Abstract
Study Objective:
To test the hypothesis that low iron availability, measured as transferrin saturation, is associated with low nocturnal hemoglobin oxygen saturation (SpO2) in children with homozygous sickle cell anemia (SCA; hemoglobin SS).
Methods:
This was a cross-sectional study of Tanzanian children with SCA who were not receiving regular blood transfusions. Thirty-two children (16 boys) with SCA (mean age 8.0, range 3.6-15.3 years) underwent motion-resistant nocturnal oximetry (Masimo Radical) and had steady state serum transferrin saturation and hematological indices assessed.
Results:
Higher transferrin saturation, adjusted for age and α-thalassemia deletion, was associated with lower nocturnal mean SpO2 (p = 0.013, r2 = 0.41), number of SpO2 dips/h > 3% from baseline (p = 0.008, r2 = 0.19) and with min/h with SpO2 < 90% (p = 0.026 r2 = 0.16). Transferrin saturation < 16% (indicative of iron deficiency) was associated with a 2.2% higher nocturnal mean SpO2.
Conclusions:
Contrary to our hypothesis, higher iron availability, assessed by transferrin saturation, is associated with nocturnal chronic and intermittent hemoglobin oxygen desaturation in SCA. Whether these associations are causal and are driven by hypoxia-inducible factor and hepcidin-mediated upregulation of demand for iron warrants further investigation.
Citation:
Cox SE; L'Esperance V; Makani J; Soka D; Prentice AM; Hill CM; Kirkham FJ. Sickle cell anemia: iron availability and nocturnal oximetry. J Clin Sleep Med 2012;8(5):541-545.
Keywords: Africa, anemia, sickle cell, hemoglobin saturation, hypoxemia, iron
Sickle cell anemia (SCA) remains one of the most common inherited diseases, with the major burden of disease in sub-Saharan Africa, where iron deficiency is also common.1 Determinants of the wide variation in the clinical expression of SCA are being investigated; current candidates include hemolytic rate2 and hemoglobin oxygen desaturation,3 both of which may be modified by iron status through effects on red cell count and mean cell hemoglobin concentrations (MCHC), thus affecting red cell oxygen carrying capacity. Iron deficiency, if severe enough, should contribute to the level of anemia in SCA and also decrease mean cell volume and MCHC. However, there is some evidence that in SCA, higher iron status is associated with increased hemolysis, lower hemoglobin, but higher MCHC.4 Higher MCHC may cause increased sickling rates as the propensity of sickle hemoglobin (HbS) to polymerize increases with concentration of HbS. Reduced MCHC in co-inherited α-thalassemia is a proposed mechanism for some beneficial effects in SCA.5 Thus red cell indices are affected by co-inherited α-thalassemia and iron status, but overall effects on hemoglobin oxygen saturations are unknown.
The prevalence of nocturnal hemoglobin oxygen desaturation is increased in SCA6 and is associated with complications including frequent painful crisis7 and vascular dysfunction.8,9 The underlying mechanisms of hemoglobin oxygen desaturation are poorly understood but likely involve anemia and red cell physiology. Reduced lung function in children with SCA does not appear to be an important factor,10,11 but interactions with cardiac function are likely in view of the high prevalence of elevated pulmonary pressures, with the potential for right-to-left shunting, as well as diastolic dysfunction.9,12
BRIEF SUMMARY
Current Knowledge/Study Rationale: Nocturnal hemoglobin oxygen desaturation in children with sickle cell disease is associated with increased rate of complications. Determinants of nocturnal hemoglobin oxygen desaturation in children with sickle cell disease are unclear, but we hypothesised that they could include iron deficiency, potentially common in African children with poor diets.
Study Impact: This study reports for the first time an association between increased iron availability measured as transferrin saturation and nocturnal hemoglobin oxygen desaturation, both sustained and intermittent, in non-transfused children with sickle cell disease. This suggests that iron deficiency may have potentially beneficial effects in sickle cell disease. It is also possible that this association is the result of reverse causality, with increased hemoglobin oxygen desaturation driving increased iron mobilization.
Limited data on iron status in SCA populations exist, partly because determination of iron status is complex due to inflammation increasing ferritin independently of iron status. Transferrin saturation may be reduced in inflammation, but values < 16% have been reported to be sensitive to response to iron supplementation in SCA.13 Iron deficiency may be underestimated in children with SCA and may be particularly common in sub-Saharan Africa. Low or absent iron stores within the bone marrow have been reported, especially in non-transfused patients.14
In children from the general population undergoing adenotonsillectomy, anemia and markers suggestive of iron deficiency are reported to be common.15 We therefore investigated the relationship between transferrin saturation, α-thalassemia genotype, and nocturnal oximetry in pediatric SCA patients with the hypothesis that those with evidence of iron deficiency would have lower SpO2.
PATIENTS AND METHODS
Ethical permission was granted by the Muhimibili University of Health – Allied Sciences ethics committee (MU/RP/AECNoI.XII/77). Written informed consent was obtained from parents or guardians in their own language and where appropriate, children's assent obtained. Data were collected between the 9 March and 19 June 2009.
Patients
Children (< 16 y) were recruited from confirmed HbSS patients enrolled in a cohort study at Muhimbili National Hospital, Dar-es-Salaam1 during a routine clinic visit when clinically well. All cohort children are routinely prescribed folate supplementation (5 mg/day) and prophylactic chloroquine. None of the children were receiving hydroxyurea or routine blood transfusions.
Nocturnal Pulse Oximetry
Motion-resistant pulse oximetry was sampled in the day at rest and over a single night using a 2-sec averaging time and 1 Hz sampling rate (Masimo Radical, Artemis UK). Sleep diaries were kept by the attending parent or guardian during the night. Data analysis was performed with Download 2001 software (Stowood Scientific, Oxford UK). Poor perfusion, low signal identification and quality, movement artifact data, and periods of wakefulness as recorded in the sleep diary were excluded manually. Only studies with a minimum of 4 h of artifact-free data were included. Analysis software yielded standard measures including mean and minimum SpO2 and desaturation index ≥ 3% from baseline.
Hematology and Iron Status Measurements
Averaged steady-state values for hemoglobin and other red cell indices from full blood pictures (Pentra 60, Horiba ABX, Kyoto, Japan) conducted at routine clinic visits in the previous year were calculated. Blood samples were collected between 08:00 and 10:00. Serum iron and total iron binding capacity were measured in stored samples (Architect C8000, Abbott, New York, USA) meeting steady-state criteria: temperature ≤ 37.4°C, negative malaria rapid test, and absence of pain or hospital admission within 90 days. Transferrin saturation was calculated from serum iron and total iron binding capacity.
Data Analysis
Variables were inspected for normality and multivariable linear regression was used (STATA 11-IC; StataCorp, College Station, TX, USA). No differences were observed with log-transformed or non-transformed variables with mild positive skew, thus results of non-transformed data are presented. P values < 0.05 were considered significant.
RESULTS
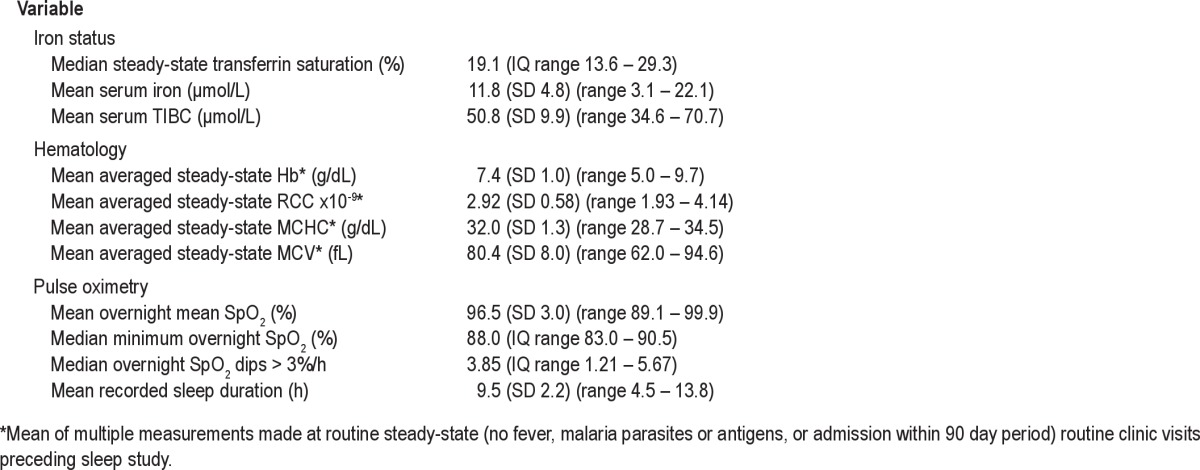
Out of 50 children with SCA in whom nocturnal oximetry was conducted, 32 (mean age 8.0 [SD 3.3; range 3.6-15.3] years, 16 boys (50%) also had transferrin saturation assessed. Descriptive statistics for transferrin saturation, averaged steady-state hematology, and pulse oximetry data are given in Table 1. There were no significant differences in age, sex, hematology, or pulse oximetry data between those who had transferrin saturation data available and those who did not (data not shown). Data from 3 (1 boy, 1 with α-thalassemia; mean age 7.0 [SD 1.4; range 5.7-8.3] years) were excluded because there were < 4 h of artifact-free data. There were no differences between transferrin saturation in those who were included (mean 22.2%, SD 10.9) and excluded (mean 17.3%, SD 3.8). Twenty-eight percent (9/32) had low transferrin saturation (< 16%) indicating probable iron deficiency (Table 1), a similar proportion to all SCA patients with data available (25%, N = 212/836, unpublished data, Cox et al.) but lower than in non-SCA Tanzanian control children (32/66, 48%). No SCA patients had high transferrin saturation (> 55%) which might indicate iron overload. Twenty-five patients (78%) had never received a blood transfusion. The remaining patients had received one (5 patients) or two (2 patients) blood transfusions in their lifetime. There was no association between transferrin saturation and age, sex, nutritional status (body mass index z-score), or blood transfusion history. Thirty of the 32 children with transferrin saturation data available had genotypes for the 3.7 α-thalassemia deletion: 11 (37%) were heterozygous and 1 was homozygous (3.3%). There were no statistically significant associations between transferrin saturation and steady-state averaged hemoglobin, red cell count, or mean cell volume in these children (data not shown). Presence of the 3.7 α-thalassemia deletion was associated with a 56% increase in transferrin saturation (p = 0.034), but was not significantly associated with any of the hematology variables in this study population (data not shown).
Table 1.
Iron status, steady-state hematology, and daytime and nocturnal pulse oximetry data in 32 Tanzanian children with sickle cell anemia
Associations with Nocturnal Hemoglobin Oxygen Saturation
Differences in nocturnal SpO2 and in the number of dips per hour in SpO2 > 3% from baseline levels according to transferrin saturation status (' or < 16%) and α thalassemia 3.7 deletion genotype, unadjusted for age are shown in Table 2. The results of multivariable linear regression analyses of the associations between nocturnal oximetry data and transferrin saturation, averaged steady-state hematology variables and α-thalassemia deletion, adjusted for age, are shown in Table 3. There was an inverse association between the number of dips per hour in SpO2 > 3% and mean nocturnal SpO2 (p < 0.001, adjusted r2 = 0.36). An increase in the number of dips per hour in SpO2 > 3% from baseline levels was associated with higher transferrin saturation (Figure 1, Table 3). However, minimum nocturnal SpO2 was not associated with transferrin saturation but was positively associated with increased red cell count and hemoglobin concentration (Table 3). Decreased mean nocturnal SpO2 was significantly associated with higher transferrin saturation, independent of age. Presence of the 3.7 α-thalassemia deletion was also significantly associated with higher transferrin saturation. However, when these variables were included in a single model, only age and transferrin saturation remained significantly associated with mean nocturnal SpO2 (Table 3).
Table 2.
Nocturnal mean SpO2 (%) and number of SpO2 dips > 3%/h according to iron status and α-thalassemia genotype.
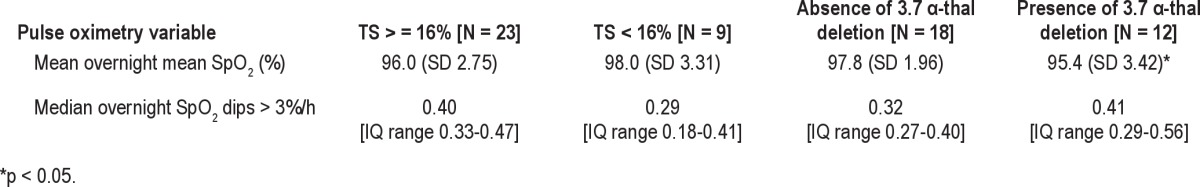
Table 3.
Relationships between nocturnal oximetry data and transferrin saturation, averaged steady-state hematology measures and α-thalassemia 3.7 deletion using linear regression, adjusted for age as indicated (N = 32, unless otherwise stated)
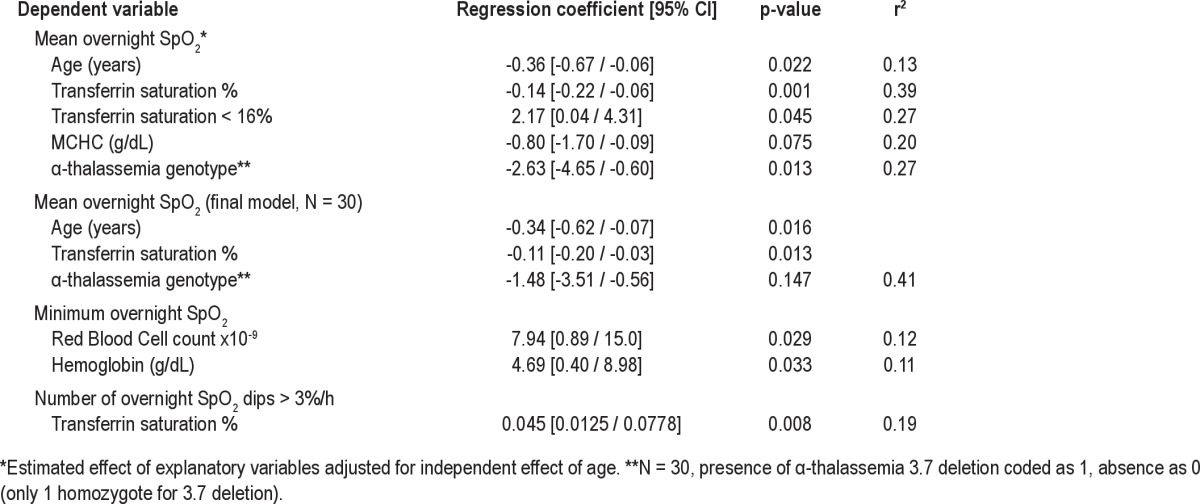
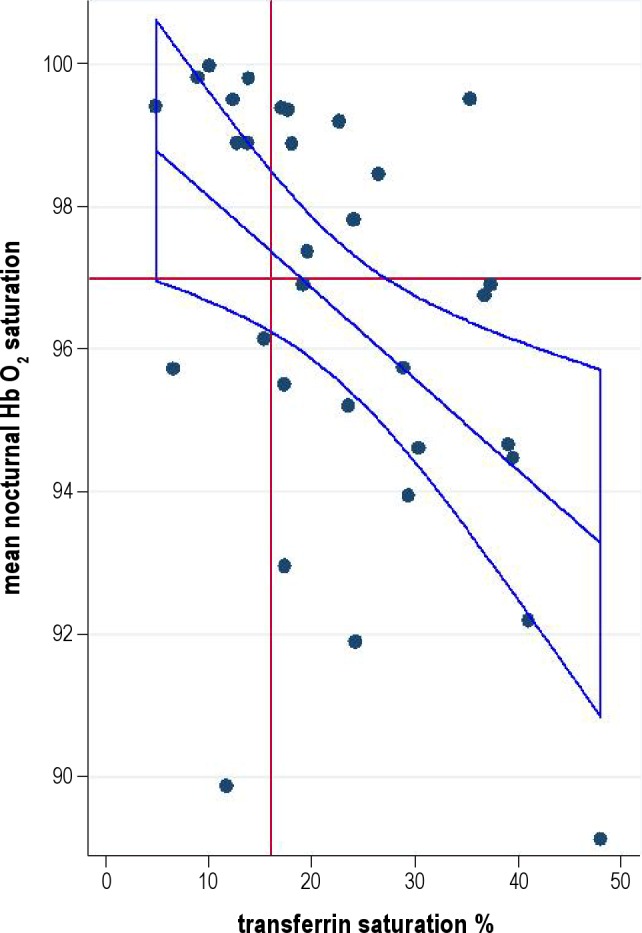
Figure 1. Association between transferrin saturation and mean nocturnal SpO2 showing the regression line and the 95% confidence interval.
DISCUSSION
In this study we report that nocturnal hemoglobin oxygen desaturation is associated with higher levels of transferrin saturation independently of the presence of the 3.7 α-thalassemia deletion in SCA, although levels were not suggestive of iron overload (unlikely in our non-transfused population). Although this relationship was unexpected and is counter to our prior hypothesis, it is in line with data from adults with obstructive sleep apnea (OSA) from the general population, in whom higher ferritin levels were associated with lower minimum overnight SpO2.16 Although this prior observation could have resulted from OSA associated inflammation as a confounding factor, in our study chronic inflammation would be expected to decrease transferrin saturation and is therefore unlikely to be a confounder.
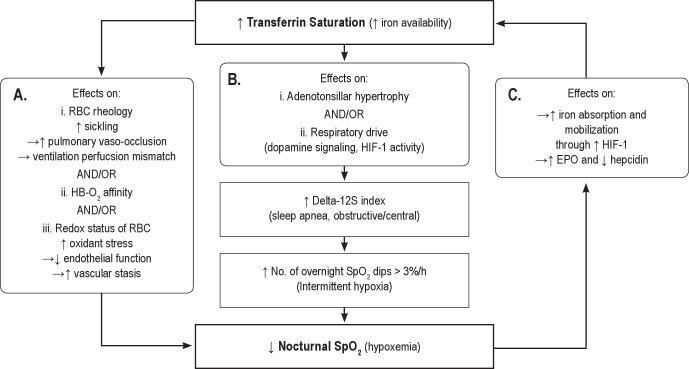
We can think of theoretical mechanisms by which higher iron availability could result in lower nocturnal SpO2 (Figure 2), but the most parsimonious explanation is that of reverse causality involving an effect of desaturation on iron metabolism through the hypoxic-inducible factors (HIF-1 and HIF-2).17 Hemoglobin oxygen desaturation causes upregulation of HIF-1 and HIF-2 levels, the downstream effects of which include increased erythropoietin, decreased hepcidin (a negative regulator of iron absorption and release from the reticuloendothelial system), and increased transferrin, increasing iron absorption and mobilization for erythropoiesis and thus higher transferrin saturation.17 The levels and exposure to chronic intermittent and sustained desaturation required to induce HIF in humans and in SCA remain to be determined. However, existing evidence from cell cultures and mouse models suggest chronic intermittent hypoxemia is a stronger and more long lasting stimulus than chronic hypoxemia.18
Figure 2. Potential mechanisms underlying the negative association between transferrin saturation and mean nocturnal SpO2.
In patients with SCA, whose hemoglobin polymerizes on deoxygenation, any decrease in mean nocturnal SpO2 or presence of significant dips in SpO2 is likely to have clinical relevance. In London children with sickle cell disease, an increase of one unit in mean nocturnal SpO2 was associated with an average fall of 0.83 in the number of days of pain per year requiring hospital treatment (p < 0.001), and the presence of any dips in SpO2 was independently associated with nearly 4 times the annual number of days admitted to hospital for pain.7
In conclusion, we have demonstrated in SCA that sustained nocturnal hemoglobin oxygen desaturation and the number of dips per hour > 3% from baseline in SpO2 were associated with higher transferrin saturation. The primary driver is probably hemoglobin oxygen desaturation, which upregulates iron absorption and erythropoiesis, with the paradoxical consequence that iron deficiency appears associated with higher SpO2. The longer-term effects of hemoglobin oxygen desaturation on iron status in children with SCA warrant further investigation in view of the clinical evidence of a link with unfavorable clinical course.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors warmly thank the patients and staff of MNH and MUHAS, Dar-es-Salaam, Tanzania who made this work possible. We also thank David Roberts and Charles Newton for reading the draft manuscript, the staff in the clinical chemistry unit at MNH central pathology laboratory for clinical chemistry and transferrin saturation analyses and Josephine Mgaya and Harvest Mariki for the hematology analyses and sample archiving. This research was supported by Wellcome Trust, UK: project grant 080025 to Dr. Cox, personal fellowship 072064; 084538 to Dr. Makani and a WT Student Elective Fellowship to Dr. L'Esperance 503831115.
Authorship contributions: Drs. Kirkham, Cox, and Hill conceived and designed the study; Dr. L'Esperance conducted the sleep studies, including manual processing of sleep data and contributed to data analysis; and Drs. Makani, Soka, and Cox collected the remaining data. Dr. Cox conducted the statistical analyses and wrote the paper. All authors contributed to critical evaluation and final drafting of the manuscript.
Ethics approval: Ethical permission was granted by the Muhimibili University of Health – Allied Sciences ethics committee (Ref: MU/RP/ AECNoI.XII/77).
ABBREVIATIONS
- SCA
sickle cell anemia
- Hb
hemoglobin
- SpO2
hemoglobin oxygen saturation
- OSA
obstructive sleep apnea
- HIF
hypoxic-inducible factor
REFERENCES
- 1.Makani J, Cox SE, Soka D, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS ONE. 2011;6:e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkham FJ, Datta AK. Hypoxic adaptation during development: relation to pattern of neurological presentation and cognitive disability. Dev Sci. 2006;9:411–27. doi: 10.1111/j.1467-7687.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro O, Poillon WN, Finke H, Massac E. Improvement of sickle cell anemia by iron-limited erythropoiesis. Am J Hematol. 1994;47:74–81. doi: 10.1002/ajh.2830470203. [DOI] [PubMed] [Google Scholar]
- 5.Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med. 2001;20:107–21. [PubMed] [Google Scholar]
- 6.Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol. 2008;140:336–9. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargrave D, Wade A, Evans J, Hewes D, Kirkham F. Nocturnal hypoxemia and painful sickle cell crises in children. Blood. 2003;101:846–8. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 8.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–5. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MC, Kirkham FJ, Redline S, et al. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood. 2010;116:16–21. doi: 10.1182/blood-2009-06-227447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Needleman JP, Franco ME, Varlotta L, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–22. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Campbell A, Minniti CP, Nouraie M, et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br J Haematol. 2009;147:352–9. doi: 10.1111/j.1365-2141.2009.07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lulla RR, Thompson AA, Liem RI. Elevated soluble transferrin receptor levels reflect increased erythropoietic drive rather than iron deficiency in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55:141–4. doi: 10.1002/pbc.22471. [DOI] [PubMed] [Google Scholar]
- 14.Koduri PR. Iron in sickle cell disease: a review why less is better. Am J Hematol. 2003;73:59–63. doi: 10.1002/ajh.10313. [DOI] [PubMed] [Google Scholar]
- 15.Kerstein R, Stimpson P, Caulfield H, Ellis G. Iron deficiency and sleep disordered breathing in children--cause or effect? Int J Pediatr Otorhinolaryngol. 2009;73:275–80. doi: 10.1016/j.ijporl.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien LM, Koo J, Fan L, et al. Iron stores, periodic leg movements, and sleepiness in obstructive sleep apnea. J Clin Sleep Med. 2009;5:525–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–9. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- 18.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–81. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]