Abstract
Introduction and Hypothesis:
The aim of the present study was in a case-control design to evaluate the association between nocturia and obstructive sleep apnea, in men and women who had nocturia ≥ 2 per night (nocturics) compared to those without nocturia (controls).
Methods:
Participants were randomly selected among respondents in a population study of 4000 elderly individuals. Nocturia was assessed using the validated Nocturia, Nocturnal Enuresis, and Sleep-interruption Questionnaire (NNES-Q). Nocturia (≥ 2 voids/night) or control (< 1 void/night) status was assessed by a 3-day frequency volume chart (FVC). Furthermore, all participants completed an overnight ambulatory polygraphic recording to identify obstructive sleep apnea (OSA).
Results:
Of 1111 eligible individuals, a total of 75 nocturics and 75 controls (13.5%) were included. Overall, the prevalence and severity of OSA among nocturics and controls was not significantly different. In a sub-analysis we found that 22 nocturics with OSA (69%) had nocturnal polyuria. This led to a significantly increased risk of having OSA (OR 2.8, 95% CI: 1.1-7.3, p < 0.05) when having nocturnal polyuria compared to other pathophysiological causes of nocturia (polyuria, low bladder capacity, a combination of nocturnal polyuria/low bladder capacity, and neither nocturnal polyuria/low bladder capacity).
Conclusions:
Nocturia twice or more was not significantly associated with OSA. However, nocturics with nocturnal polyuria had a significantly higher risk of having OSA than nocturics with other pathophysiologies.
Citation:
Bing MH; Jennum P; Moller LA; Mortensen S; Lose G. Obstructive sleep apnea in a danish population of men and women aged 60-80 years with nocturia. J Clin Sleep Med 2012;8(5):515-520.
Keywords: Case-control study, elderly, nocturia, nocturnal polyuria, obstructive sleep apnea.
Sleep apnea patients often have nocturia, mainly due to nocturnal polyuria,1,2 possibly because of increased plasma and urine levels of atrial natriuretic peptide (ANP), leading to increased natriuresis and diuresis.3,4 Treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) treatment may reduce the nocturia.5–7
The frequency of sleep disordered breathing (SDB), including obstructive sleep apnea (OSA) and central sleep apnea (CSA), increases with age.8 The main cause of SDB is OSA, a repetitive partial or complete obstruction of the upper airway during sleep leading to repetitive hypoxic episodes.9 If OSA is combined with excessive daytime sleepiness (EDS), the condition is termed obstructive sleep apnea syndrome (OSAS). The prevalence of OSA in Denmark is 10% to 15% of men and 5% to 8% of women 30-60 years old10; however, a minority of persons with OSA have OSAS, estimated at 2% to 4% of men and 1% to 2% of women.11 Despite the common occurrence of OSA in older adults, its clinical significance remains unclear12,13; it is especially unclear whether there is an association between nocturia and OSA in a general population. Data regarding this issue have mostly emerged from sleep clinic populations14,15; results from unselected populations are sparse.12,26 However, data demonstrate that older adults with severe sleep disordered breathing have an increased number of nocturia episodes.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Nocturia and OSA are common conditions in the elderly population. Is there an association between the two conditions in the general population?
Study Impact: The study showed no overall difference in prevalence of OSA among nocturics and controls. However, nocturics having nocturnal polyuria, had a significant increased risk of having OSA.
Nocturia, defined as waking up at night to void,2 is highly prevalent in the elderly population.16–18 The pathophysiological causes of nocturia are polyuria, nocturnal polyuria, low bladder capacity, sleep disorders, or combinations of these. Age-related changes in the lower urinary tract (including prostatic disease) may cause nocturia, but factors unrelated to the lower urinary tract may also play a role.19,20 Factors like excessive fluid before bedtime, alcohol, caffeine, diuretics, and pathological conditions such as hypertension, congestive heart failure, diabetes mellitus, neurological diseases, and OSA may be involved.2,19,21
Data regarding nocturia and OSA emerging from a general population are sparse; however, such data might elucidate the overall risk of having OSA in nocturics. This might support the clinician in selecting individuals with nocturia who should be referred for further work-up in a sleep clinic.
The aim of the present study was in a case-control design to evaluate the association between nocturia and obstructive sleep apnea by comparing men and women who had nocturia ≥ 2 per night (nocturics) to those without nocturia (controls).
MATERIALS AND METHODS
Nocturia was defined as waking up at night to void. Nocturnal polyuria was defined as nocturnal urine volume larger than 33% (in the elderly) of the total 24-h urine volume. All definitions were in accordance with ICS terminology.2
Nocturia was assessed in a population study using the new and validated Nocturia, Nocturnal Enuresis, and Sleep-interruption Questionnaire (NNES-Q).18,22 Daytime sleepiness was evaluated using Epworth Sleepiness Scale (ESS).23 The ESS is designed to provide a measurement of an individual's general level of daytime sleepiness. Respondents are asked to rate on a scale of 0-3 how likely they would be to doze off or fall asleep in 8 situations, based on their usual way of life in recent times.
Except for gender-specific items (e.g., deliveries, hormonal treatment, prior gynecological surgery, prior prostate surgery) the questionnaire as a whole, was similar to all. The total number of items in the questionnaire was 98 for women and 94 for men.
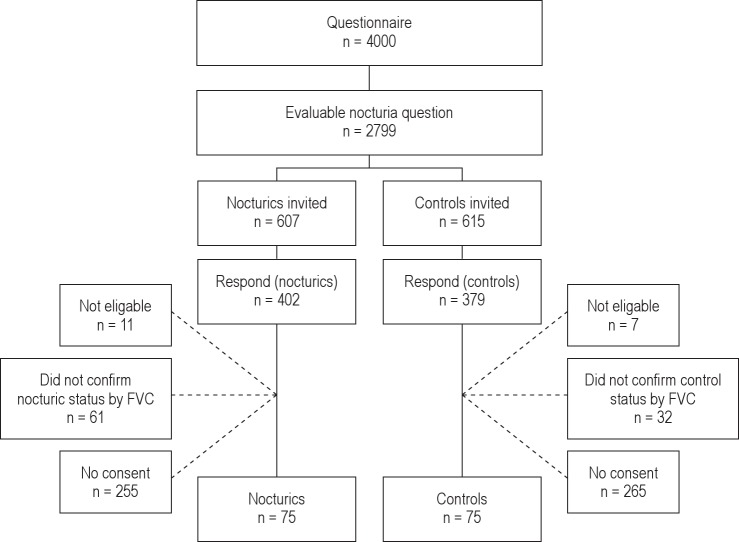
Participants were randomly recruited among respondents in a population study of 4000 men and women aged 60-80 years. Details from this study have previously been reported.18 The inclusion criterion was if participants had completed a question on nocturia. Exclusion criteria were night shift work, severe physical disability, or mental impairment (dementia) and inability to follow instructions. Eligible for inclusion were participants who confirmed nocturic or control status by using a 3-day frequency volume chart (FVC), which recorded volumes and time of each micturition over 3 representative days. Voids that occurred between the time recorded for initiation of nighttime sleep and wake time in the morning were considered nocturia episodes; all other voiding was diurnal. Based on the FVC findings, individuals were categorized as nocturics if they had an average ≥ 2 nocturia episodes/night; controls had an average < 1 nocturia (less than one) episode/night over a 3-day period. Participants were asked to report urinary incontinence episodes in FVCs (Figure 1).
Figure 1. Flow-chart of participants.
Medical history was obtained through semi-structured interviews. Physical examination was performed, and height and weight measured. All participants completed an overnight ambulatory polygraphic recording identifying chest and abdominal movements, airflow through the nose, and pulse oximetry (Embletta PDS, Flaga, Iceland), which is a valid standard method of screening for SDB.24
Participants were given oral and written instructions on how to use the polygraphic equipment on the day of the sleep study. The sleep recording equipment was returned within 1-2 days and data downloaded. Sleep data was evaluated for technical quality; if insufficient, the study was repeated.
Data analysis was performed by initial automatic scoring, supervised by an experienced polygraphic technician and lastly evaluated by one of the authors (PJ) who was blinded for knowledge of nocturic or control status. Technically adequate recordings of ≥ 5 h sleep were required for the recording to be scored. When scoring the sleep recordings the following conventional definitions were used: Apnea was defined as the cessation of airflow through the nose ≥ 10 sec, and hypopnea was defined as the reduction in airflow ≥ 50% associated with a decrease in the pulse oximetry reading (desaturation) ≥ 4%.9 The severity of obstructive sleep apnea was described by number of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]). A central apnea event occurred if no abdominal movements were registered during an apnea event. OSA was defined as an AHI ≥ 5, and we defined OSAS as an AHI ≥ 5 and excessive daytime sleepiness (ESS score > 12).
Statistical Analysis
A total of 46 participants in each group were needed to detect a difference ≥ 20% in frequency of OSA, with a statistical power of 80% and a 2-tailed type 1 error rate of 0.05. Fisher exact test or χ2 test was used to compare frequency data. Unpaired t-test or Wilcoxon rank sum test were used to compare continuous data. A logistic regression model was used to analyze the relationship between nocturic and control status, and the odds of having OSA. Prevalence of potential confounding factors in nocturic and control group was analyzed. Only factors in which a priori χ2 test (categorical data) or ANOVA (continuous data) was significant were included in the model. In all analyses, a 5% significance level was used.
The study was approved by the local ethics committee, Copenhagen County (KA 02049).
In all analyses, the significance level was chosen to be 0.05. The statistical packages JMP, Version 5 (SAS Institute Inc., http://www.jmpdiscovery.com) and SAS, Version 8.2 were used in the calculations.
RESULTS
Recruitment of Participants
Of 4,000 questionnaires sent, 2,799 (70.0%) answered affirmatively the question regarding nocturia, thereby becoming potential candidates for participation in the study. Of these, 1,022 participants were initially classified as nocturics and 1,777 participants as controls.18
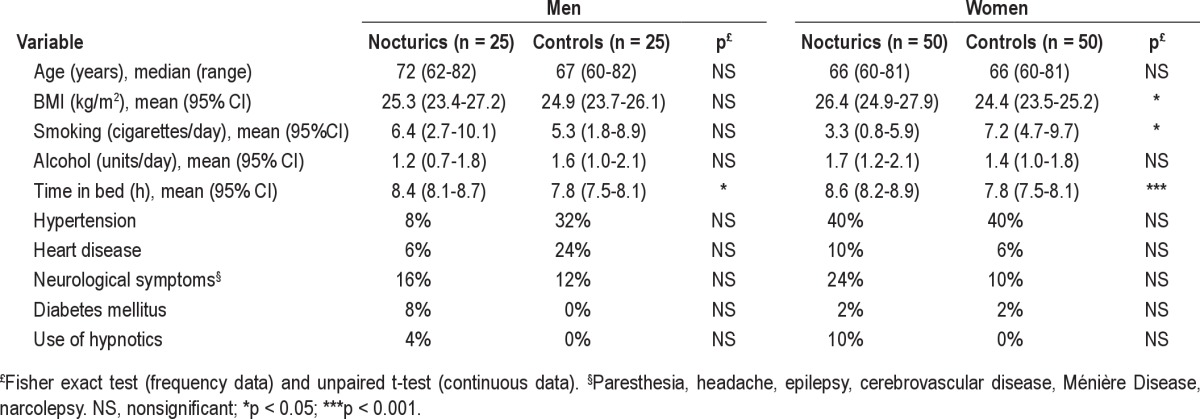
A total of 607 nocturics and 615 controls were invited to participate; 93 (7.7%) respondents did not fulfill inclusion criteria according to FVC findings. Another 18 individuals were not eligible for inclusion (1 had died, 6 had concomitant medical diseases prohibiting their participation, 7 had moved away, and 4 withdrew consent). A total of 1,111 individuals were eligible for inclusion. We included 50 women in each group; however, we were only able to include 25 men within the time period from January 2003 to January 2005. Thus, a total of 75 nocturics and 75 controls (13.5%) were included. Median age was 71 years (range: 60-82) in nocturics and 66 years (range: 60-82) in controls (Table 1). Participants in this study were slightly younger (median age 67 years [range: 60-82]) than participants in the population study (median age 70 years [range: 60-80]).18,22
Table 1.
Characteristics of participants
Frequency Volume Charts
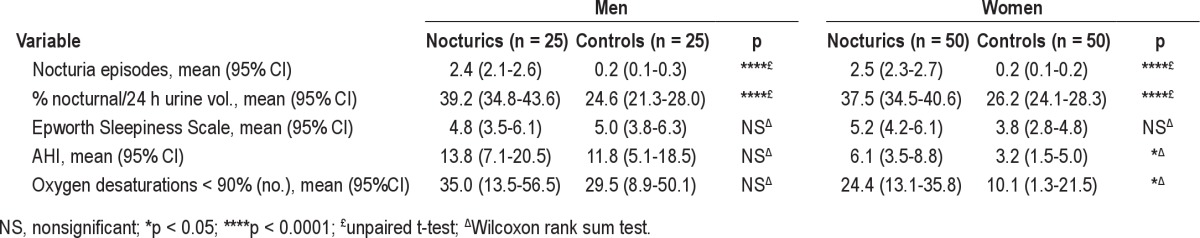
Mean (95% CI) number of nocturia episodes in nocturics was 2.5 (2.3-2.6), and in controls 0.2 (0.1-0.2), with no significant difference between genders (Table 2). Frequency of individuals having nocturnal polyuria was 55% in nocturics vs. 20% in controls (p < 0.0001).
Table 2.
Nocturia, Epworth Sleepiness Scale (ESS) score, and sleep variables in participants
Obstructive Sleep Apnea
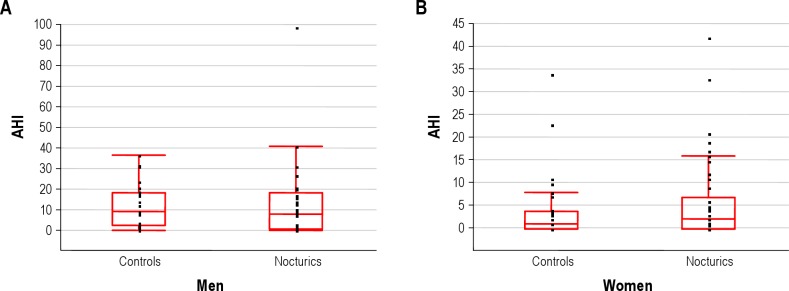
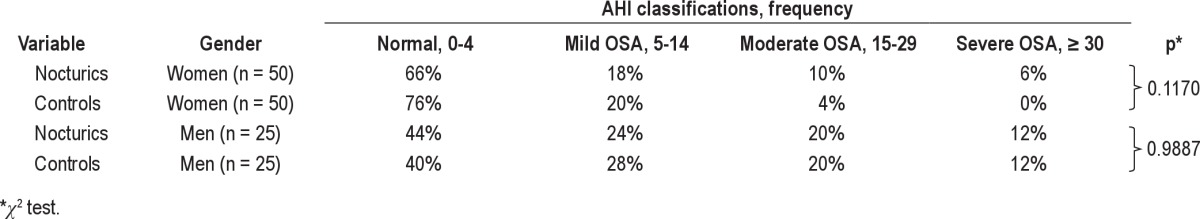
Eleven initial sleep recordings (7.3 %) required repeated evaluation. Two participants with apneas/hypopneas had missing oxygenation recording even after repeated measurements; however they were classified according to their AHI. Further, more women nocturics had oxygen desaturation < 90% compared to controls (Table 2), whereas there was no difference in men. As seen in Figure 2 and Table 2, mean AHI in women was significantly higher in nocturics than controls, whereas no difference in men was observed. In terms of the Epworth Sleepiness Scale, score no significant difference between nocturics and controls was observed.
Figure 2. AHI index in relation to nocturic and control status in men (A) and women (B).
The solid line is the median, the box the interquartile range, the bars the 10th and 90th percentile and the points the outliers.
In nocturics and controls, no significant difference in prevalence and severity of OSA was observed (Table 3). In women, 34% of nocturics had AHI ≥ 5 vs. 24% of controls; in men 56% of nocturics had AHI ≥ 5 vs. 60% of controls. With a more strict definition of OSA based on AHI ≥ 15, the prevalence among nocturics dropped to 16% of nocturics vs. 4% of controls (women) and 32% of nocturics vs. 32% of controls (men). Only one nocturic had OSAS, and this patient had been previously diagnosed with narcolepsy.
Table 3.
Obstructive sleep apnea (OSA) distribution according to nocturic or control status, stratified by gender
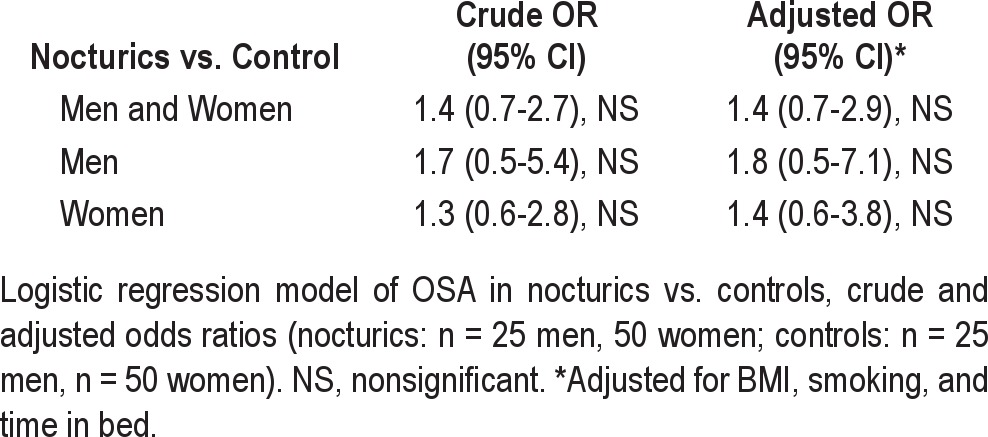
Overall, no significant association between OSA and nocturia in general was demonstrated (OR 1.4, 95% CI: 0.7-2.9; Table 4). One participant had both OSA and central apnea events, and one had predominantly central apnea events. Exclusion of these participants did not change the result. Among those with nocturnal polyuria, there was no significant association between OSA in nocturics vs. controls (OR 1.3 [95% CI: 0.4-4.3]).
Table 4.
In a sub-analysis among nocturics only, we found that 22 individuals with OSA (69%) had nocturnal polyuria. This gives a significant increased risk of having OSA (OR 2.8, 95% CI: 1.1-7.3) when a nocturic had nocturnal polyuria compared to other pathophysiological causes of nocturia (polyuria, low bladder capacity, a combination of nocturnal polyuria/low bladder capacity, or neither nocturnal polyuria/low bladder capacity). In addition, only 7 controls with OSA (27 %) had nocturnal polyuria, and there was no significant increased risk of having OSA when nocturnal polyuria occurred in controls (OR 1.9, 95% CI: 0.6-6.0).
We found no significant difference in the prevalence of potential confounders such as hypertension, cardiac disease, neurological disease, use of hypnotics, diabetes, or alcohol intake between nocturics and controls (Table 1). Only time spent in bed differed between the groups. BMI and smoking differed only between women nocturics and controls (Table 1).
DISCUSSION
Our study assesses the association between nocturia and OSA in an unselected elderly population of nocturics and controls. We used the validated Nocturia, Nocturnal Enuresis and Sleep-interruption Questionnaire (NNES-Q) for assessing nocturia22; nocturia was defined according to the International Continence Society2 and OSA according to the American Academy of Sleep Medicine Task Force.9 Assessing voiding and sleep related respiratory variables within the same sample, selected from a general population have only been addressed in few studies.12,26 These studies demonstrated that older adults with severe sleep disordered breathing have an increased number of nocturia episodes. Most previous studies have been performed in patients attending sleep clinics.37,39
The protocol included an intensive examination program, which may partly explain the low participation rate (13.5%). It is likely that the subgroup studied represents a more mobile and healthier population than the total population, which may limit the external validity of the study.
Except from time spent in bed, and BMI, and smoking in women, the frequency of potential confounders did not differ between nocturics and controls (Table 1). This is in contrast with results from our earlier population study, which showed that several morbidities were associated with increasing nocturia severity.29 It has previously been demonstrated that time spent in bed differed between nocturics and controls,25 which was confirmed in our study, and we thus adjusted for this effect in the analyses (Table 4).
Frequency Volume Charts
We found that significantly more nocturics than controls had nocturnal polyuria (p < 0.0001), with no difference between genders.41 This finding corresponds with studies by Swithinbank et al. (women),30 Rembratt et al. (men and women),31 and Massolt et al. (incontinent women)32 who found a markedly higher frequency of nocturnal polyuria in subjects having nocturia (≥ 2 voids) compared to no nocturia.
Obstructive Sleep Apnea
We found no significant association between nocturia per se and obstructive sleep apnea (Table 4). An explanation could be that the association between nocturia and OSA is only found in severe OSA as previously described in a study of community dwelling elderly.12 Further, a type II error may influence the results.
The finding of nocturnal polyuria in the vast majority of nocturics with OSA emphasizes the importance of elucidating the pathophysiology of nocturia in the individual person with nocturia twice or more. The present study shows that AHI was significantly higher in women with nocturia (Table 2); however, the AHI level was relatively low in both nocturics and controls, and the clinical impact of this gender difference remains unclear.
In a cross-sectional study of community older adults, Endeshaw et al.12 observed that elderly with an AHI of 25 or greater had significantly more nocturia episodes. This finding agreed with reports on sleep clinic patients.33 Endeshaw et al.12 noted that subjects were not randomized, which could have biased the results.
OSA may be associated with increased renal sodium and water excretion mediated by plasma atrial natriuretic peptide (ANP) levels.7,34,35 However, whether ANP level is associated with nocturnal polyuria in the elderly remains unclear.36 In a recent study by Svatikova et al.,37 the effect of moderate-to-severe OSA (AHI ≥ 20) on plasma ANP levels was examined. The authors concluded that acute untreated OSA and subsequent treatment with CPAP significantly altered the levels of ANP. Among contributing factors to nocturnal polyuria may be an age-related reduction in the diurnal variation in the secretion of plasma arginine vasopressin (AVP)38; AVP is often undetectable in elderly with nocturia. However, studies of elderly persons have failed to find any correlation between nocturnal polyuria and AVP levels.42
Mechanisms other than those mentioned above may be involved in the pathogenesis of nocturia. Sagaya et al.40 studied biochemical and body composition differences between elderly with nocturia ≥ 2 with young and elderly control groups. The main finding was that in elderly persons with nocturia, a sleep disorder related to a decrease of melatonin may be considered, and a sleep disorder may decrease the threshold for awakening by the desire for voiding.
The above studies demonstrate that the mechanism explaining the association between nocturia and obstructive sleep apnea in the elderly remains unclear.
In conclusion, nocturia twice or more was not significantly associated with OSA in the present study. However, female nocturics had significantly higher AHI and more desaturations < 90% than female controls, indicating a trend towards women having more severe obstructive sleep apnea if they had nocturia. Furthermore, nocturics with nocturnal polyuria had a significantly higher risk of having OSA than nocturics with other pathophysiologies.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was financially supported mainly by Lions Club (Scandinavia), Augustinus Foundation, Director Jacob Madsen and Olga Madsens Foundation, AP Moeller Foundation and Ferring Pharmaceuticals. Research Centre for Prevention and Health, Glostrup County Hospital, Denmark has contributed with advice regarding design of the study and layout of the questionnaire, and technical support.
REFERENCES
- 1.Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev. 2003;7:403–11. doi: 10.1053/smrv.2002.0273. [DOI] [PubMed] [Google Scholar]
- 2.van Kerrebroeck P, Abrams P, Chaikin D, et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:179–83. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 3.Krieger J, Laks L, Wilcox I, et al. Atrial natriuretic peptide during sleep in patients with obstructive sleep apnea before and during treatment with nasal continuous positive airway pressure. Clin Sci. 1989;77:407–11. doi: 10.1042/cs0770407. [DOI] [PubMed] [Google Scholar]
- 4.Ichioka M, Hirata Y, Inase N, et al. Changes of circulating atrial natriuretic peptide and antidiuretic hormone in obstructive sleep apnea syndrome. Respiration. 1992;59:164–8. doi: 10.1159/000196049. [DOI] [PubMed] [Google Scholar]
- 5.Rodenstein DO, D′Odemont JP, Pieters T, Aubert-Tulkens G. Diurnal and nocturnal diuresis and natriuresis in obstructive sleep apnea. Effects of nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1992;145:1367–71. doi: 10.1164/ajrccm/145.6.1367. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Huang X, Li X, Wu Q. Alterations in renal function in patients with obstructive sleep apnea syndrome and effects of continuous positive airway pressure. Chin Med J (Engl) 1997;110:915–8. [PubMed] [Google Scholar]
- 7.Umlauf M, Kurtzer E, Valappil T, Burgio K, Pillion D, Goode P. Sleep-disordered breathing as a mechanism for nocturia: preliminary findings. Ostomy Wound Manage. 1999;45:52–60. [PubMed] [Google Scholar]
- 8.Young T, Shahar E, Nieto FJ, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 9.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 10.Jennum P, Sjol A. Epidemiology of snoring and obstructive sleep apnoea in a Danish population, age 30-60. J Sleep Res. 1992;1:240–4. doi: 10.1111/j.1365-2869.1992.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 11.Jennum P, Thonnesen P, Rasmussen N, Norregaard O. Sleep disordered breathing: definition, prevalence, pathophysiology and consequences. Ugeskr Laeger. 2004;167:2380–4. [PubMed] [Google Scholar]
- 12.Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep-disordered breathing and nocturia in older adults. J Am Geriatr Soc. 2004;52:957–60. doi: 10.1111/j.1532-5415.2004.52264.x. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 14.Pressman MR, Figueroa WG, Kendrick-Mohamed J, Greenspon LW, Peterson DD. Nocturia. A rarely recognized symptom of sleep apnea and other occult sleep disorders. Arch Intern Med. 1996;156:545–50. doi: 10.1001/archinte.156.5.545. [DOI] [PubMed] [Google Scholar]
- 15.Dahlstrand C, Hedner J, Wang YH, Pettersson S. Snoring - a common cause of voiding disturbance in elderly men. Lancet. 1996;347:270–1. doi: 10.1016/s0140-6736(96)90452-3. [DOI] [PubMed] [Google Scholar]
- 16.Jackson S. Lower urinary tract symptoms and nocturia in men and women: Prevalence, aetiology and diagnosis. BJU Int. 1999;84:5–8. doi: 10.1046/j.1464-410x.84.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 17.Middelkoop H, Smilde-van den Doel D, Neven AK, Kamphuisen HAC, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50-93: effect of gender and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med. 1996;51:M108–15. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 18.Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Prevalence and bother of nocturia, and causes of sleep-interruption in a Danish population of men and women aged 60-80 years. BJU Int. 2006;98:599–604. doi: 10.1111/j.1464-410X.2006.06390.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JP, Blaivas JG, Stember DS, Brooks MM. Nocturia in adults: etiology and classification. Neurourol Urodyn. 1998;17:467–72. doi: 10.1002/(sici)1520-6777(1998)17:5<467::aid-nau2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Homma Y, Yamaguchi O, Kageyama S, et al. Nocturia in the adult: Classification on the basis of largest voided volume and nocturnal urine production. J Urol. 2000;163:777–81. doi: 10.1016/s0022-5347(05)67802-0. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura K, Terada N, Matsui Y, Terai A, Kinukawa N, Arai Y. Prevalence of and risk factors for nocturia: Analysis of a health screening program. Int J Urol. 2004;11:282–7. doi: 10.1111/j.1442-2042.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 22.Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Validity and reliability of a questionnaire for evaluating nocturia, nocturnal enuresis and sleep-interruptions in an elderly population. Eur Urol. 2006;49:710–9. doi: 10.1016/j.eururo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Tosteson T, Boucher MA, et al. Measurement of sleep-related breathing disturbances in epidemiologic studies. Assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100:1281–6. doi: 10.1378/chest.100.5.1281. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K, Terai A. Classification and distribution of symptomatic nocturia with special attention to duration of time in bed: a patient-based study. BJU Int. 2005;95:1259–62. doi: 10.1111/j.1464-410X.2005.05515.x. [DOI] [PubMed] [Google Scholar]
- 26.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–44. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 27.Kaynak H, Kaynak D, Oztura I. Does frequency of nocturnal urination reflect the severity of sleep-disordered breathing? J Sleep Res. 2004;13:173–6. doi: 10.1111/j.1365-2869.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 28.Hajduk IA, Strollo PJ, Jr, Jasani RR, Atwood CW, Jr, Houck PR, Sanders M. Prevalence and predictors of nocturia in obstructive sleep apnea-hypopnea syndrome - a retrospective study. Sleep. 2003;1:61–4. [PubMed] [Google Scholar]
- 29.Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Nocturia and associated morbidity in a Danish population of men and women aged 60-80 years. BJU Int. 2008;102:808–14. doi: 10.1111/j.1464-410X.2008.07813.x. [DOI] [PubMed] [Google Scholar]
- 30.Swithinbank LV, Vestey S, Abrams P. Nocturnal polyuria in community dwelling women. Neurourol Urol. 1998;17:314–5. doi: 10.1111/j.1464-410x.2003.04683.x. [DOI] [PubMed] [Google Scholar]
- 31.Rembratt A, Norgaard JP, Andersson KE. Differences between nocturics and non-nocturics in voiding patterns: an analysis of frequency-volume charts from community-dwelling elderly. BJU Int. 2003;91:45–50. doi: 10.1046/j.1464-410x.2003.03079.x. [DOI] [PubMed] [Google Scholar]
- 32.Massolt ET, Wooning MM, Stijnen T, Vierhout ME. Prevalence, impact on the quality of life and pathophysiological determinants of nocturia in urinary incontinent women. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:132–7. doi: 10.1007/s00192-004-1239-4. [DOI] [PubMed] [Google Scholar]
- 33.Krieger J, Follenius M, Sforza E, Brandenberger G, Peter JD. Effects of treatment with nasal continuous positive airway pressure on atrial natriuretic peptide and arginine vasopressin release during sleep in patients with obstructive sleep apnoea. Clin Sci (Lond) 1991;80:443–9. doi: 10.1042/cs0800443. [DOI] [PubMed] [Google Scholar]
- 34.Matthiesen TB, Rittig S, Norgaard JP, Pedersen EB, Djurhuus JC. Nocturnal polyuria and natriuresis in male patients with nocturia and lower urinary tract symptoms. J Urol. 1996;156:1292–9. [PubMed] [Google Scholar]
- 35.Yalkut D, Lee LY, Grider J, Jorgensen M, Jackson B, Ott C. Mechanism of atrial natriuretic peptide release with increased inspiratory resistance. J Lab Clin Med. 1996;128:322–8. doi: 10.1016/s0022-2143(96)90034-7. [DOI] [PubMed] [Google Scholar]
- 36.Ouslander J, Johnson T, Nasr S, Schnelle J, Miller M. Atrial natriuretic peptide levels in geriatric patients with nocturia and nursing home residents with nighttime incontinence. J Am Geriatr Soc. 1999;47:1439–44. doi: 10.1111/j.1532-5415.1999.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 37.Svatikova A, Shamsuzzaman AS, Wolk R, Phillips BG, Olson LJ, Somers VK. Plasma brain natriuretic peptide in obstructive sleep apnea. Am J Cardiol. 2004;94:529–32. doi: 10.1016/j.amjcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Asplund R, Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. J Intern Med. 1991;229:131–4. doi: 10.1111/j.1365-2796.1991.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson TM, 2nd, Miller M, Pillion DJ, Ouslander JG. Arginine vasopressin and nocturnal polyuria in older adults with frequent nighttime voiding. J Urol. 2003;170:480–4. doi: 10.1097/01.ju.0000071406.18453.5f. [DOI] [PubMed] [Google Scholar]
- 40.Sugaya K, Nishijima S, Oda M, Morozumi M, Hatano T, Ogawa Y. Biochemical analysis of nocturia in the elderly. Neurourol Urodyn. 2001;20:458. doi: 10.1002/nau.20492. (abstract 55) [DOI] [PubMed] [Google Scholar]
- 41.Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Pathophysiological aspects of nocturia in a danish population of men and women age 60-80 years. J Urol. 2007;178:552–7. doi: 10.1016/j.juro.2007.03.141. [DOI] [PubMed] [Google Scholar]