Abstract
Study Objectives:
Adherence to CPAP therapy is low in patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). The purpose of the present study was to evaluate the utility of measures of sleep architecture and sleep continuity on the CPAP titration study as predictors of both short- and long-term CPAP adherence.
Methods:
93 patients with OSAHS (RDI 42.8 ± 34.3/h) underwent in-laboratory diagnostic polysomnography, CPAP titration, and follow-up polysomnography (NPSG) on CPAP. Adherence to CPAP was objectively monitored. Short-term (ST) CPAP adherence was averaged over 14 days immediately following the titration study. Long-term (LT) CPAP adherence was obtained in 56/93 patients after approximately 2 months of CPAP use. Patients were grouped into CPAP adherence groups for ST (< 2 h, 2-4 h, and > 4 h) and LT adherence (< 4 h, > 4 h). Sleep architecture, sleep disordered breathing (SDB) indices, and daytime outcome variables from the diagnostic and titration NPSGs were compared between CPAP adherence groups.
Results:
There was a significant relationship between ST and LT CPAP adherence (r = 0.81, p < 0.001). Neither ST nor LT adherence were related to demographic variables, baseline severity of untreated SDB, sleep architecture, or measures of daytime impairment. Good CPAP adherence groups had significantly lower %N2 and greater %REM on the titration NPSG. A model combining change in sleep efficiency and change in sleep continuity between the diagnostic and titration NPSGs predicted 17% of the variance in LT adherence (p = 0.006).
Conclusions:
These findings demonstrate that characteristics of sleep architecture, even on the titration NPSG, may predict some of the variance in CPAP adherence. Better sleep quality on the titration night was related to better CPAP adherence, suggesting that interventions to improve sleep on/prior to the CPAP titration study might be used as a therapeutic intervention to improve CPAP adherence.
Citation:
Somiah M; Taxin Z; Keating J; Mooney AM; Norman RG; Rapoport DM; Ayappa I. Sleep quality, short-term and long-term CPAP adherence. J Clin Sleep Med 2012;8(5):489-500.
Keywords: CPAP therapy, CPAP adherence, obstructive sleep apnea, OSAHS treatment, sleep disordered breathing, sleep stages
Untreated obstructive sleep apnea/hypopnea syndrome (OSAHS) significantly affects quality of life and cardiovascular/cerebrovascular morbidities and mortality.1–4 CPAP therapy has been shown to be effective in treating sleep disordered breathing (SDB) by reducing the apnea/hypopnea index (AHI)5 and by reducing excessive daytime somnolence (EDS).6 Despite this, CPAP acceptance and adherence are disappointingly low. A significant number of patients (ranging from 30% to 80% in various studies) demonstrate an average CPAP usage of less than 4 hours per night.7,8 Several reviews have emphasized the need to identify patients who are at the greatest risk for non-adherence, with the goal of developing techniques to maximize overall adherence.9,10
Studies have suggested that CPAP adherence can be correlated to characteristics of patients at baseline, such as the severity of OSAHS,11–13 the level of EDS,14 and anatomical factors (smaller nasal cross-sectional area, reduced nasal volume, and high nasal resistance15,16), but the strength of these correlations has been weak. Drake et al. showed that patients whose sleep efficiency on the CPAP titration night improved most had the greatest CPAP compliance at 47 days.17 More recently, studies using social cognitive theory18–20 and health behavior models21 demonstrate that psychological factors (e.g., outcome expectations, self-efficacy, risk perception) significantly influence CPAP adherence. Interventions such as education (centered around OSAHS, CPAP treatment, and machine management22), supportive phone calls,23 group sessions,24 and frequent office visits,22,25 as well as technologies aimed at reducing pressure intolerance (auto-titrating devices,26 bilevel CPAP, and expiratory pressure reduction27,28), have all been inconsistent in improving long-term adherence. Nevertheless, the consistent observation has been that early adherence and acceptance of CPAP has a relatively significant predictive value for long-term adherence.7,13,29,30
Brief Summary
Current Knowledge/Study Rationale: Despite its efficacy, adherence to CPAP therapy is highly variable amongst patients with obstructive sleep apnea with large numbers of patients demonstrating inadequate adherence to CPAP. The present study is aimed at identifying early predictors of CPAP adherence from variables obtained at the time of diagnosis and titration of CPAP, thereby providing potential for early intervention.
Study Impact: Our data show that better sleep quality (greater % REM) was seen in patients with higher CPAP adherence and confirm that long term adherence was largely predicted by short term adherence. This suggests that interventions that improve sleep during or prior to the CPAP titration study may be useful in improving CPAP adherence.
The purpose of the present study was to evaluate the utility of measures of sleep architecture and continuity on the CPAP titration study as predictors of both short-term and long-term CPAP adherence. In addition to traditional metrics of sleep such as total sleep time, sleep efficiency, and time in sleep stages and wake, we also examined sleep continuity using survival (of sleep) analysis, which has been shown to be useful in characterizing sleep in OSAHS.31 These data were collected as part of a larger research study examining the relationship of SDB to daytime function, in which patients with OSAHS underwent standardized evaluation and management of their SDB, objective monitoring of CPAP adherence, and evaluation of daytime outcomes.
METHODS
For the present study, 106 patients (72 male/34 female; > 18 years of age) were prospectively recruited from all patients seen at the NYU Sleep Disorders Center between 2006 and 2009 who presented with complaints of EDS and/or snoring and were all eligible for a clinical trial of CPAP based on usual medical criteria. All patients had a primary diagnosis of OSAHS or upper airway resistance syndrome. In 99 patients, the respiratory disturbance index (RDI; see definition in data analysis) was > 10/h from an in-laboratory full-night diagnostic nocturnal polysomnography (NPSG). In 7 patients, RDI was < 10/h, but these patients had long periods of inspiratory flow limitation and snoring, EDS, or REM-related or supine RDI > 15/h, and were prescribed a therapeutic CPAP trial. We excluded subjects who were pregnant, had medically unstable conditions, congestive heart failure, change in medications during the trial, recent or confirmed history of alcohol or recreational drug abuse, inability to provide informed consent, or inability to perform the psychomotor vigilance test (PVT). Ninety-three (61 male/32 female) of 106 recruited patients completed the initial recruitment protocol that included the CPAP titration study and objective evaluation of CPAP adherence. Six patients did not return for the CPAP titration study; CPAP adherence data were not available in 3 patients due to technical problems with the CPAP machine; 3 patients withdrew from the study after their CPAP titration; one patient was removed from the protocol due to the patient's inability to follow instructions. This protocol was approved by NYU School of Medicine Institutional Review Board, and all patients signed informed consent.
Protocol Summary
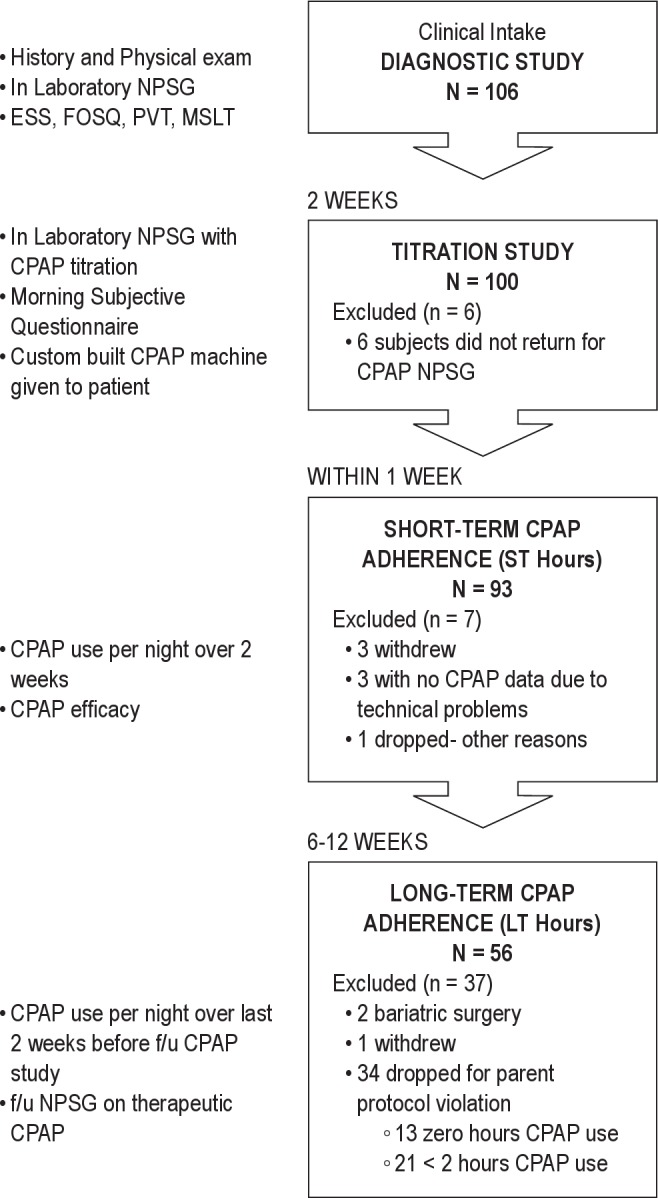
The study was divided into 3 phases: diagnostic evaluation, titration, and follow-up. First, all patients underwent a full clinical evaluation for sleep disorders, which included a sleep-specific interview and a physical exam performed by a sleep physician at the NYU Sleep Disorders Center. Following this, all patients underwent a full in-laboratory diagnostic NPSG, including objective and subjective assessment of sleepiness and a separate standard in-laboratory CPAP titration study. On the morning after CPAP titration, patients filled out a questionnaire assessing their subjective response to the CPAP therapy. Patients were then given a custom CPAP machine (Fisher – Paykel HealthCare, NZ) to use at home with enhanced adherence monitoring capability (see below). A follow-up NPSG at therapeutic CPAP and concurrent daytime testing were repeated at the end of an average of 9 weeks of CPAP use (range 6 weeks- 3 months). Adherence to CPAP use in the home was assessed using data from the CPAP machine download over 2 distinct 14-day periods. The short-term (ST) adherence was evaluated in the 14-day period immediately following dispensing the CPAP machine (usually within one week of the laboratory CPAP titration). Long-term (LT) adherence was evaluated for the 2 weeks prior to the follow-up study. Figure 1 shows a flow chart of the protocol.
Figure 1. Flow chart of study protocol.
Procedures
In-Laboratory Polysomnography (NPSG)
All in-laboratory NPSGs were performed according to AASM guidelines and included full sleep and respiratory montages.32 During the Diagnostic NPSG, respiratory airflow was assessed with a nasal cannula connected to a pressure transducer (Protech PTAF2) and an oral thermistor, whereas during CPAP titration NPSG respiratory airflow was assessed from the CPAP analog output.
The CPAP titration study was performed manually by an experienced sleep technician according to AASM guidelines during a separate full-night NPSG. Pressure was raised until all SDB events (including obstructive apneas, hypopneas, and runs of inspiratory flow limitation) were eliminated. A single optimal pressure was identified for each patient following review of the study by a physician. Per protocol, patients were educated about the function, purpose, and maintenance of CPAP and given a custom CPAP machine (Fisher – Paykel Healthcare) that was capable of continuously recording the raw signals of delivered airflow and pressure at 50 Hz on a portable USB memory card for prolonged periods. This machine provided heated humidification but did not provide bilevel pressure, expiratory pressure relief, or a pressure ramp. Using this CPAP machine, data were collected and downloaded, after which the raw tracings could be visualized and manually scored for respiratory events. The device could also be programmed to change pressure at a predetermined date and time. All patients had a scheduled return visit with the research coordinator after 2 weeks on CPAP, during which time CPAP use was reviewed and problems with the equipment or mask fitting were addressed. Patients then used the CPAP machine set at their fixed therapeutic pressure for the remainder of the study. Follow-up thereafter consisted of a phone call once a month by the research coordinator to discuss CPAP use and schedule additional visits. Patients were encouraged to call the research coordinator at any time during the study period if they experienced problems with therapy.
In 74 patients, the CPAP device was programmed to deliver CPAP in a sequence that began with an initial period of 2 nights at the prescribed therapeutic pressure. This was followed by multiple nights at fixed pressures covering a total range of 2-3 cm H2O above and below the prescribed therapeutic pressure. Pressures were changed every 2 days over 2 weeks in a sequence that alternated increases or decreases of pressure with a return to prescribed therapeutic pressure. In 12 patients, the initially prescribed pressure was low, and hence pressures below therapeutic were not tested. Data files containing the CPAP pressure and airflow were downloaded and reviewed. From these, we confirmed or modified the prescription of CPAP based on the efficacy of each pressure to obtain an optimal setting, which was used thereafter. ST adherence was evaluated over the entire 2-week period (during which time pressures were varying). In 19 patients, only one pressure (optimal determined by physician) was used for the entire 2-week period due to unavailability of the CPAP device with programming capabilities. CPAP adherence was defined as the total time that airflow was observed visually at the prescribed pressure.
In a minority of patients (n = 14), side effects such as improper fitting of mask, dryness requiring humidification, or travel preventing CPAP use needed to be addressed, and collection of ST data was initiated only after these complaints were addressed. Despite the fact that pressure fluctuated around the optimal pressure, “ST adherence” was defined as the average hours of use from all nights, regardless of pressure, including nights in which the patient chose to not use CPAP. In order to evaluate the effect of varying CPAP pressure across nights in a patient (in those subjected to these changes), we also calculated the hours of use at each separate pressure. Figure 2 plots hours of use against the deviation from the optimal pressure and shows there was no trend for an effect of pressure on CPAP adherence. This allowed us to pool these data, providing a single value per subject over all pressures to define ST adherence. After ST data collection, patients were switched to a commercial CPAP generator (with conventional adherence monitoring) set to the therapeutic CPAP pressure, and no further changes were made in CPAP pressure delivered.
Figure 2.
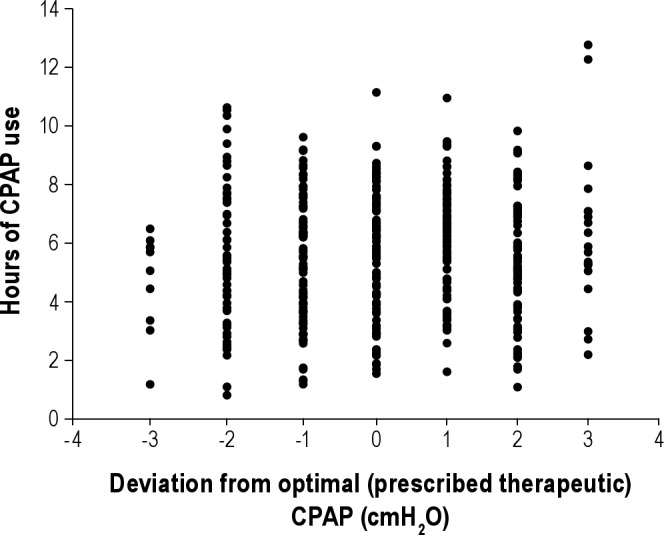
Graph plots data of pressure deviation from therapeutic pressure on the x-axis versus CPAP use (h) at that pressure in patients who were subjected to multiple pressures (± 2-3 cm H2O above or below) during the short-term adherence period. No relationship was seen between adherence and pressure (r = 0.07, p = ns).
LT adherence was obtained in only 56 patients due to the design of the parent study, which was intended to evaluate for follow-up only those patients who showed adequate ST CPAP use. Patients without LT adherence data collected include one patient who withdrew from the study, 2 patients who underwent bariatric surgery and 34 patients who were excluded because they showed zero hours of use (13 patients) or < 2 h average use per night (21 patients) on the ST adherence monitoring. Towards the end of the study enrollment period, these criteria were relaxed, and 4 patients with ST adherence < 2 h average per night were restudied at LT. All statistical tests were run with and without these 4 patients included, with no effect on the results. All of the LT data reported in the results section includes the 4 patients.
Measurement of Sleepiness
Subjective assessment of sleepiness consisted of the Epworth Sleepiness Scale (ESS),33 and the Functional Outcomes of Sleep Questionnaire (FOSQ).34 Objective tests consisted of a 20-min psychomotor vigilance task (PVT)35 and the multiple sleep latency test (MSLT).36 The objective tests were administered 4 times across the day at 2-h intervals beginning at 09:00. Average PVT lapses (transformed as √lapses + √(lapses + 1) and mean sleep latency were calculated as the average from the 4 tests.
Data Analysis
Sleep stages, arousals, periodic leg movements, and respiratory events (apneas, hypopneas, respiratory effort-related arousals [RERAs]) were scored by a single technician (in order to minimize interscorer variability) using American Academy of Sleep Medicine guidelines.32 In particular, hypopnea was defined by the “preferred” AASM definition: airflow reduction > 30% of baseline and associated with a 4% drop in oxygen saturation. AHI4% was defined as the sum of apneas and hypopneas divided by the total sleep time (TST). RDI was defined as the sum of apneas, hypopneas, and RERAs divided by the TST. Summary data included conventional methods for quantifying the quality of sleep (e.g., TST, sleep efficiency, time and %TST spent in sleep stages and wake). We also evaluated sleep continuity using the survival analysis technique described by Norman et al. and calculated the average run length of continuous sleep defined as the time (in minutes) between the first occurrence of stage 1 and the return to stage wake or stage 1 (if stage N2 sleep was observed).31
Comparisons between diagnostic, titration, and follow-up NPSG parameters were made using paired t-tests. ST and LT adherence were compared by Pearson correlation analysis. Significance of this relationship was tested at the 0.05 level, as it was the primary predetermined variable of the study.
For other continuous variables, we examined the relationship between each variable and ST adherence, and similarly each variable and LT adherence, using Pearson correlation coefficients. In addition, the 93 patients who underwent ST assessment were divided into 3 groups as per usual clinical practice based on the average ST hours of CPAP use (< 2 h, 2-4 h, > 4 h) and compared using ANOVA. Data are presented as mean ± standard deviation and data analyses were conducted using SPSS 17. The patients who underwent LT adherence assessment were divided into 2 groups (< 4 h and > 4 h), as there were only 4 patients with LT adherence < 2 h, and differences between groups were examined using independent sample t-tests. We applied χ2 tests to compare frequencies and proportions. For all comparisons (other than between ST and LT adherence), we used a significance level of < 0.005 due to the large number of variables examined. The term “trend” is used when the p values were between 0.05 and 0.005.
The total data set (n = 93) consisted of 61 male and 32 female patients, with body mass index (BMI mean ± SD): 35.7 ± 9.6 kg/m2, age: 47.9 ± 11.7 years (range = 56). Racial breakdown was as follows: 40% of patients were White, 27% Black, 7% Asian, and 26% not reported/other. Ethnicity was 19% Hispanic.
RESULTS
Table 1 shows summary data for the entire group for SDB indices and sleep architecture variables obtained from the diagnostic, CPAP titration (before short-term compliance assessment) and final follow-up (after long-term compliance assessment) laboratory NPSG on therapeutic CPAP. As expected, SDB and sleep architecture variables improved significantly from the baseline diagnostic NPSG to the CPAP titration NPSG. In addition, from the CPAP titration NPSG to the final CPAP NPSG, AHI4% and RDI decreased and changes were observed in %N2 and %N3 sleep. We did not analyze TST or change in TST among the 3 NPSGs, as this variable is confounded by the design of our protocol: on the CPAP titration NPSG patients were allowed to leave as early as 07:00 (at their discretion), whereas on the diagnostic and final follow-up CPAP NPSG, they were encouraged to sleep ad libitum because they were expected to remain in the laboratory for daytime testing. Thus, any prolongation of TST on the final NPSG could have reflected the longer time in bed than the CPAP titration night.
Table 1.
Demographic, sleep, and SDB variables in all subjects from diagnostic, titration, and follow-up NPSGs (Mean ± SD)
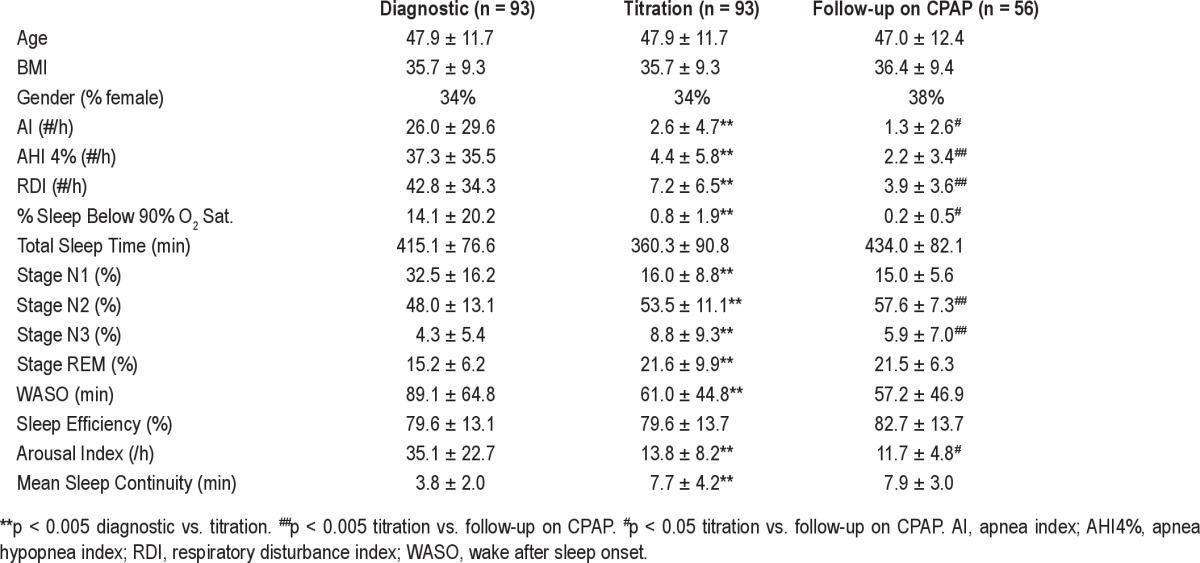
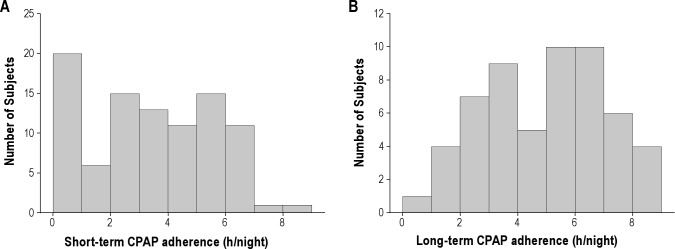
Table 2 and Figure 3A show average ST CPAP adherence data in the 93 patients. The average ST adherence was 3.2 ± 2.3 h (range = 8.6 h). Thirteen of these patients had zero hours of short-term use. Average ST adherence excluding these 13 patients was 3.9 ± 1.1 h/night. The number of days on which CPAP was used (> 0 h) was correlated (r = 0.73, p < 0.001) to the average hours of use per day, supporting the use of the latter as our metric of adherence. Subjects with poor (< 2 h), moderate (2-4 h), or adequate (> 4 h) CPAP usage did not differ in age, BMI, gender, race, or level of therapeutic CPAP. Table 2 and Figure 3B also show average LT CPAP adherence in the 56 patients who completed this part of the protocol. The average LT adherence was 4.8 ± 2.1 h (range = 8.6 h). For these same 56 patients, the ST adherence had been 4.4 ± 1.8 hours. Subjects with moderate (< 4 h) and adequate (> 4 h) CPAP usage did not differ in age, BMI, or gender.
Table 2.
Short-term and long-term adherence
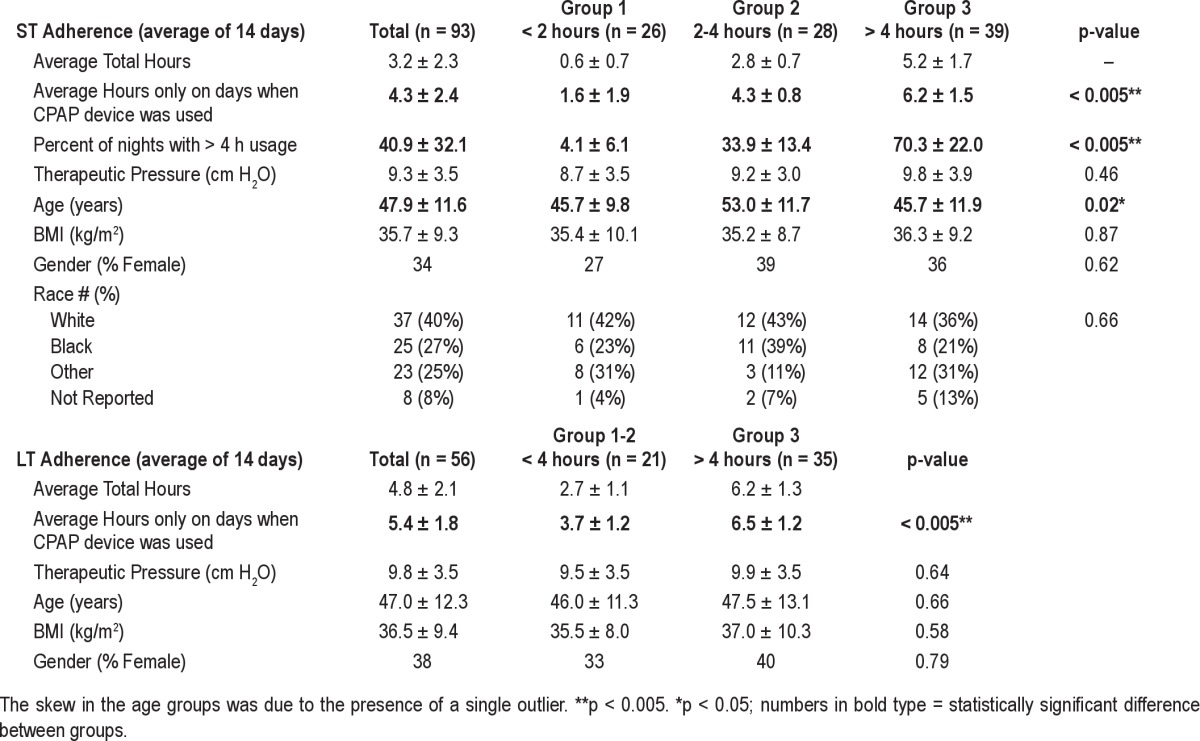
Figure 3.
Number of patients grouped by CPAP adherence is shown for (A) short-term (n = 93) and (B) long-term (n = 56) data.
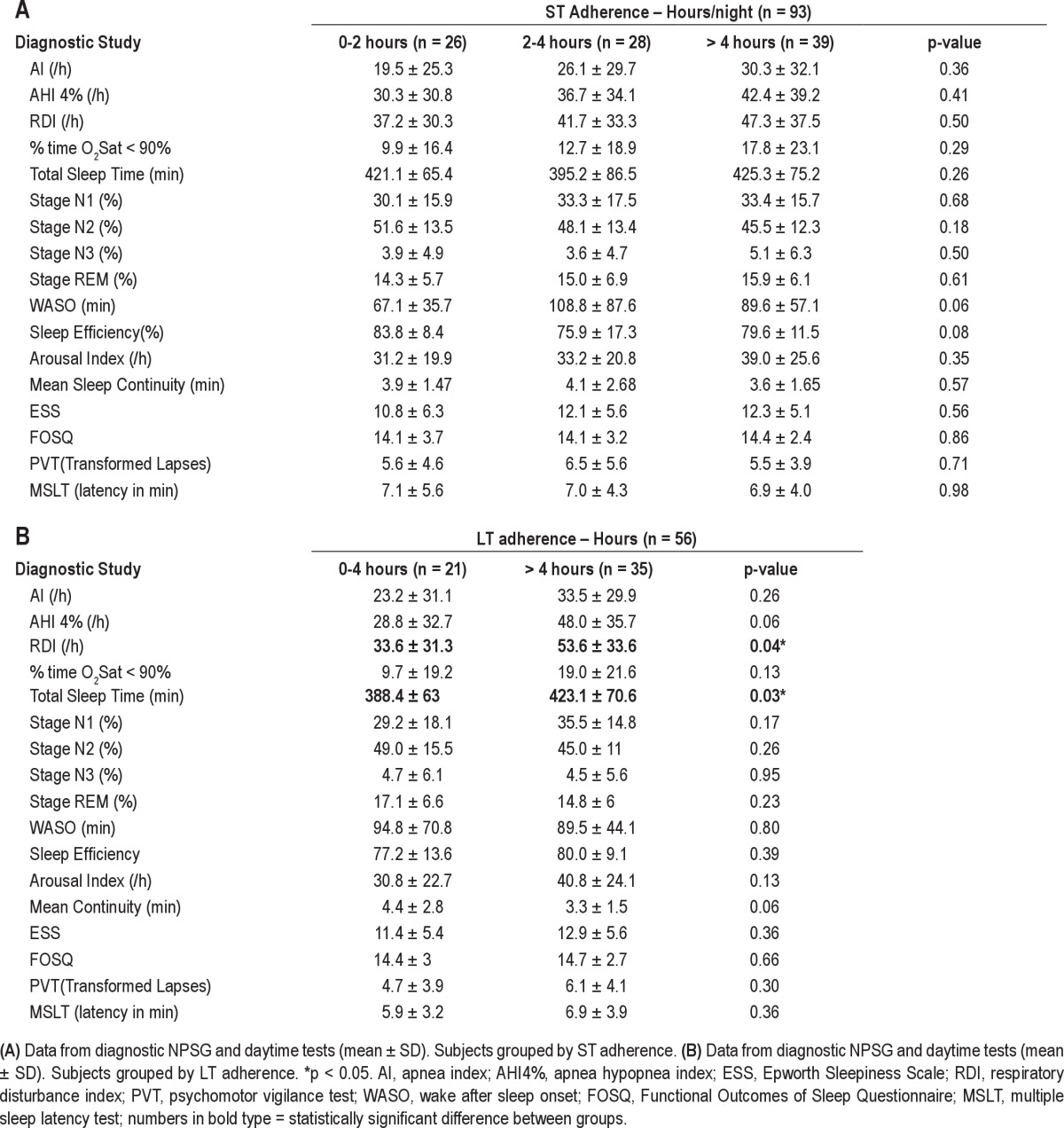
Table 3A shows data from the initial diagnostic NPSG and the concurrent daytime assessments. Groups of poor, moderate, and adequate ST users did not show statistically significant differences in SDB, sleep architecture, or daytime outcome variables. Moderate and adequate LT CPAP users are compared in Table 3B. Although no statistically significant differences (assessed at p < 0.005) were seen for SDB, sleep architecture, or daytime outcome variable, an interesting trend (p = 0.03) suggests that poor LT adherence was associated with shorter TST and lower RDI (p = 0.04) on the diagnostic laboratory NPSG.
Table 3.
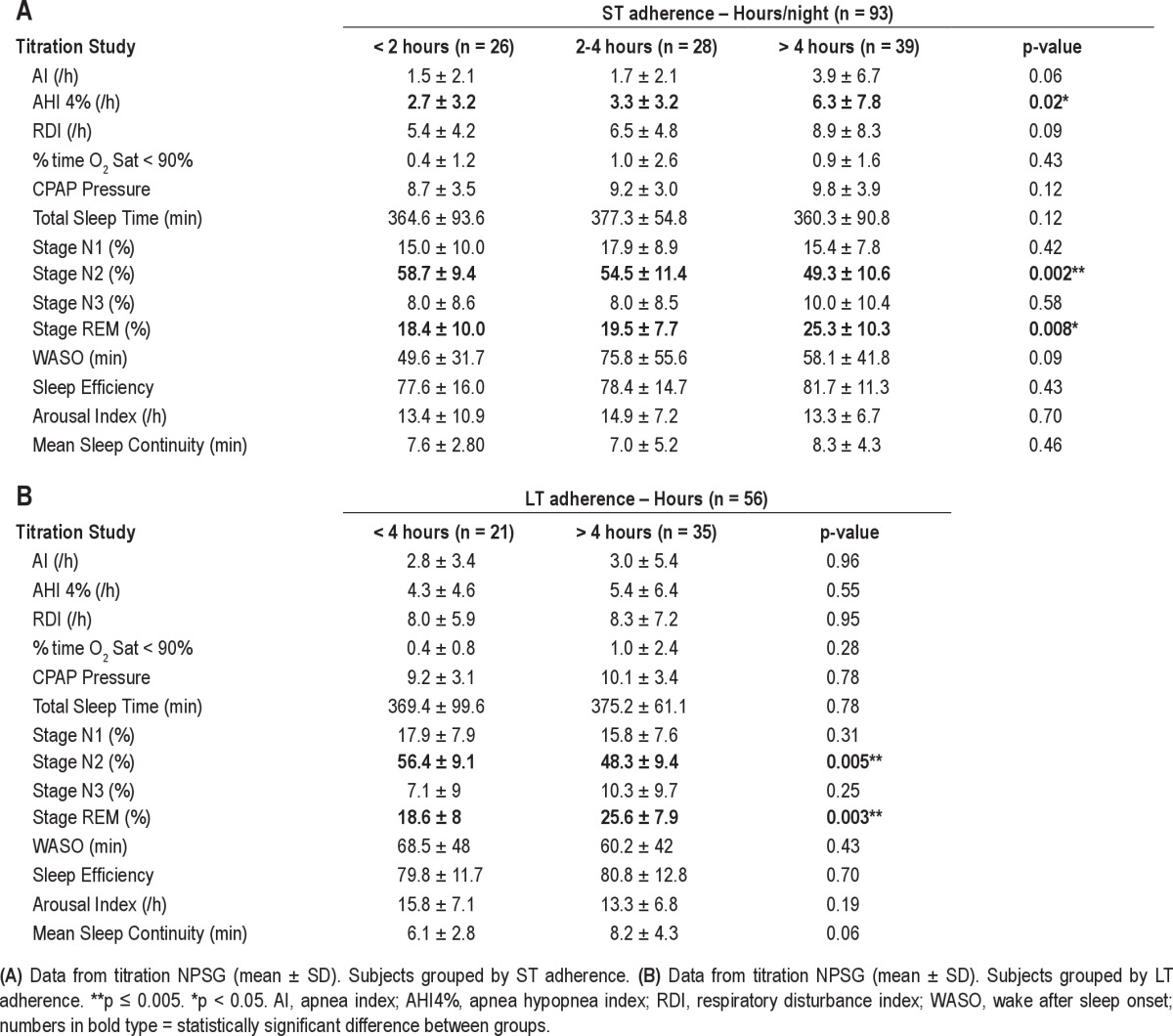
Tables 4A and 4B show data from the titration NPSG for poor, moderate, and adequate CPAP user groups in the short-term, and moderate and adequate user groups in the long-term, respectively. These data show there was no difference between the groups in TST on the titration NPSG, despite the differences in their home usage, suggesting that under supervision all patients were able to use CPAP therapy similarly, and that this did not clearly reflect variation in their usage in the home. However, there was a statistically significant difference observed between the adherence groups for %time in stage N2 sleep (p = 0.002) on the titration NPSG. There was also a trend for adequate CPAP users to have a higher %time in REM sleep (p = 0.008) on the titration NPSG. Table 4B shows the same titration NPSG data broken down when the patients were grouped by their long-term CPAP usage. Differences between LT adherent groups in %Stage N2 (p = 0.005) and in %REM sleep (p = 0.003) on the titration NPSG are still evident. Sleep continuity (mean duration to arousal) on the titration NPSG was greater in the adequate LT adherence group than the moderate adherence group, but this did not reach statistical significance (p = 0.061). All results were unchanged when the ST adherence data were analyzed combining poor and moderate ST adherence groups (n = 54) compared to subjects with adequate CPAP use (n = 39).
Table 4.
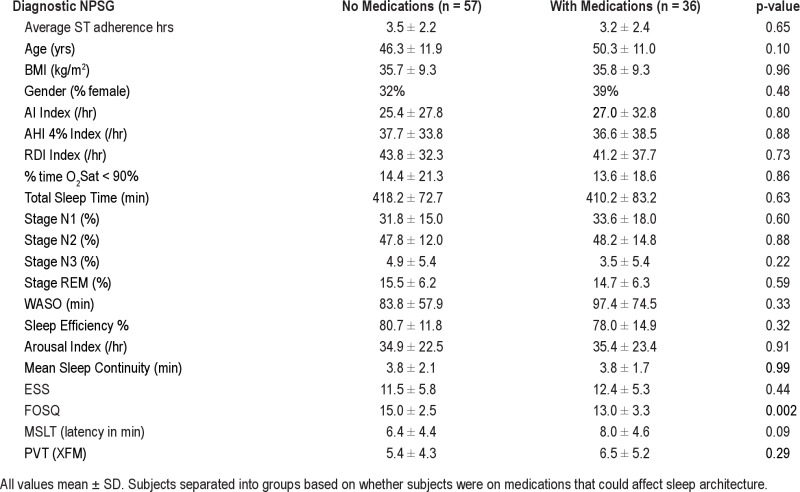
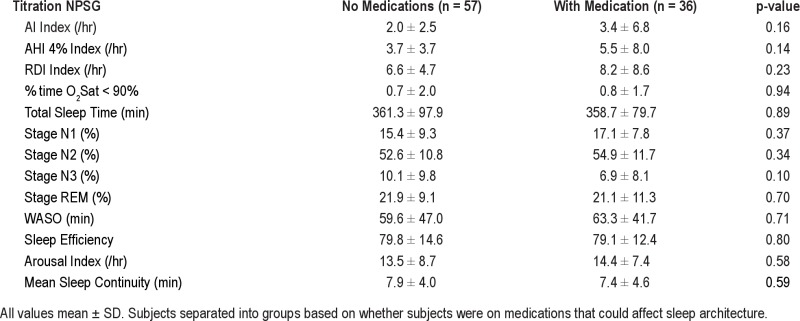
Thirty-six of the 93 subjects were on medications that could have affected sleep architecture (SSRIs, β-blockers, anti-psychotic agents, and anti-epileptic agents), particularly the duration of REM sleep. However, we found no difference between the 3 ST adherence groups, in the number of subjects who were on medications (46%, 36%, and 36%, respectively, p = 0.45). Interestingly, and contrary to our expectation, we also found no significant differences in any sleep variables between the subjects with or without medications on the diagnostic and titration NPSGs (data in supplement Tables S2A and S2B). The only medication-related significant finding in our data was that those subjects on medication had a worse FOSQ score than those without medications. Despite our inability to show a difference in sleep architecture related to medication use, we did re-analyze our data excluding the 36 subjects who were on medications; for the remaining 57 subjects we showed the same findings (or trends) in sleep percentages between ST adherence groups (lower %N2 and trend for higher %REM, higher sleep efficiency and sleep continuity on the CPAP titration night in the adequate users compared to the poor CPAP users data in supplement Tables S3A and S3B).
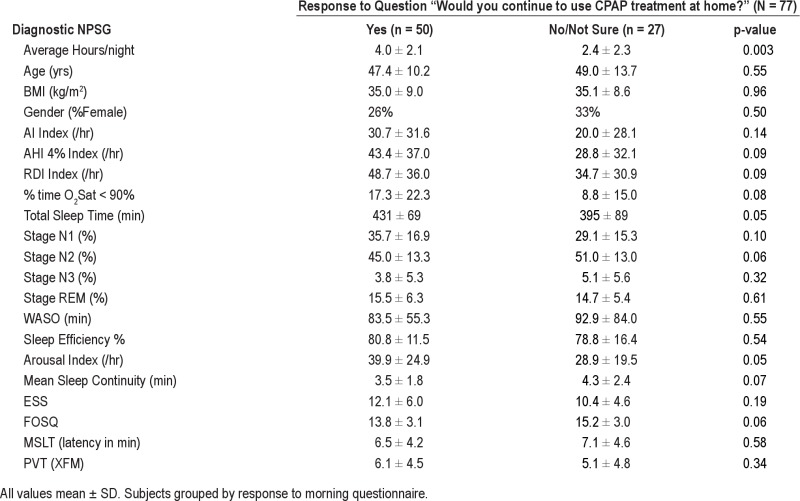
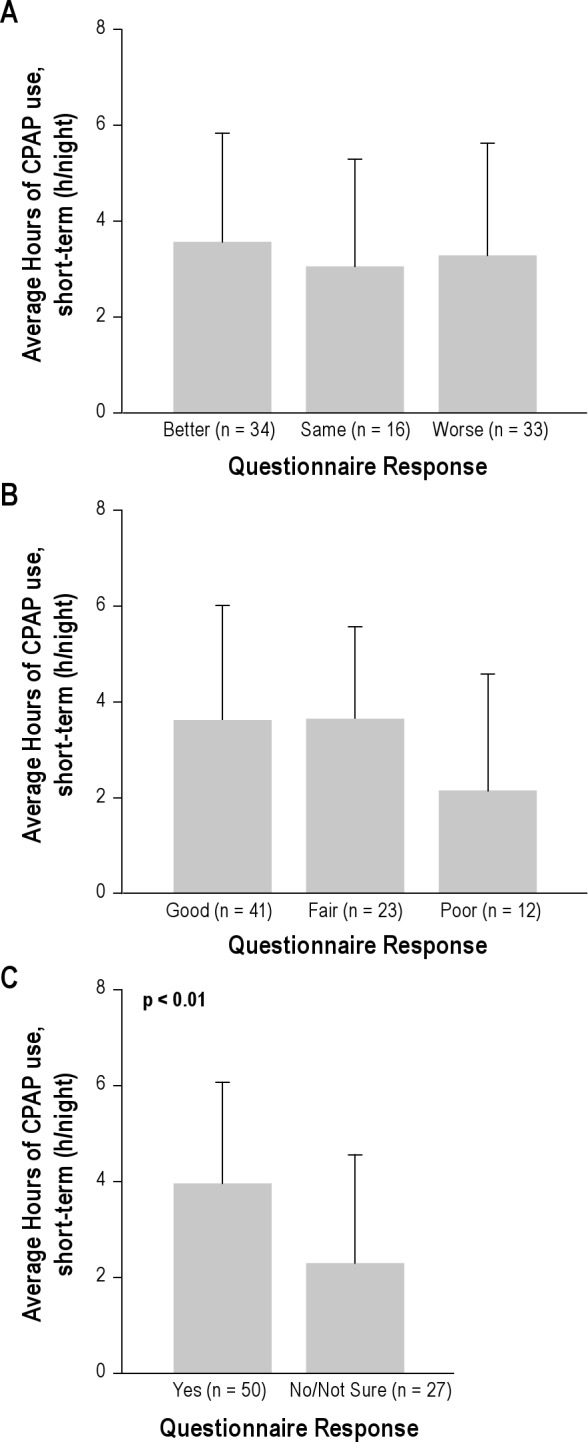
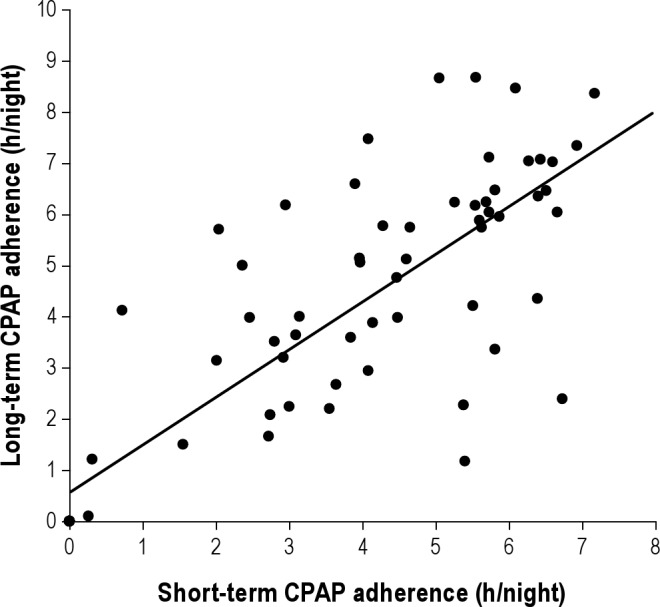
Figure 4 examines the impact of subjective patient assessment of satisfaction with CPAP on the morning after the CPAP titration on ST CPAP adherence in the 85/93 patients who filled out the morning questionnaire. ST CPAP adherence was not different when patients were separated into groups by their answers to 2 questions “How would you rate your sleep after using CPAP?” and “How would you rate your CPAP treatment?” However, answering “yes” to “Would you like to continue to use CPAP therapy at home?” was associated with higher ST CPAP adherence (4.0 ± 2.1 h/night vs. 2.1 ± 2.2 h/night, p < 0.001). When subjects were grouped based on response to this question (Tables S4A and S4B in supplement) no differences were observed in demographics or on the diagnostic NPSG. Those subjects who reported willingness to use CPAP also had significantly longer TST, greater %REM, and better sleep efficiency on the CPAP titration NPSG. Figure 5 shows that the better short-term CPAP users tended to continue on to become better long-term users; i.e., there was a significant correlation between ST and LT home CPAP adherence (r = 0.81, p < 0.001). Because this correlation was performed including subjects with zero CPAP usage, we repeated the analysis with these subjects excluded and were still able to show a significant relationship (r = 0.61, p = 0.05; not shown on figure).
Figure 4.
Graph shows short-term CPAP adherence (x-axis) plotted against long-term adherence (y-axis) (r = 0.81, p < 0.001). Each data point represents one subject.
Figure 5.
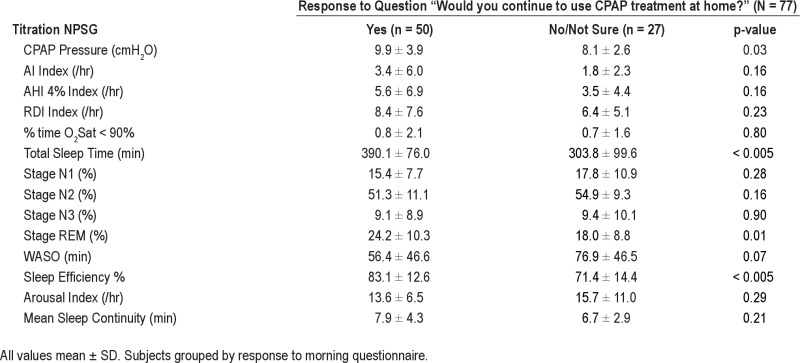
(A) The graph shows the ST adherence grouped by subject responses to the question “How would you rate your sleep after using CPAP?” the morning after the titration study. No significant differences were seen between groups. (B) The graph shows the ST adherence grouped by subject responses to the question “How would you rate your CPAP treatment?” the morning after the titration study. No significant differences were seen between groups. (C) The graph shows the ST adherence grouped by subject responses to the question “Would you like to continue to use CPAP therapy at home?” The group that answered “yes” had a significantly (p < 0.001) higher CPAP adherence that the group who answered “No/Not Sure.” The graphs shows the mean and SD of ST CPAP adherence in each group.
In order to assess the relationship between CPAP adherence and the change in sleep parameters from diagnostic to titration NPSG (Δ = titration NPSG value minus diagnostic NPSG value), we examined these for each CPAP adherence group. In the ST data, no Δvariable was significantly different between groups, although ΔSleep_Efficiency showed a trend (−6.2% ± 16.4% for < 2-h adherence, +2.5% ± 16.4% for 2- to 4-h adherence, +2.1% ± 11.7% for > 4-h adherence, p = 0.045). In the LT data, only Δ%REM (+1.5% ± 10.0% for < 4 h, +10.4% ± 9.8% for > 4 h, p = 0.002) was significantly different between groups, with the adequate users showing a bigger change in %REM between diagnostic and titration NPSG.
We also examined the strength of a linear regression model predicting ST and LT adherence from all sleep and SDB variables obtained on the titration NPSG. We found that the %N2 sleep on the titration NPSG had a significant correlation to both ST adherence (r = 0.32, p = 0.002) and LT adherence (r = 0.38, p = 0.001), and addition of other variables did not improve the fit beyond that of a model with %N2 alone. We further examined models using the Δ in sleep variables between titration and diagnostic NPSGs. No significant models could be found that adequately predicted ST adherence. A model combining ΔSleep_Continuity (r = 0.29) and ΔSleep_Efficiency (r = 0.23) predicted LT adherence (r2 = 0.17, p = 0.006), although it did not satisfy the null-hypothesis α limit of 0.005 for significance.
DISCUSSION
Our data confirm that there is a significant relationship between short-term and long-term CPAP adherence,7,29,30 and that short-term CPAP use observed in the first two weeks at home is largely predictive of long-term adherence. Importantly, we were able to demonstrate a new finding: variables addressing aspects of sleep architecture (%N2 and %REM) on the titration NPSG were correlated to short-term and long-term CPAP home use. Sleep continuity on the titration NPSG did not correlate with either ST or LT adherence. However, in subjects with LT adherence data, a regression model combining change in sleep continuity and change in sleep efficiency between the diagnostic and titration nights was predictive of LT adherence (r2 = 0.17, p = 0.006). These findings are in accord with and supplement published findings,17,37 which demonstrate that characteristics of sleep, even on the CPAP titration NPSG, can help predict long-term adherence.
Despite observing the expected improvement in SDB and all sleep parameters on CPAP, the overall mean ST adherence was 3.2 h and the LT adherence (which excluded most of the patients with ST adherence < 2 h) was 4.8 hours. Although these values are slightly lower than average adherence in some studies27,28 they are in the same range as most studies reporting CPAP adherence.19,38,39
The only differences we found between adequate and poor ST CPAP adherents were that the good users had lower %N2 sleep and higher %REM sleep on the titration laboratory NPSG. At the present time there is no consensus as to the underlying purpose of sleep, and thus it is difficult to define “better” sleep quality without assessing a behavioral outcome. In the sleep literature it is often assumed that increases in %REM and %N3 sleep, even within the normal range, may suggest “better” sleep. Both REM disruption and a reduction in %REM and %N3 sleep are generally seen in diseases that reduce the well-being of a subject through sleep-disrupting mechanisms, with corresponding increases in other stages of sleep. This pattern has been invoked as a measure of poor sleep quality in aging40 and insomnia literature,41 and also when describing the “first-night” effect in the laboratory. Furthermore in CPAP-treated OSA, increases in %REM and/or %N3 sleep are generally interpreted as indicating a beneficial effect of CPAP on sleep. The effect of CPAP on %REM sleep was seen in our own data (Table 1 comparing CPAP NPSGs to the diagnostic NPSG). The average %REM sleep on the titration CPAP night was within the normal range, but was highest in the subjects with the highest ST CPAP adherence. We interpret this as consistent with the idea that these particular subjects may have had better sleep on CPAP than those who eventually showed lower CPAP adherence.
With the above assumptions about sleep quality, our data suggest “better sleep” on the titration night is related to better home CPAP adherence, but this observation is consistent with at least two interpretations: (1) Subjects with a “better” first night on CPAP may be more likely to continue using CPAP because of the good quality of their first exposure to CPAP (or, stated conversely, poor initial sleep caused by CPAP discomfort during early use limits CPAP adherence). Alternatively, (2) Subjects with better sleep pre-CPAP may be less susceptible to the discomfort of CPAP use (or, stated conversely, preexisting poor sleep limits CPAP adherence). Because we cannot separate the impact of CPAP on quality of sleep from CPAP's effect on the underlying SDB, our data do not allow us to distinguish between these two possible explanations. Only knowing the patients' quality of sleep prior to their having SDB would directly address this issue.
Our findings are in agreement with the Drake et al.17 who reported that a patient's initial experience with CPAP (and the degree of improvement in sleep efficiency on the titration) was a significant predictor of future CPAP adherence. Lewis et al.42 also showed that those patients who reported problems on the first night of CPAP showed worse CPAP adherence. In support of the hypothesis that better sleep on the titration night predicts better adherence, Colleen et al.43 and Lettieri et al.37 showed that use of a sedative hypnotic on the titration study night was associated with longer TST and higher sleep efficiency on that night and was also a significant predictor of higher short-term CPAP adherence. These authors suggest that “medication use may improve the patients' overall experience in the sleep laboratory setting.”
Previous studies have produced conflicting data about the relationship of age, gender, degree of sleepiness, and severity of SDB with short or long-term CPAP use.13,20,44–48 Our data, which include subjects with a wide range of SDB severity and sleepiness as well as wide ranging racial and gender distribution, are in agreement with studies that showed no relationship between these demographic variables and short-term or long-term CPAP use. In our dataset with 27% Black subjects, we failed to show a difference in ST CPAP adherence based on race (Black subjects: 3.2 ± 2.0 h/night vs White subjects: 3.2 ± 2.4 h/night, p = 0.99). Other studies have showed significantly reduced CPAP adherence in black subjects,30,46,47 although most of these studies did not report socioeconomic status, which has been shown to be an independent predictor of CPAP adherence regardless of race.45,49
In our dataset, patients who stated that they would not or were not sure they would use CPAP following their titration night had significantly lower ST adherence: adherence in these subjects was almost 50% less than in those who stated that they would use CPAP (2.1 vs. 4.0 h/night). Of note, these same patients had significantly lower TST and sleep efficiency on the titration laboratory NPSG (sleep efficiency 71% ± 14% for “no/not sure” vs. 83% ± 13% for “yes,” p < 0.001). These data show that the initial subjective, as well as objective, experience with CPAP (positive or negative) is associated with subsequent adherence. The questionnaire responses may be related to the domain of self-efficacy and our finding is in keeping with some recent studies examining psychological factors that include patient's beliefs and perceptions of OSA and CPAP (risk perception, outcome expectancies, and self-efficacy) that have been shown to explain up to 25% of the variance in CPAP adherence. However, we are not able to distinguish whether poor sleep while on CPAP caused poor adherence or a patient's preconception against CPAP caused both poor sleep on the titration night and poor adherence. One potential implication of the relationship between sleep quality on the CPAP titration night and CPAP adherence is that home CPAP titration might produce “better” sleep and therefore enhance CPAP adherence. However, there are conflicting results regarding whether CPAP adherence is higher or lower when home titration is performed compared to in-laboratory titration,50–52 and the present data do not allow us to address this issue directly.
The strengths of our study are that data were obtained in a group of subjects with a wide range of severity of SDB, with objective monitoring of both the efficacy of the pressure and of usage. All subjects were given comprehensive education about their need for CPAP and were encouraged to use it with intense feedback and follow-up from a research coordinator. Changes to masks and need for humidity were attended to promptly prior to data collection. While there is some evidence in the literature that sub-therapeutic pressures can influence adherence, it is also possible that excessive pressure may impact adherence.44 In our study, we titrated therapeutic CPAP to the lowest effective pressure using multiple full nights of home monitoring at varying pressures above and below the therapeutic prescription pressure in the 2 weeks following the in-laboratory CPAP titration NPSG. Thus “optimal” CPAP was ensured for the long-term treatment and poor titration is not likely to have affected adherence. In a subset of patients there was a delay in implementation of CPAP that could have affected ST adherence results (n = 14 subjects where delay was > 2 weeks). However, there were no demonstrable differences in age, BMI, gender, ST, and LT hours between this subset and the remaining subjects.
Our study also had limitations. First, we did not obtain information on psychosocial factors that could have impacted adherence. Social factors such as socioeconomic status, social support, and partner involvement have been shown in some studies to affect adherence, although not in a simple manner.42,45,53 Similarly, psychological factors have also been shown to predict adherence in many studies,18–20 although not in all.47,49 Most of the current literature focuses on the use of psychological interventions to promote CPAP usage (CBT,54 motivational interviewing55), but these are time and resource intensive, so that identifying who would or would not benefit from these interventions may be useful. Our data focus on the earliest possible identification of these subjects using measurements routinely collected in a sleep laboratory for clinical purposes (e.g., the CPAP titration NPSG).
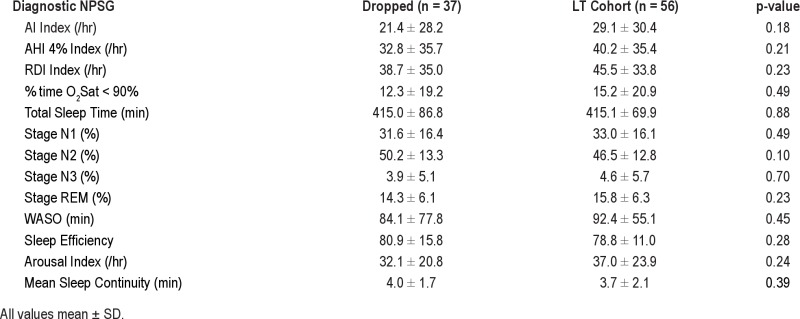
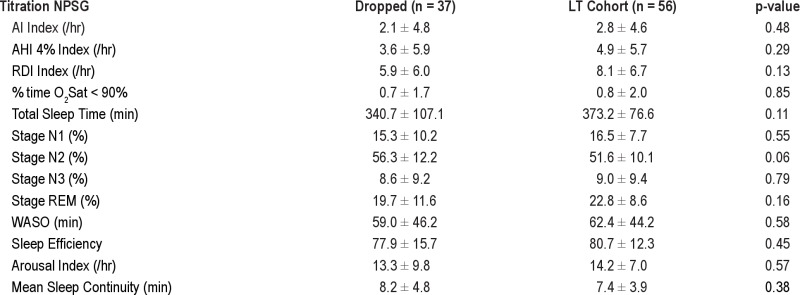
Second, LT data were not collected in all patients due to the study design of the parent protocol. However, there were no differences in demographic or diagnostic NPSG data between those subjects who did not have LT data and those who were studied long term (see supplemental data Table S1A and S1B). On the titration night, there was also a trend to worse sleep (more %N2 and less %REM) in those who did not complete the LT data collection; this would be consistent with our adherence result if all of these ST dropout subjects went on to be poor LT users (as opposed to just not being restudied).
Although we show a statistically significant correlation of adherence to sleep variables on the titration NPSG, the proportion of explained variance of this finding is small (5% to 15%). Thus, adherence to CPAP appears to be driven primarily by additional factors beyond those seen on the PSG. This is in accord with work on adherence to other medical therapies, suggesting that physiological disease may be less important in determining adherence to therapy than psychosocial factors.56 It is difficult to say from our data whether good sleepers become good CPAP users or whether initial CPAP tolerance and good sleep on the titration night promotes LT adherence. If therapeutic interventions to improve adherence are to be considered, separating these causal pathways to CPAP adherence has implications beyond making a prediction of LT adherence. In the second scenario, an intervention could be targeted at improving sleep during the titration night (e.g., giving sedatives or anxiolytics), whereas in the former, the goal would be to improve sleep independent from the CPAP intervention (e.g., by treating insomnia itself, as by using CBT prior to a CPAP trial or to rescue poor CPAP users). In either case, the close correlation between ST and LT adherence and the difficulty in predicting who will be poorly compliant before the first exposure to CPAP suggests that improvements to CPAP adherence are most likely to come from interventions before, rather than after a patient identifies him/herself as poorly adherent. This, of course, does not apply to interventions aimed at specific conditions related to CPAP discomfort that appear when therapy is initiated (e.g., mask fit, nasal congestion), but, by the above reasoning, the data we and others have collected suggest that interventions after the first exposure to CPAP may not be the only method to impact on LT CPAP adherence.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Norman holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher – Paykel Healthcare, Advanced Brain Monitoring and Tyco (Health C'Aire). Dr. Rapoport has received support for research from Fisher – Paykel Healthcare, Ventus Medical; speaking and consulting engagements for Fisher – Paykel Healthcare. He holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher – Paykel Healthcare, Advanced Brain Monitoring and Tyco (Health C'Aire). Dr. Indu Ayappa has received support for research Fisher – Paykel Healthcare and Ventus Medical. She holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher – Paykel Healthcare and Advanced Brain Monitoring. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of Rakhil Kanevskaya and Ming Chen. Support for this study was provided by NIH grants R01HL81310, 1 UL1RR029893, and grants from the Foundation for Research in Sleep Disorders.
ABBREVIATIONS
- OSAHS
obstructive sleep apnea hypopnea syndrome
- SDB
sleep disordered breathing
- AHI
apnea hypopnea index
- EDS
excessive daytime somnolence
- CPAP
continuous positive airway pressure
- NPSG
nocturnal polysomnogram
- RDI
respiratory disturbance index
- AI
apnea index
- N1
sleep stage N1
- N2
sleep stage N2
- N3
sleep stage N3
- WASO
wake after sleep onset
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- PVT
psychomotor vigilance test
- MSLT
multiple sleep latency test
- TST
total sleep time
- ST
short-term
- LT
long-term
- BMI
body mass index
SUPPLEMENTAL MATERIAL
Diagnostic NPSG data comparing subjects included in the LT cohort versus the subjects who dropped out
Titration NPSG data comparing subjects included in the LT cohort versus the subjects who dropped out
Data from diagnostic NPSG and daytime tests
Data from titration NPSG
Data from Diagnostic NPSG and daytime tests in 57 subjects with no medications
Data from Titration NPSG in 57 subjects with no medications
Data from Diagnostic NPSG and daytime tests
Data from Titration NPSG
REFERENCES
- 1.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20:608–13. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 7.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 8.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149:149–54. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 9.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal L, Gerhardstein R, Lumley A, et al. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med. 2000;1:215–20. doi: 10.1016/s1389-9457(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 12.Yetkin O, Kunter E, Gunen H. CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep Breath. 2008;12:365–7. doi: 10.1007/s11325-008-0188-4. [DOI] [PubMed] [Google Scholar]
- 13.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier-Fleury N, Rakotonanahary D, Fleury B. The age and other factors in the evaluation of compliance with nasal continuous positive airway pressure for obstructive sleep apnea syndrome. A Cox's proportional hazard analysis. Sleep Med. 2001;2:225–32. doi: 10.1016/s1389-9457(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 15.Li HY, Engleman H, Hsu CY, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28:1554–9. doi: 10.1093/sleep/28.12.1554. [DOI] [PubMed] [Google Scholar]
- 16.Nakata S, Noda A, Yagi H, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43:296–9. [PubMed] [Google Scholar]
- 17.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- 18.Aloia MS, Arnedt JT, Stepnowsky C, Hecht J, Borrelli B. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med. 2005;1:346–53. [PubMed] [Google Scholar]
- 19.Stepnowsky CJ, Jr., Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3:239–47. doi: 10.1016/s1389-9457(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 20.Sawyer AM, Canamucio A, Moriarty H, Weaver TE, Richards KC, Kuna ST. Do cognitive perceptions influence CPAP use? Patient Educ Couns. 2011;85:85–91. doi: 10.1016/j.pec.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen S, Smith S, Oei T, Douglas J. Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J. 2008;32:710–7. doi: 10.1183/09031936.00127507. [DOI] [PubMed] [Google Scholar]
- 22.Jean Wiese H, Boethel C, Phillips B, Wilson JF, Peters J, Viggiano T. CPAP compliance: video education may help! Sleep Med. 2005;6:171–4. doi: 10.1016/j.sleep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997;20:284–9. doi: 10.1093/sleep/20.4.284. [DOI] [PubMed] [Google Scholar]
- 24.Likar LL, Panciera TM, Erickson AD, Rounds S. Group education sessions and compliance with nasal CPAP therapy. Chest. 1997;111:1273–7. doi: 10.1378/chest.111.5.1273. [DOI] [PubMed] [Google Scholar]
- 25.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med. 1999;159:1096–100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 26.Massie CA, McArdle N, Hart RW, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 27.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33:523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepin JL, Muir JF, Gentina T, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest. 2009;136:490–7. doi: 10.1378/chest.08-2646. [DOI] [PubMed] [Google Scholar]
- 29.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5:229–40. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- 30.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–4. [PubMed] [Google Scholar]
- 31.Norman RG, Scott MA, Ayappa I, Walsleben JA, Rapoport DM. Sleep continuity measured by survival curve analysis. Sleep. 2006;29:1625–31. doi: 10.1093/sleep/29.12.1625. [DOI] [PubMed] [Google Scholar]
- 32.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 35.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh Res Method Instr Comp. 1985;17:652–5. [Google Scholar]
- 36.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 37.Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136:1263–8. doi: 10.1378/chest.09-0811. [DOI] [PubMed] [Google Scholar]
- 38.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushida CA, Berry RB, Blau A, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34:1083–92. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 41.Riemann D, Spiegelhalder K, Nissen C, Hirscher C, Baglioni C, Feige B. REM sleep instability-a new pathway for insomnia? Pharmacopsychiatry. 2012;45:167–76. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- 42.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 43.Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest. 2009;135:704–9. doi: 10.1378/chest.08-2182. [DOI] [PubMed] [Google Scholar]
- 44.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21:419–26. doi: 10.1111/j.1365-2869.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 49.Bakker JP, O'Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep. 2011;34:1595–603. doi: 10.5665/sleep.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Means MK, Edinger JD, Husain AM. CPAP compliance in sleep apnea patients with and without laboratory CPAP titration. Sleep Breath. 2004;8:7–14. doi: 10.1007/s11325-004-0007-5. [DOI] [PubMed] [Google Scholar]
- 51.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 52.Cross MD, Vennelle M, Engleman HM, et al. Comparison of CP AP titration at home or the sleep laboratory in the sleep apnea hypopnea syndrome. Sleep. 2006;29:1451–5. doi: 10.1093/sleep/29.11.1451. [DOI] [PubMed] [Google Scholar]
- 53.Baron KG, Smith TW, Berg CA, Czajkowski LA, Gunn H, Jones CR. Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep Breath. 2011;15:525–34. doi: 10.1007/s11325-010-0374-z. [DOI] [PubMed] [Google Scholar]
- 54.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30:635–40. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 55.Olsen S, Smith SS, Oei TP, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol. 2012;80:151–63. doi: 10.1037/a0026302. [DOI] [PubMed] [Google Scholar]
- 56.Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162:412–24. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic NPSG data comparing subjects included in the LT cohort versus the subjects who dropped out
Titration NPSG data comparing subjects included in the LT cohort versus the subjects who dropped out
Data from diagnostic NPSG and daytime tests
Data from titration NPSG
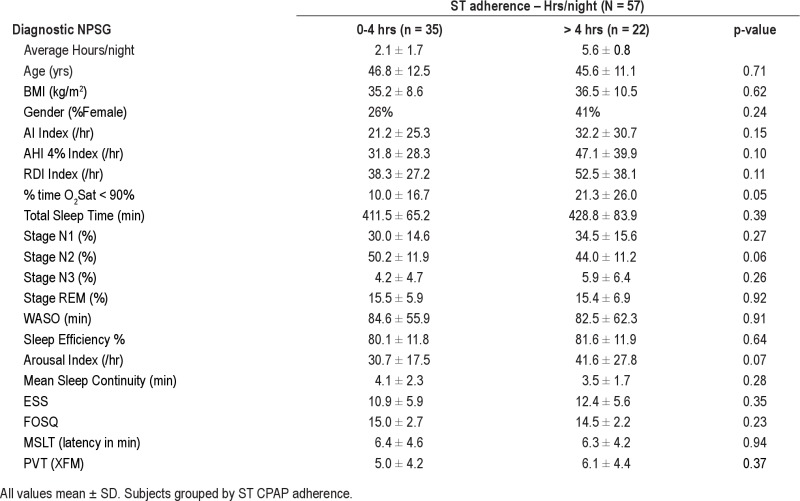
Data from Diagnostic NPSG and daytime tests in 57 subjects with no medications
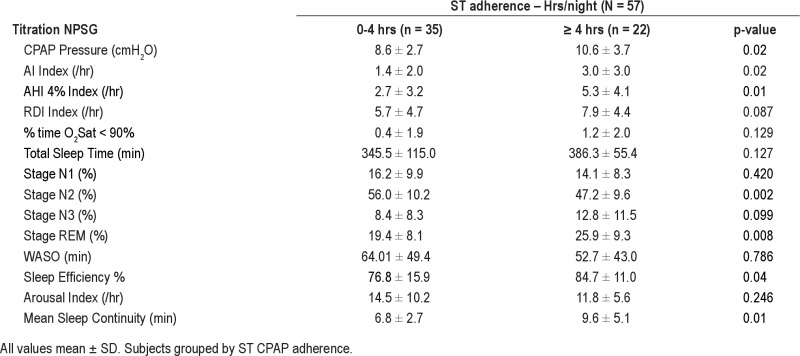
Data from Titration NPSG in 57 subjects with no medications
Data from Diagnostic NPSG and daytime tests
Data from Titration NPSG