Abstract
Background:
Obstructive sleep apnea (OSA) is prevalent in the surgical population, and it has been suggested that preoperative patients should be screened and treated for OSA. However, it remains unclear whether patients diagnosed with OSA in the preoperative period adhere to prescribed CPAP therapy.
Objective:
Our aim was to objectively quantify CPAP adherence, investigate predictors of poor CPAP adherence, and to establish an optimal CPAP setting in a cohort of presurgical patients diagnosed with OSA as part of the preoperative work-up.
Methods:
In a retrospective observational study, we collected data on all adult presurgical patients seen by the Anesthesia Perioperative Medicine Clinic (APMC) who screened positive for OSA on the STOP-Bang questionnaire and underwent an in-laboratory diagnostic polysomnogram (PSG) before surgery. CPAP was offered to patients with moderate or severe OSA. Objective CPAP adherence was recorded during the perioperative period. Factors associated with reduced CPAP adherence were delineated. Patient characteristics were compared between those with STOP-Bang scores of 3-4 and those with higher scores (STOP-Bang score ≥ 5).
Results:
During a 2-year period, 431 patients were referred and 211 patients completed a PSG. CPAP therapy was required in 65% of patients, and the optimal level was 9 ± 2 cm H2O. Objective CPAP adherence was available in 75% of patients who received CPAP therapy; median adherence was 2.5 h per night, without any significant difference between the STOP-Bang subgroups. African American race, male gender, and depressive symptomatology were independent predictors of reduced CPAP adherence. Severe OSA was significantly more prevalent in patients with a STOP-Bang score ≥ 5 than those whose score was 3-4 (55.1% versus 34.4%, p = 0.005). However, optimum CPAP pressure levels and adherence to therapy did not differ between the 2 STOP-Bang groups.
Conclusions:
Adherence to prescribed CPAP therapy during the perioperative period was extremely low. African American race, male gender, and depressive symptoms were independently associated with reduced CPAP usage. Further research is needed to identify and overcome barriers to CPAP acceptance and adherence in the perioperative setting.
Citation:
Guralnick AS; Pant M; Minhaj M; Sweitzer BJ; Mokhlesi B. CPAP adherence in patients with newly diagnosed obstructive sleep apnea prior to elective surgery. J Clin Sleep Med 2012;8(5):501-506.
Keywords: Obstructive sleep apnea, continuous positive airway pressure, CPAP, adherence, compliance, perioperative, STOP-Bang
Since its initial description more than 40 years ago, OSA has been gaining more interest among anesthesiologists, and there is accumulating evidence that moderate to severe OSA can increase perioperative complications.1–3 Given the important implications of untreated OSA,4–9 the American Society of Anesthesiologists (ASA) recommends screening presurgical patients for OSA and implementing treatment if significant OSA is present.10 There is lack of consensus on the most appropriate method of screening, diagnosis, and timing of therapy implementation.11 Although most studies have focused on screening and diagnosis of OSA, less is known about CPAP therapy and adherence during the perioperative period.
In this study we aimed to objectively quantify perioperative CPAP adherence and optimal CPAP pressure settings in a cohort of patients diagnosed preoperatively with moderate or severe OSA. We also examined patient characteristics associated with reduced CPAP adherence. We tested the hypothesis that patients with higher scores on the STOP-Bang screening questionnaire, a validated screening tool for the preoperative setting, would have a higher prevalence of moderate or severe OSA than those with lower, yet positive scores and require a higher level of CPAP pressure for successful treatment.
Brief Summary
Current Knowledge/Study Rationale: Moderate to severe obstructive sleep apnea (OSA) may increase postoperative complications and there are recommendations suggesting that preoperative patients should undergo screening for OSA and be treated during the perioperative period. To date, it has not been established whether patients newly diagnosed with OSA prior to elective surgery are adherent with CPAP therapy. This study sought to objectively quantify perioperative CPAP adherence in patients with moderate or severe OSA.
Study Impact: Pre-surgical patients with newly diagnosed OSA had very poor adherence to CPAP therapy. These results should encourage future investigations to determine barriers to CPAP adherence in the perioperative period.
METHODS
Between March 2009 and February 2011, adult presurgical patients who were identified at high risk for OSA by the STOP-Bang screening survey administered during their visit to the Anesthesia Perioperative Medicine Clinic (APMC) were recommended to undergo a diagnostic in-laboratory PSG prior to elective surgery.12,13 Patients with prior diagnosis of OSA were excluded from our cohort. The STOP-Bang surveys were completed by the patients; APMC medical assistants measured the neck circumference, weight, and height, and the body mass index (BMI) was calculated by the preoperative computerized program used by the APMC. A request for an expedited in-laboratory PSG was submitted by the APMC staff to the sleep laboratory, and an appointment for the PSG was made within 3 days of the initial APMC visit as long as the patient was willing to undergo the PSG. In order to avoid any delay in surgery, split-night PSGs (first half of the study to establish diagnosis and the second half dedicated to CPAP titration) were performed on those patients who demonstrated at least moderate OSA (AHI ≥ 15).14 Given the long wait times in the sleep clinic and in order to avoid delaying surgery, the patients were not evaluated by a sleep physician preoperatively. However, patients were seen in consultation by a sleep physician 6 to 8 weeks after surgery.
PSGs were staged and scored according to the 2007 American Academy of Sleep Medicine Manual for the Scoring of Sleep and Related Events.15 Hypopneas were scored if the magnitude of ventilation signal decreased by ≥ 50% of the baseline amplitude of the nasal pressure transducer ≥ 10 sec and were associated with either ≥ 3% drop in oxygen saturation as measured by finger pulse oximetry, or an electroencephalographic microarousal.15 The 3% oxygen desaturation index (ODI) indicated the number of 3% desaturations per hour of sleep. A patient was considered not to have OSA if the AHI was < 5, to have mild OSA if the AHI was 5-14, moderate OSA if the AHI was 15-29, and severe OSA if the AHI was ≥ 30. CPAP titration was considered successful if, on optimal CPAP pressure, the residual AHI was < 5 and included supine REM sleep.16 The sleep studies were reviewed by sleep specialists the day after the PSG, and those with moderate or severe OSA were prescribed and received an auto-titrating CPAP device. The pressure range of the auto-titrating device was determined based on the optimal CPAP level obtained during the titration portion of the PSG. As an example, if the optimal CPAP pressure was 9 cm H2O, the minimum pressure setting of the auto-CPAP device would be set at 2 cm H2O below the optimal pressure and the maximum pressure would be set at 3 cm H2O above the optimal pressure (e.g., 7 to 12 cm H2O). The patients were systematically educated by a trained respiratory therapist on the importance of adherence to therapy during the perioperative period, underwent appropriate mask fitting, and were instructed on how to use their auto-titrating CPAP device. Patients received the auto-titrating CPAP devices the day after the in-laboratory PSG and were instructed to bring their auto-CPAP devices with them on the day of surgery. All patients were contacted by a sleep physician the day after the sleep study to briefly discuss the findings of the polysomnogram and the importance of adhering to CPAP therapy before and after surgery. There were no additional communication between the sleep laboratory staff and the patient until the sleep clinic consultation 6-8 weeks after surgery.
Ethics
The study was approved by the Institutional Review Board of the University of Chicago (IRB protocol # 11-0120). Given the retrospective nature of the study, the need for informed consent was waived.
Patient Characteristics
On the night of the PSG, patients completed a routine questionnaire, which included demographics, medical history, Epworth Sleepiness Scale (ESS),17 and the Center for Epidemiologic Studies Depression Scale (CES-D), a validated depression questionnaire.18 BMI was calculated using weight and height measurements obtained on the day of the PSG. The patient's neck circumference was obtained from the measurement done in the APMC. The patient's age and sex were obtained from the electronic medical record. Race was self-reported and was categorized into African American and non-African American. Self-reported education level was categorized into “high school degree or less” and “more than high school degree.”
CPAP Adherence
CPAP adherence during the first 30 days of therapy was determined objectively when patients were seen in consultation by a sleep physician 6 to 8 weeks after surgery.
Statistical Analyses
Means or proportions of all variables were calculated for the entire cohort and then separately for those with STOP-Bang scores of 3-4 and those with STOP-Bang scores ≥ 5. The distribution of each continuous variable was examined. Continuous variables that were normally distributed were summarized as mean ± standard deviation (SD) and were compared using Student's t-test. Continuous variables that were not normally distributed were summarized as median and interquartile range (IQR) and were compared using the Mann-Whitney nonparametric test. Differences in proportions were tested using the χ2 test. We performed a linear regression model to adjust for possible confounding variables in order to identify independent predictors of CPAP adherence. Differences were considered statistically significant when p value was ≤ 0.05. All analyses were performed using PASW Statistics (v.18.0, SPSS, Inc., Chicago, IL).
RESULTS
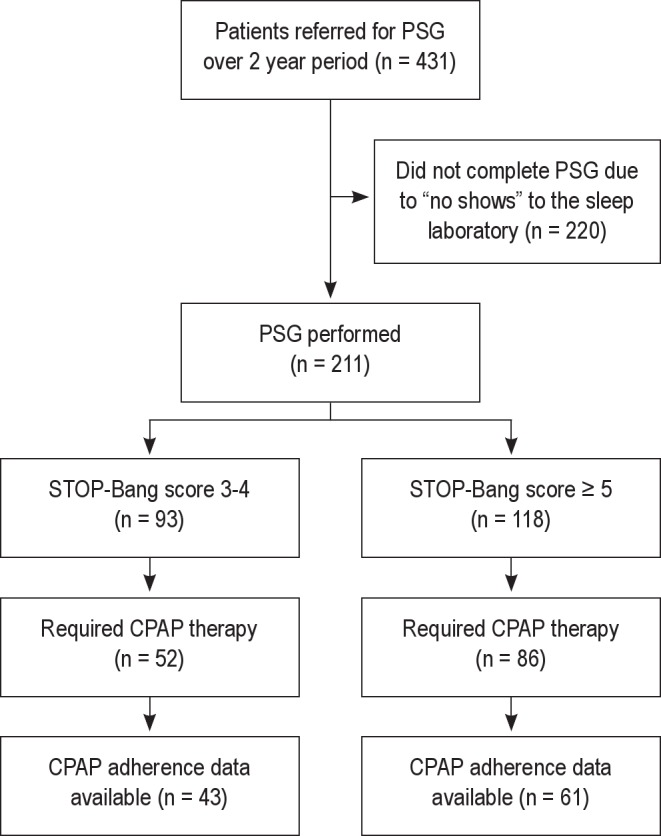
Over a 2-year period, 431 patients who screened at high risk for OSA were referred for an in-laboratory PSG. Of the 431 referred patients, 211 scheduled and completed the in-laboratory PSG. The remainder of the patients did not show up for the scheduled polysomnogram (Figure 1). Our population of patients who had a PSG included those undergoing elective orthopedic surgery (29%), genitourinary surgery (18%), general surgery (12%), vascular surgery (8%), head and neck surgeries (7%), gynecologic surgery (5%), thoracic surgery (3%), and all other surgeries (18%). None of the patients underwent a sleep consultation prior to the diagnostic polysomnogram.
Figure 1. Flow diagram of patients in the study that were referred to the sleep laboratory.
Of the 211 patients who screened high risk for OSA and underwent a diagnostic PSG, 6% did not have OSA, 20% had mild OSA, 28% had moderate OSA, and 46% had severe OSA. However, the prevalence of severe OSA increased from 36% in patients with STOP-Bang score of 3 to 79% in patients with STOP-Bang scores of 7-8 (Figure 2). We divided the cohort into 2 STOP-Bang subgroups with similar sample sizes. Among the 211 patients who underwent PSG, we compared 93 patients (44%) with STOP-Bang scores of 3-4 to 118 patients (56%) with STOP-Bang scores of 5-8 (Tables 1, 2). Severe OSA was significantly more prevalent in patients with a STOP-Bang scores of 5-8 (55.1% vs. 34.4, p = 0.005) (Table 1). The group with STOP-Bang scores of 5-8 included a significantly higher proportion of men and had a larger neck circumference, higher BMI, and more severe indices of OSA as measured by AHI, ODI, and percent of total sleep time below oxygen saturation of 90% (T90) (Table 2, Figure 3).
Figure 2. Prevalence of moderate or severe OSA across all STOP-Bang scores in 211 patients who underwent in-laboratory polysomnogram.
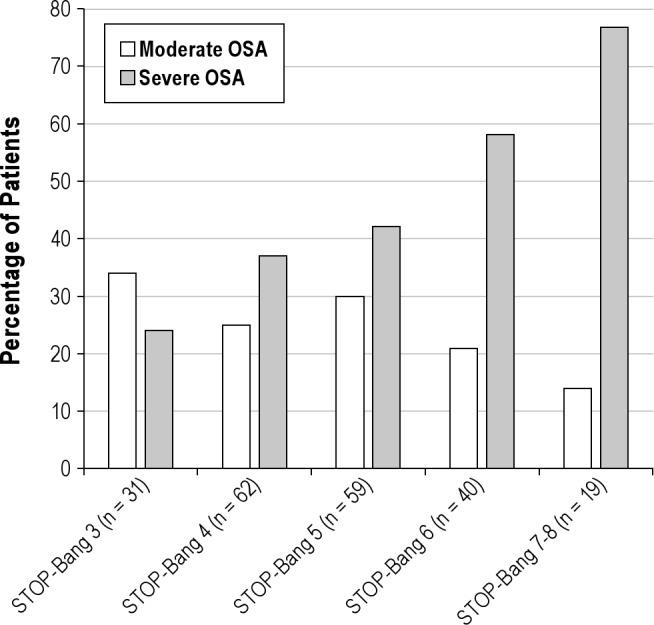
Table 1.
Prevalence of OSA severity in the two STOP-Bang categories
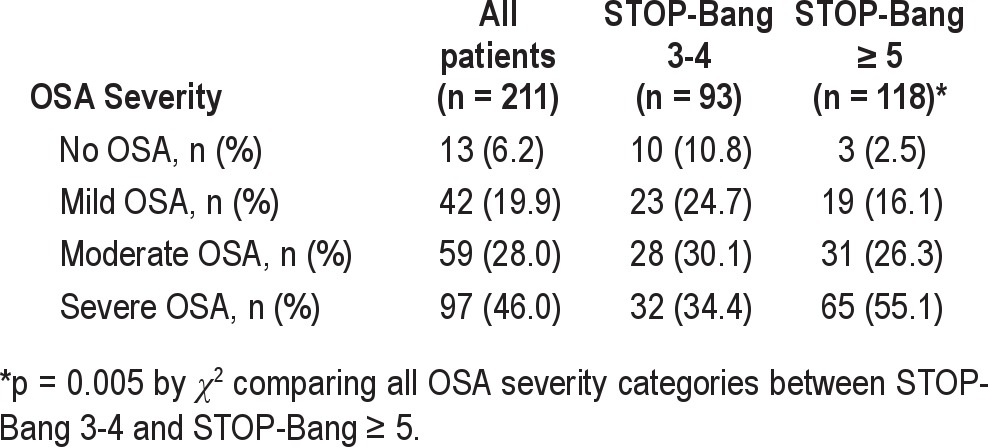
Table 2.
Patient characteristics
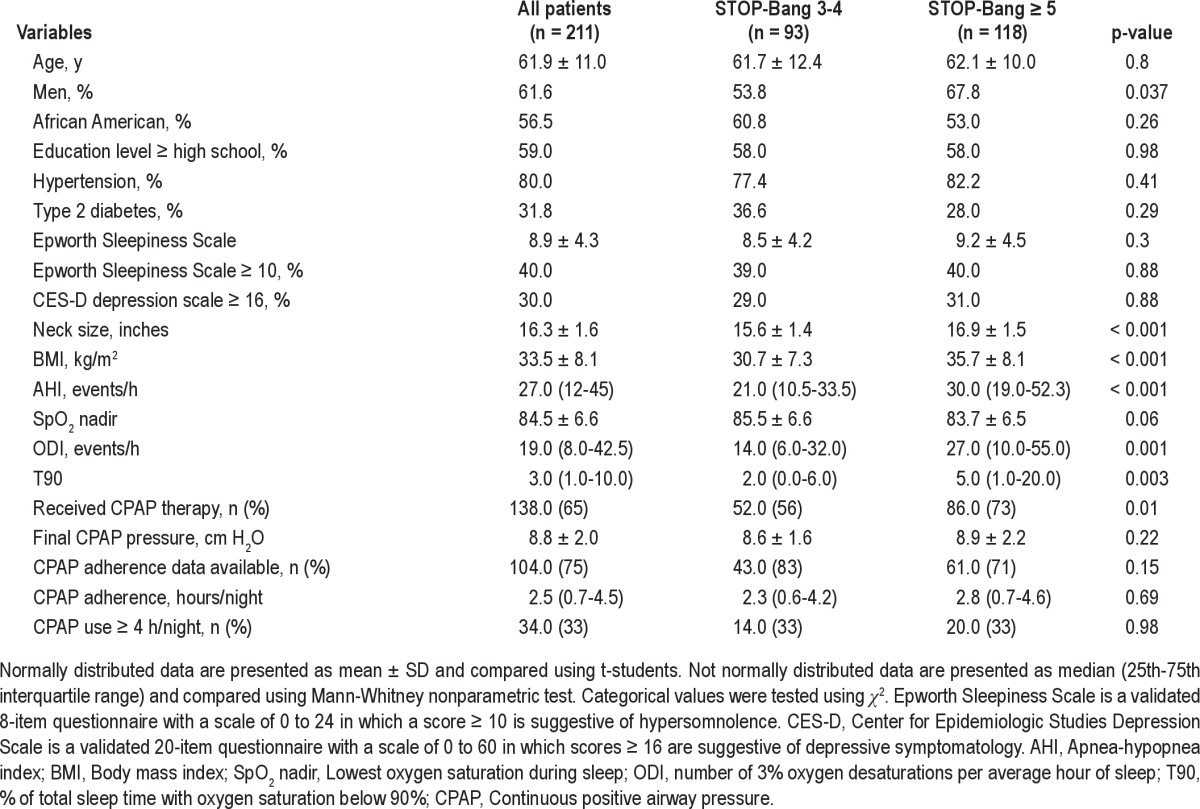
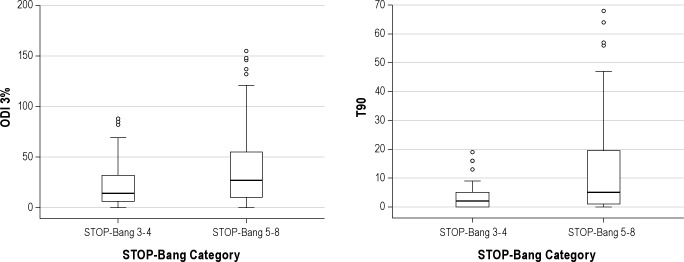
Figure 3. Box plots showing the distribution of and differences in 3% oxygen desaturation index (ODI 3%) and percent of total sleep time below oxygen saturation of 90% (T90) across the two STOP-Bang categories.
Lower and upper boundaries of the box indicate 25th and 75th percentages. A solid line within the box marks the median, and vertical lines indicate the 10th and 90th percentages. Circles are outliers. ODI 3% and T90 were significantly higher in patients with STOP-Bang scores of 5-8 (p = 0.001 and p = 0.003, respectively).
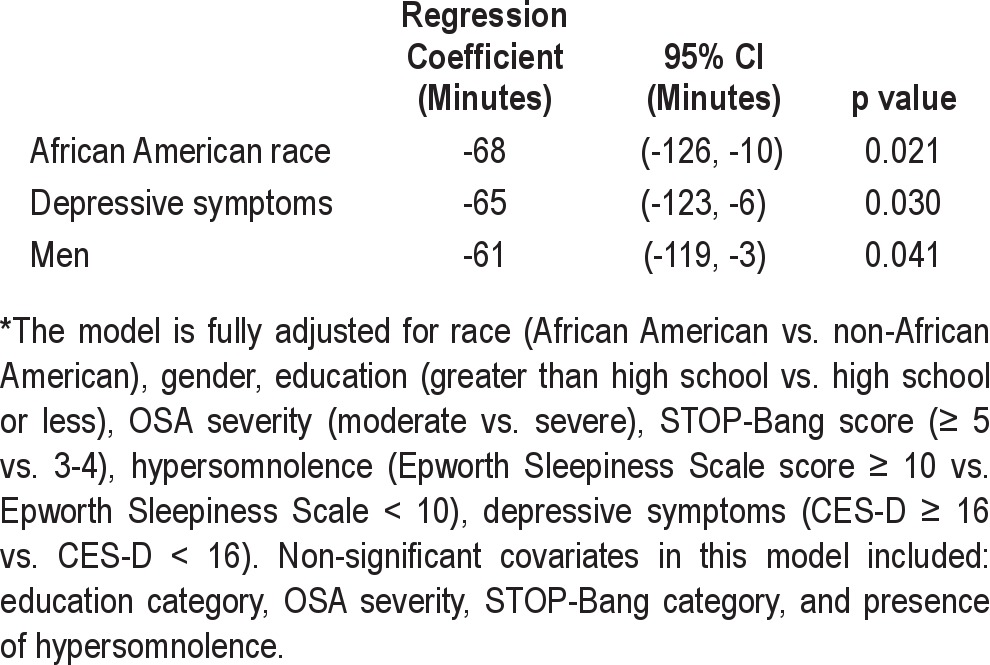
CPAP therapy was required and provided to a total of 138 out of 211 patients (65%). On average, patients were started on CPAP therapy 4 days before the date of surgery. Not surprisingly, a higher proportion of patients with STOP-Bang scores ≥ 5 required CPAP therapy (73% vs. 56%, p = 0.01). However, among patients who underwent CPAP titration in the 2 groups, there was no significant difference in the CPAP levels required to optimally treat OSA (Table 2). Objective CPAP adherence data during the first 30 days of therapy was available in 104 of the 138 patients who received CPAP therapy (75%). There was no significant difference in the proportion of patients with CPAP adherence data available in the 2 STOP-Bang categories (p = 0.15). CPAP adherence was quantified as a continuous variable (mean daily usage over 30 days of therapy) as well as a categorical variable (percentage of patients using CPAP ≥ 4 h/night). The overall median adherence to CPAP during the first 30 days of therapy was quite suboptimal at 2.5 h/night (interquartile range of 0.7-4.5 h/night), and there was no significant difference in CPAP adherence between the 2 STOP-Bang categories. Based on this distribution, only 25% of patients prescribed CPAP therapy were using their CPAP devices for ≥ 4.5 h/night. As expected, adherence was also poor when examined as a categorical variable since only 33% of patients used CPAP ≥ 4 h/night (Table 2). In a fully adjusted linear regression model, African American race, male gender, and the presence of depressive symptomatology were each independently associated with approximately 1 h less of CPAP use per night after adjustment for OSA severity, STOP-Bang category, hypersomnolence, and education level (Table 3). We also found that the majority of patients were optimally treated with CPAP pressure of 9 ± 2 cm H2O, and there was no significant difference in the optimal CPAP pressure between the 2 STOP-Bang groups.
Table 3.
Significant predictors of mean CPAP adherence over the first 30 days of therapy in minutes from a fully adjusted multiple linear regression model*
DISCUSSION
Our study demonstrates that in an urban tertiary care academic medical center, a high proportion of presurgical patients that underwent polysomnographic evaluation had severe OSA. Overall CPAP adherence during the first 30 days of perioperative period was extremely low compared to known CPAP adherence at our institution, as well as reported national averages of 4.7 hours/night.19–23 Indeed, only a third of patients were using CPAP at least 4 hours/night. Moreover, African American race, male gender, and the presence of depressive symptoms were significant predictors of reduced CPAP adherence. These are novel findings that have not been previously investigated in this patient population.
Further investigation into why perioperative patients are less adherent to CPAP and interventions to overcome noncompliance is needed if successful therapy is desired. While we have previously reported that OSA patients seen by sleep physicians are more adherent to CPAP therapy,19 none of the patients underwent a sleep consultation prior to the diagnostic polysomnogram. It remains to be elucidated whether a sleep consultation in the preoperative period would lead to improvement in CPAP adherence. In a recent study, 88 patients identified as having OSA who received CPAP therapy during the preoperative period were surveyed with a mailed questionnaire two years after surgery to assess “subjective” CPAP adherence.24 Although the study reported 45% of patients being adherent with therapy, the findings were limited by not having objective CPAP adherence data, as well as lack of information about the more immediate perioperative CPAP use. It is important to recognize that CPAP adherence is frequently overestimated by patients.20,22 Notwithstanding these limitations, the study reported that patients adherent with CPAP therapy experienced greater symptomatic benefit.24 Although long-term CPAP non-adherence is associated with increased cardiovascular morbidity and mortality,4,9 the impact of CPAP non-adherence in the perioperative period has not been evaluated in a systematic fashion.
We also found that despite removing administrative barriers and ensuring that there would be full availability of resources from the sleep laboratory to obtain a confirmatory in-laboratory PSG in a timely fashion without delaying surgery, a large proportion of patients were unable or unwilling to undergo a PSG. It is noteworthy that others have also reported a low patient acceptance rate of in-laboratory PSG in either a clinical or research setting.13,25 Our study was not designed to elucidate the reasons for which patients refused or deferred a PSG. However, we speculate that the inconvenience of an overnight in-laboratory PSG, cost, under-appreciation of the implications of OSA, lack of understanding of the consequences of untreated OSA or unwillingness to use CPAP may have all contributed.
Our data also demonstrates that the average CPAP level required to optimally treat patients is approximately 9 ± 2 cm H2O. Therefore an auto-titrating CPAP device with a minimum pressure setting of 7 cm H2O and a maximum pressure setting of 12 cm H2O adequately treated the vast majority of our patients. In practice, we have noted that some anesthesiologists at our institution start empiric CPAP at 5 cm H2O in the postoperative period in patients with suspected OSA. Most likely, this treatment approach will not give full resolution of upper airway obstruction; based on our findings, higher pressure needs to be prescribed for successful treatment. This is an important finding, given that the vast majority of patients with OSA are not typically diagnosed preoperatively, but can be suspected to have OSA based on patient characteristics, by using the STOP-Bang screening tool, or by nocturnal oximetry.26–28
Our study has several limitations. Although we objectively measured CPAP adherence, we were unable to obtain adherence data on all the patients. However, objective CPAP adherence data were available in 75% of patients who received CPAP therapy. There is a possibility of a selection bias leading to a high proportion of patients having moderate or severe OSA in our study. Patients with more severe symptoms may have been more willing to undergo a PSG. We cannot calculate sensitivity or specificity for the STOP-Bang cutoffs used in our study, since we did not systematically test patients who screened at low risk for OSA on the questionnaire (scores below 3). Given that overall serious postoperative complications due to OSA are rare, our study was neither powered nor designed to ascertain rates of postoperative complications between patients with higher and lower STOP-Bang scores or between patients that were adherent and non-adherent to CPAP therapy. Finally, the findings in our inner-city urban cohort may not be applicable to other populations. As such, further studies are needed to confirm our findings.
In summary, the willingness of patients who screen at high risk for OSA to submit to an in-laboratory PSG during the immediate preoperative period is low. However, those identified as high risk by STOP-Bang scores of 5-8 have a high likelihood of moderate-severe OSA. The adherence to prescribed CPAP therapy in the perioperative period in our population was extremely low. Further research is needed to identify barriers to CPAP adherence in this patient population, or efforts directed towards diagnosis are likely to be wasted. Ultimately, large multicenter randomized trials with clinically important and relevant end points are needed to test the validity and cost-effectiveness of various approaches in diagnosing and treating OSA in the perioperative setting.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141:436–41. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 2.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–21. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 3.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116:788–96. doi: 10.1097/ALN.0b013e31824b94fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 10.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. quiz 1117-1088. [DOI] [PubMed] [Google Scholar]
- 11.Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–21. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 12.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 13.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 14.Khawaja IS, Olson EJ, van der Walt C, et al. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med. 2010;6:357–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson AL, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 16.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Pamidi S, Knutson KL, Ghods F, et al. The impact of sleep consultation prior to a diagnostic polysomnogram on continuous positive airway pressure adherence. Chest. 2012;141:51–57. doi: 10.1378/chest.11-0709. [DOI] [PubMed] [Google Scholar]
- 20.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 21.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149:149–54. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta V, Subramanyam R, Shapiro CM, Chung F. Health effects of identifying patients with undiagnosed obstructive sleep apnea in the preoperative clinic: a follow-up study. Can J Anaesth. 2012;59:544–55. doi: 10.1007/s12630-012-9694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fidan H, Fidan F, Unlu M, Ela Y, Ibis A, Tetik L. Prevalence of sleep apnoea in patients undergoing operation. Sleep Breath. 2006;10:161–5. doi: 10.1007/s11325-006-0067-9. [DOI] [PubMed] [Google Scholar]
- 26.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114:993–1000. doi: 10.1213/ANE.0b013e318248f4f5. [DOI] [PubMed] [Google Scholar]
- 27.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farney RJ, Walker BS, Farney RM, et al. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med. 2011;7:459–465B. doi: 10.5664/JCSM.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]