemic control (mean of 3 highest fasting glucose and hemoglobinA1C values) at 3-6 months (T1) and 9-12 months (T2) following treatment compared to pretreatment. A generalized estimating equation model was used with adjustment for potential confounders: demographics, body mass index (BMI), OSA severity, Charlson comorbidity index, and pharmacologic treatment for hypertension and diabetes. Sustained independent effects of OSA treatment (mean change [95% CI]) were noted in both systolic BP (T1; −7.44 [-10.41 to −4.47] and T2; −6.81 [-9.94 to −3.67]) and diastolic BP (T1; −3.14, [-4.99 to −1.29] and T2; −3.69, [-5.53 to −1.85]). Diabetes control measures did not change with OSA treatment.
Conclusions:
Treatment of OSA improves office blood pressure in hypertensive men. Prospective studies are necessary to better characterize specific populations with OSA that benefit from treatment with respect to progression of hypertension and type 2 diabetes.
Citation:
Prasad B; Carley DW; Krishnan JA; Weaver TE; Weaver FM. Effects of positive airway pressure treatment on clinical measures of hypertension and type 2 diabetes. J Clin Sleep Med 2012;8(5):481-487.
Keywords: Effectiveness, OSA, hypertension
Considerable evidence from observational studies implicates obstructive sleep apnea (OSA) as an independent risk factor for systemic hypertension1 and abnormal glucose metabolism.2 The results of experimental studies evaluating effects of OSA treatment in hypertension reveal modest benefits,3,4 and the data regarding improvement in glucose homeostasis in type 2 diabetics from small clinical trials are inconsistent.5,6 This discrepancy is partly due to the size and type of cohorts examined or the specific outcomes assessed.7 Several studies examined populations with differential baseline characteristics (presence or absence of preexisting hypertension/diabetes) or variable OSA disease severity.7–12 Additional sources of variability relate to duration of follow-up and levels of treatment adherence.13 However, frequently the outcomes examined are not routinely available in clinical practice, and the applicability of these findings to usual care settings is unclear.
The objective of this study was to examine the effectiveness of treatment of OSA on routine clinical measures of hypertension and diabetes control in a primary care practice setting. We examined the long-term effects of OSA treatment on diurnal office BP, fasting glucose, and hemoglobin A1C (HbA1C) in veterans with newly diagnosed OSA and comorbid systemic hypertension and/or type 2 diabetes.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Clinical trials indicate management of sleep apnea maybe efficacious in improving cardiometabolic outcomes. We examined whether the management of sleep apnea improves these outcomes in clinical practice.
Study Impact: CPAP treatment effectively reduces blood pressure, in clinical practice, in men with hypertension. Future studies that identify treatment responsive populations with respect to key clinical outcomes are needed to guide healthcare providers and to optimize healthcare delivery for sleep apnea.
METHODS
Subjects
We reviewed national Veterans Health Administration databases and clinical records at 2 Veterans Affairs Medical Centers (VAMC1 and VAMC2, IL) for fiscal years 2005-2006 and identified a cohort with new diagnosis and treatment of OSA (Figure 1). Individuals were included in the analysis if they met the following 2 criteria: (1) new diagnosis and treatment of OSA; and (2) presence of preexisting hypertension or diabetes, identified by ICD9 codes. New diagnosis of OSA with initiation of treatment was defined as: (1) a CPT code for a diagnostic sleep procedure (polysomnography; PSG or unattended level 3 portable monitoring, Stardust II, Philips-Respironics; PM) followed by an ICD-9 code for OSA within 3 months; (2) prosthetics records indicating CPAP or APAP device provision within 6 weeks of the diagnostic procedure; (3) no OSA ICD-9 code in administrative and clinical records for 6 months prior to the diagnostic procedure date. The exclusion criterion was CPT codes for or history of surgical or dental device treatment for OSA. Subjects received either laboratory titrated fixed-CPAP treatment (Remstar Pro, Philips-Respironics, Inc; Murrysville, PA, USA) or APAP device treatment set at 4 to 20 cm H2O upon initiation (Remstar Auto with C-Flex, Philips-Respironics). The veterans with both fixed CPAP and APAP treatments had similar outpatient follow-up with sleep medicine physicians, and the APAP device pressure was adjusted to ≥ 90th percentile after treatment initiation at the treating physician's discretion. This study was approved by the institutional review board at both participating facilities.
Figure 1. Cohort Identification.

Analysis
The index date for the study was the date of CPAP or APAP device distribution. Three months preceding the index date was defined as the baseline period (T0), 3-6 months following the index date as T1 (first follow-up), and 9-12 months following the index date as T2 (second follow-up). The outcomes assessed (final cohort, n = 221) at T1 and T2 were: (1) Outpatient office visit systolic and diastolic BP (from the same recordings) in the subjects diagnosed with hypertension. For conservative treatment-related effect estimates, an average of the highest 3 values was taken to represent the systolic and diastolic BP within each time interval. (2) Outpatient fasting glucose values extracted from clinical records. If multiple values were noted, the last value within each time interval (T0, T1, and T2) was recorded. If the lab did not specifically indicate fasting glucose in the remarks, an outpatient blood draw that occurred between 07:00 to 08:30 was considered a fasting sample. (3) Outpatient hemoglobin A1C (HbA1C) extracted from clinical records. If multiple values were noted, the last value within each time interval (T0, T1, and T2) was recorded.
Several potential confounders were assessed, including: demographic data (age, race, BMI), concurrent medical illnesses per ICD9 codes, drug treatment of hypertension and diabetes, objective OSA treatment adherence (SmartCard download to EncorePro software), and self-reported daytime sleepiness documented in clinician notes. Demographic, comorbid medical disorders, and pharmacy data were extracted from administrative records. Data regarding vital signs, OSA disease severity, OSA treatment adherence, and symptom of sleepiness were extracted from clinical records. OSA disease severity was defined by the apnea hypopnea index (AHI) per published criteria for PSG.14 For both PSG and PM, the criterion for hypopnea was ≥ 50% airflow reduction and ≥ 3% desaturation (or associated arousal for PSG). The Charlson comorbidity index (CCI) was calculated and used to indicate general health status.15 The pharmacy data provided the total number and dosage of medications used for treatment of hypertension and diabetes. Three pharmacologic treatment measures were considered as covariates: changes in number of drugs, changes in dose of drugs, and adherence to drugs. Adherence to medications for hypertension and diabetes was defined by the medication possession ratio (MPR).16 MPR calculation results in a ratio < 1.0 if there are lapses in prescription refilling. The MPR was truncated at the maximum value of 1.0 (indicating potentially perfect adherence). An MPR was calculated for each 90-day interval (T1 and T2) and for each drug. The mean MPR for each drug class (antihypertensive and oral plus injectable hypoglycemics) within T1 and T2 individually were used as a measure of adherence to pharmacologic treatment. Total number of antihypertensive and antihyperglycemic medications as well as dose changes was recorded. Change in total number of medications during each follow up interval compared to baseline was coded as −3 to +3 (0 being no change in number of medications). Change of medications within the same class (e.g., ACE inhibitors) and replacement of one drug with another was not considered. Dose changes of individual medications was recorded categorically: none (0), increase (+1), and decrease (-1).
Statistical Analysis
Comparisons of sociodemographic characters of the final cohort to those lost to follow-up (Figure 1) were performed with χ2 or t-tests. Primary outcomes were examined for treatment-related changes in outcomes at T1 and T2 using the generalized estimating equation (GEE) model, where the final model was adjusted for time and the potential covariates described above. OSA disease severity was treated as a categorical variable with 2 levels: mild (AHI or RDI 5-15) and moderate to severe (AHI or RDI > 15). Daytime sleepiness symptom was coded as a categorical variable (yes/no/missing). Multicollinearity amongst the covariates was checked with variance inflation factor (VIF, < 5 accepted). In the stratified analyses (by type of treatment; CPAP vs. APAP and by race; European Americans vs. African Americans), similar adjusted GEE model was used with added variables of groups as defined above and time-group interaction terms. Adherence to OSA device treatment (CPAP or APAP) was treated as a continuous variable (average nightly use in hours and minutes). Due to significant missing data, adherence to OSA treatment was excluded as a covariate in the final GEE model. The effect of adherence to OSA treatment on individual outcomes was separately examined with general linear regression. All analyses were performed using SAS 9.2, and a p-value ≤ 0.05 was considered significant.
RESULTS
Cohort Lost to Follow-Up (Figure 1)
As total of 650 veterans were diagnosed with OSA, but only 302 were identified as receiving treatment (CPAP or APAP device) within 6 weeks of diagnosis. Veterans who did vs. did not receive treatment were not different by race (p = 0.81), marital status (p = 0.54), or age (p = 0.57).
Baseline Measurements (Table 1)
Table 1.
Baseline characteristics of cohort (N = 221)

Ninety-four percent of participants had a diagnosis of hypertension, while 40% had type 2 diabetes. Approximately one-fourth had mild sleep apnea, and more than half reported no daytime sleepiness. A third of the subjects were treated with APAP long-term in the autoadjusting mode. Compared to those treated with CPAP, the APAP treated subjects had higher BMI (33.9 ± 6.3 vs. 35.9 ± 5.7, p = 0.02) and were more frequently ethnic minorities (28% vs. 68%, p < 0.0001). The preponderance of African Americans veterans among those treated with APAP reflects the practice patterns at the 2 study sites (i.e., the urban VAMC utilizes APAP more frequently). Due to this difference of treatment modality with regards to race, we examined the effect of race on baseline characteristics of the cohort. African Americans (AA) compared to European Americans (EA) and Hispanics/other were not different in OSA disease severity, self-reported sleepiness, or the prevalence of hypertension or diabetes at baseline. However, AA veterans were younger (59.5 ± 10.9 vs. 64.7 ± 10.5, p = 0.007), more obese (BMI 36.5 ± 5.9 vs. 33.9 ± 6.3, p = 0.004), and had lower adherence to pharmacologic treatment (MPR) for hypertension (0.96 ± 0.09 vs. 0.98 ± 0.03, p = 0.01) and diabetes (0.93 ± 0.13 vs. 0.97 ± 0.05, p = 0.05).
Effects of OSA Treatment (Table 2): Primary Outcomes
Table 2.
Changes in outcomes with OSA treatment at Time 1 and Time 2

Both systolic and diastolic BP decreased significantly with initiation of OSA treatment at both T1 and T2. No significant change in fasting glucose or HbA1C was noted at either T1 or T2 after initiation of PAP treatment. Of the other factors (explanatory variables) considered in the model, age was significant with regard to BP outcomes. Specifically, increasing age was associated with a greater reduction in both systolic BP (p = 0.04) and diastolic BP (p < 0.0001). A second significant factor was change in dose of antihypertensive medications. An increase in dose of antihypertensive medications was related to less reduction in diastolic BP (p = 0.03; systolic not significant at p = 0.08), likely reflecting a response rather than a causal effect.
For glucose control measures, OSA treatment had no independent effect on fasting glucose or HbA1C. Of the other predictors considered for fasting glucose, a greater reduction in fasting glucose was seen in older veterans (p = 0.05). Medical comorbidity (measured by CCI) was the only significant predictor of HbA1C, where a higher number of diagnosed medical conditions was associated with an increase in HbA1C over time (p = 0.001).
Notably, large standard deviations were noted for reductions in both systolic (-10.41 to −4.47 at T1) and diastolic BP (-9.94 to −3.67 at T1), indicating a heterogeneity of treatment effects (HTE) within the cohort. To examine potential explanatory variables for this HTE (Table 3), we examined changes in systolic and diastolic BP outcomes by type of treatment (CPAP vs. APAP) and by race (EA vs. AA). Systolic and diastolic BP improved in veterans with either CPAP or APAP. African Americans exhibited significant reductions in both systolic and diastolic BP, while among the European Americans, only systolic BP showed statistically significant change.
Table 3.
Changes in blood pressure with type of treatment and by race

Adherence to OSA Treatment
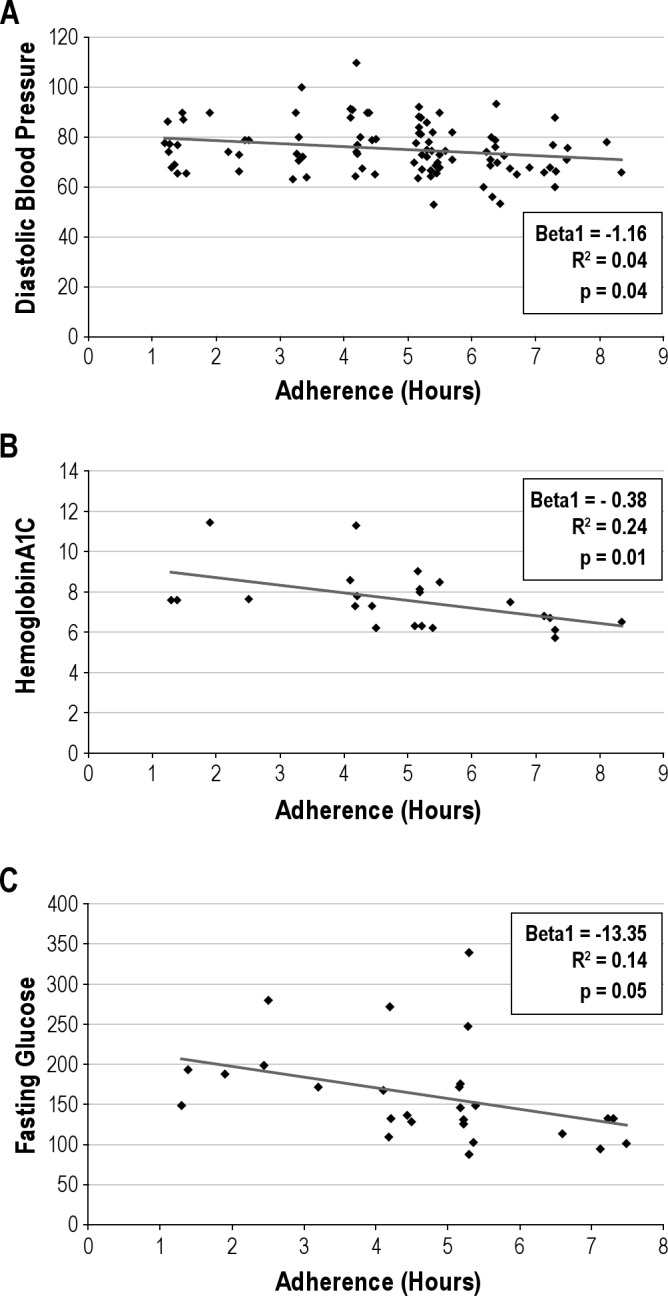
As expected in a naturalistic dataset, we encountered missing data (39% at T1 and 54% at T2) with regards to OSA treatment adherence. Missing data for adherence to OSA treatment was not significantly different by age, race, OSA severity, or symptoms at T1. In the subset of the cohort where adherence to OSA treatment data was available, adherence was objectively assessed for each time interval (SmartCard reports extracted in average daily hours of use). The adherence was stable over time (T1; mean ± SD = 4.90 ± 1.59, T2; mean ± SD = 4.44 ± 1.57, p = 0.15). Adherence to OSA treatment did not correlate with adherence to pharmacologic treatment (MPR for hypertension; p = 0.77 or MPR for diabetes; p = 0.67). Examination of the effect of adherence to OSA treatment on primary outcomes at T1 with general linear regression showed results consistent with previous reports, i.e., adherence to OSA treatment was significantly associated with lower diastolic BP, HbA1C, and fasting glucose (Figures 2A-C).
Figure 2. (A) OSA Treatment Adherence and Diastolic Blood Pressure. (B) OSA Treatment Adherence and HemoglobinA1C. (C) OSA Treatment Adherence and Fasting Glucose.
DISCUSSION
This is the first study to our knowledge to examine the effectiveness of treatment of OSA on routine clinical measures of hypertension and diabetes control in a real-world setting. In this cohort of hypertensive men on drug therapy, we found OSA treatment was associated with improvement in diurnal office systolic and diastolic blood pressure up to 1 year after initiation of PAP treatment. The finding was not related to the type of device (CPAP and APAP) used for treatment. Despite their comparatively lower adherence to pharmacologic treatment, we noted significant reductions in both systolic and diastolic BP among African Americans, while among the European Americans, only systolic BP showed a statistically significant change. We failed to discern any independent effects of OSA treatment in veterans with type 2 diabetes on clinically utilized glucose homeostasis markers: fasting glucose and HbA1C.
Published evidence largely supports a causal association between OSA and elevated blood pressure,17,18 and OSA is recognized as a treatable cause of hypertension.19 Efficacy studies support modest beneficial effects of fixed-CPAP and APAP treatments on blood pressure.9,20 This effect may be more robust on 24-hour and nocturnal BP, which are better predictors of cardiovascular risk.21 Clinical trials and meta-analyses indicate that populations with higher levels of obesity (BMI) and severity of OSA22–24 with preexisting systemic hypertension, and those receiving antihypertensive pharmacologic therapy maybe more likely to benefit from CPAP treatment.25,26 The magnitude of systolic and diastolic BP reduction noted in this study is higher than has been previously reported with CPAP treatment.13,27 Studies that recruited participants without hypertension may have encountered floor effects. Other characteristics of this naturalistic cohort, which may explain these findings include; older men (mean age 61-63 years), with a high prevalence of moderate-severe OSA (60% to 80% with AHI/RDI > 15/h) and obesity, a large proportion with resistant hypertension28 (use of ≥ 3 antihypertensive medications; 59/209, 28%) and multiple cardiovascular comorbidities (more than half had at least one of the following: heart failure, atrial fibrillation, hypertensive heart disease). These population characteristics have individually been associated with higher therapeutic effects of CPAP on BP.4,20,29,30 Age and OSA interact as risk factors for hypertension31: the importance of age as an effect modifier of treatment response is illustrated in this study; that is, older men showed greater OSA treatment-related response in both systolic and diastolic BP. The effect size (Cohen's d for repeated measures) when considered by type of treatment and race varied from small to large, indicating that type of treatment and race may also be significant determinants of HTE. For systolic BP changes the effect size were; CPAP = 0.49, APAP = 0.56, European Americans = 0.39, and African Americans = 0.80. For changes in diastolic BP observed, the effect size were; CPAP = 0.28, APAP = 0.50, European Americans = 0.27, and African Americans = 0.67.
The duration of treatment effect of OSA on BP is unknown, with most studies examining outcomes at a few weeks.23 We noted a sustained response of BP in this cohort to OSA treatment up to 12 months of follow-up. These results are consistent with a recent randomized trial of CPAP in a population with coexistent OSA and systemic hypertension. Barbe et al., reported significant but comparatively modest therapeutic effects of CPAP on systolic (-1.89 mm Hg) and diastolic BP (-2.19 mm Hg).13 Less than half of the participants in this study were on any drug treatment and all were asymptomatic, while the average number of antihypertensive medications per subject in this cohort was 2.67, and 40% reported daytime sleepiness.
Small efficacy studies indicate the cardiovascular risk reduction with use of APAP devices for treatment may not be equivalent to fixed-CPAP treatment.32–34 Moreover, due to lack of data, the use of APAP therapy in populations with comorbid cardiorespiratory illnesses is not recommended.35 The current cohort included subjects with comorbid conditions—known chronic obstructive pulmonary disease (45/221; 20%), congestive heart failure (20/221; 9%), and stroke (12/221; 5%)—were included, a previously understudied group.35,36 Our results indicate reduction in systemic BP with both APAP and CPAP treatments. These data provide a rationale to prospectively test the effectiveness of APAP treatment for titration and long-term therapy in a broader population, particularly as this technology advances.
A third of this cohort was African American, a population that suffers a higher burden of hypertension37 and in whom treatment of OSA is recommended as adjunctive therapy.38 We found significant effects on both systolic and diastolic BP that are clinically highly significant. Such treatment-related effects, if prospectively confirmed in a similar population, would have a considerable impact on cardiovascular disease.39
Our data do not support the effectiveness of OSA treatment in improving clinical glycemic control indices in type 2 diabetes. This maybe related to sample size or inability to adequately control for adherence in this cohort. Despite the demonstrated independent detrimental effect of OSA on the full spectrum of abnormal glucose homeostasis,40–43 data from a single randomized sham-CPAP controlled trial of CPAP (mean CPAP adherence 3.6 h nightly) in subjects with OSA and diabetes are negative.6 In contrast, studies with a less robust design in a similar population demonstrate a therapeutic effect of CPAP on HbA1C at 3-4 months post-treatment, suggesting an important modification of effects of CPAP on glucose homeostasis by level of adherence.44,45
The impact of OSA treatment adherence on intermediate cardiovascular risk markers noted in this study is consistent with published reports.24,45–47 Nevertheless, sizable missing data on device treatment adherence limit conclusions in this study. Prospective examination of the effect modification of OSA treatment adherence on systemic BP and glucose metabolism by objective and unobtrusive measurement is crucial. A second limitation of this study pertains to selection bias (Figure 1), where the final cohort assessed for OSA treatment effects is about half of all eligible veterans. The veterans who did not follow-up at the study sites were not different with regard to available sociodemographic data, i.e. race, age, or marital status. However, there are other potential uncontrolled sources of bias that limit our results. In addition, the retrospective design imposed a burden of attrition of sample size due to missing data for key variables.
CONCLUSIONS
OSA treatment lowers blood pressure in a clinical population of men with hypertension. This study extends the known efficacy to real-world effectiveness of OSA management on hypertension. Prospective effectiveness research examining changes in cardiovascular outcomes with treatment interventions for OSA is necessary to confirm these findings, to identify traits associated with a positive therapeutic response, and to inform clinical practice.
DISCLOSURE STATEMENT
Disclaimer: The findings of this research do not reflect the policy of the Department of Veterans Affairs. This was not an industry supported study. Dr. Terri Weaver has received equipment for research from Philips Respironics, Inc., grant support from Cephalon, Inc., and remuneration for consultation from Apnex Medical, Inc. She also has FOSQ license agreements with Nova Som, Apnex Medical, Inc., GlaxoSmithKline, Philips Respironics, Inc., Cephalon, Inc., and Innovaderm Research, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Samuel Kuna for his review and valuable advice during the preparation of this manuscript. Work for this study was performed at Center for Management of Complex Chronic Care, Hines, Edward J Hines Jr. VAMC and University of Illinois at Chicago. Funding was provided by Center for Management of Complex Chronic Care, Edward Hines Jr. VA Hospital
REFERENCES
- 1.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 2.Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ. 2009;108:263–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 4.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 5.Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–42. [PMC free article] [PubMed] [Google Scholar]
- 6.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. New Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 8.Kushida CA, Berry RB, Blau A, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34:1083–92. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrone O, Salvaggio A, Bue AL, et al. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: relationship with nocturnal sympathetic activity. Clin Exp Hypertens. 2011;33:373–80. doi: 10.3109/10641963.2010.531853. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Zoumakis E, Bixler EO, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–95. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 12.Borgel J, Sanner BM, Keskin F, et al. Obstructive sleep apnea and blood pressure. Interaction between the blood pressure-lowering effects of positive airway pressure therapy and antihypertensive drugs. Am J Hypertens. 2004;17:1081–7. doi: 10.1016/j.amjhyper.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson AL, Quan SF, et al. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–57. [PubMed] [Google Scholar]
- 17.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 18.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–55. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 21.Duran-Cantolla J, Aizpuru F, Martinez-Null C, Barbe-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13:323–31. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 23.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 24.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 25.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 26.Thomopoulos C, Michalopoulou H, Kasiakogias A, Kefala A, Makris T. Resistant hypertension and obstructive sleep apnea: the sparring partners. Int J Hypertens. 2011:947246. doi: 10.4061/2011/947246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calhoun DA. Obstructive sleep apnea and hypertension. Curr Hypertens Rep. 2010;12:189–95. doi: 10.1007/s11906-010-0112-8. [DOI] [PubMed] [Google Scholar]
- 28.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–7. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 31.Chao CC, Wu JL, Chang YT, Lin CY. Combined effect of obstructive sleep apnea and age on daytime blood pressure. Eur Arch Otorhinolaryngol. 2012;269:1527–32. doi: 10.1007/s00405-011-1800-y. [DOI] [PubMed] [Google Scholar]
- 32.Dursunoglu N, Dursunoglu D, Cuhadaroglu C, Kilicaslan Z. Acute effects of automated continuous positive airway pressure on blood pressure in patients with sleep apnea and hypertension. Respiration. 2005;72:150–5. doi: 10.1159/000084045. [DOI] [PubMed] [Google Scholar]
- 33.Drummond M, Winck J, Guimaraes J, Santos AC, Almeida J, Marques J. Long term effect of autoadjusting positive airway pressure on c-reactive protein and interleukin-6 in men with obstructive sleep apnoea syndrome. Arch Bronconeumol. 2009;45:577–84. doi: 10.1016/j.arbres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–9. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 35.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31:141–7. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesson AL, Jr., Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 38.Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 39.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9. [PubMed] [Google Scholar]
- 40.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 2009;64:834–9. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 41.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 42.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–80. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 45.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 46.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 47.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27:249–53. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]


