Abstract
Study Objectives:
Adaptive servoventilation (ASV) is often used to treat central sleep apnea (CSA) and complex sleep apnea syndrome (CompSAS). Both CompSAS and CSA may occur in the setting of CHF and with the use of chronic opioids. We hypothesized that ASV would be less successful in treatment of CSA and CompSAS secondary to opioid use than in CHF patients.
Methods:
Consecutive patients were studied between January and December 2009 who underwent ASV titration for CSA or CompSAS due to CHF (defined as EF < 45%, or > 50% with evidence for diastolic dysfunction on echocardiogram) and chronic opioid users (defined by the use of opioids > 6 months).
Results:
Study included one hundred and eight patients with 77 males (71.3%) and 31 females (28.7%). Subjects had severe sleep apnea at baseline (AHI 45.6 ± 27.4) and inadequate control of sleep disordered breathing on CPAP (AHI 50.0 ± 32.2, CAI 36.6 ± 32). No significant differences were found between the groups in overall ASV success, defined as AHI < 10/h (p = 0.236). ASV was successful in 28 (59.6%) of those in the opioid group, compared to 43 (70.5%) of those in the CHF group. When ASV success was defined as AHI < 5/h at optimum EEP, there was again no significant difference between the groups (p-value = 0.812). Logistic regression showed unit increases in BMI, unit increases in HCO3, and presence of CSR were each associated with decreased likelihood of ASV success.
Conclusion:
We did not find a statistically significant difference in the effectiveness of ASV between CHF patients and chronic opioid users, with the overall success rate approaching 70%, as defined by an AHI < 10/h.
Commentary:
A commentary on this article appears in this issue on page 577.
Citation:
Ramar K; Ramar P; Morgenthaler TI. Adaptive servoventilation in patients with central or complex sleep apnea related to chronic opioid use and congestive heart failure. J Clin Sleep Med 2012;8(5):569-576.
Keywords: Adaptive servoventilation, central sleep apnea, complex sleep apnea, chronic opioid use, congestive heart failurex
Sleep physicians are increasingly confronted with patients who suffer with centrally mediated sleep disordered breathing. This is in part due to two trends. Since the consensus statement on liberalization of opioids for chronic pain use by the American Academy of Pain Medicine and the American Pain Society, the total doses of prescribed fentanyl, hydromorphone, morphine, and oxycodone have increased dramatically in the last decade.1 With continued increasing use of chronic opioids, there is greater risk and prevalence of central and obstructive sleep disordered breathing that may result in increased morbidity and mortality.2,3 Similarly, congestive heart failure (CHF) is on the rise, with a reported prevalence of 2% among the general population, increasing to 10% among patients 65 years or older, and it also is associated with increased presence of sleep disordered breathing, including central sleep apnea syndromes.4
Adaptive servoventilation (ASV) is often used to treat central sleep apnea (CSA) due to Cheyne-Stokes respiration (CSR) in the setting of CHF5–7 and complex sleep apnea syndrome (CompSAS).8,9 CompSAS is described as the development of central sleep apnea with the application of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA). Both CompSAS and CSA may occur in the setting of CHF and with the use of chronic opioid medications.10–13 The predominant pattern of CSA in CHF patients is CSR, which is a highly periodic breathing pattern. In contrast, both periodic and non-periodic breathing patterns of CSA or CompSAS are described with chronic opioid users.10–13 The periodic CSA breathing pattern in chronic opioid users is called cluster breathing, while the non-periodic or irregular form of CSA is called ataxic or Biot breathing pattern. The periodic cluster breathing pattern in chronic opioid users is considerably shorter in length than CSR. The non-periodic ataxic/Biot breathing pattern seen in opioid users is characterized by irregular frequency and variable respiratory rate and tidal volume. Though previous studies have shown ASV to be an effective treatment for CSA-CSR in CHF patients, studies assessing the effectiveness of ASV in chronic opioid users have shown conflicting results.14,15 One study showed it to be highly effective,15 while another reported it as insufficient to treat the sleep disordered breathing.14 The study samples in these two studies were small (5 and 22 patients, respectively). In addition, there are no studies to date that compare the effectiveness of ASV between the CHF and opioid users. This study compares the efficacy of ASV in the treatment of CSA and CompSAS secondary to opioid use with those secondary to CHF.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Based on its internal proprietary algorithm, we hypothesized that adaptive servoventilation (ASV) might function better in the presence of a highly regular and periodic central sleep disordered breathing (such as Cheyne-Stokes respiration (CSR) in the setting of CHF) compared to the non-periodic (such as irregular or ataxic/Biot breathing patterns in chronic opioid users) central sleep disordered breathing.
Study Impact: ASV was as effective in the treatment of central and complex sleep apnea in chronic opioid users as it is in patients with CHF, with the overall success rate of 70% as defined by an AHI ≤ 10/hour. Lower body mass index, but higher bicarbonate values and absence of CSR, were predictors for ASV success in both groups.
Adaptive servoventilation (ASV) is a pressure preset, volume or flow cycled form of closed-loop mechanical ventilator that monitors the patient's breathing pattern over a certain period of time. It uses an internal algorithm to provide breath-by-breath dynamic adjustment of inspiratory pressure support with a back-up respiratory rate to normalize breathing patterns to a predetermined target of either minute ventilation or peak flow, thereby stabilizing the central sleep disordered breathing pattern. Embedded computer-based algorithms use logic to determine whether a given hypopneic breath is towards the beginning of a hyperpnea or leading towards an apnea so that they may adjust the delivered support accordingly. Since ASV, based on its internal proprietary algorithm might function more optimally in the presence of a regular or periodic form of central sleep disordered breathing such as CSR, we considered, as was suggested by Farney et al., that it may prove less effective for the non-periodic, more irregular breathing pattern such as ataxic/Biot breathing pattern seen in chronic opioid users.14 We therefore hypothesized that ASV would be less successful in treatment of CSA and CompSAS secondary to opioid use than in those secondary to CHF.
METHODS
Overview and Study Design
The study was approved by the Mayo Clinic Institutional Review Board (IRB Application #:11-007500). Retrospectively, we screened consecutive patients referred to our sleep center who underwent ASV titration between January and December 2009. Patients were selected if their baseline diagnostic polysomnography (PSG) showed an AHI ≥ 5/h and subsequent continuous positive airway pressure (CPAP) titration showed control of obstructive events but a residual AHI > 5/h or the persistence of CSA and/or CSR on CPAP. All of our patients were identified based upon split-night studies. They were allotted in the CHF group if they had a transthoracic echocardiogram that showed an ejection fraction (EF) ≤ 45%, or if they had an EF > 50% but had documentation of heart failure with preserved EF (diastolic dysfunction). The chronic opioid group included those who had been treated with opioid medications > 6 months. Patients were excluded from analysis if they did not meet our definition for CHF or chronic opioid use.
Setting and Participants
One hundred and eight patients were found eligible based on the above screening criteria. Patient records from 2009 contained data on the titration study to assess the optimum AHI at the prescribed end-expiratory pressure (EEP) on ASV titration. EEP was also titrated to a maximum of 15 cm of water, if needed.
Clinical records of consults and follow-up visits were reviewed, and data were collected including age, gender, body mass index (BMI), EF from echocardiogram, and presence or absence of atrial fibrillation. Polysomnographic data on both the diagnostic and titration studies were also collected as stated below. The main reason for the use of chronic opioids was for chronic pain syndrome.
Polysomnography
Polysomnography (PSG) was performed using a digital polygraph (Nicolette, San Diego, CA). We used the electroencephalogram ([EEG] 2 channels), electroculogram ([EOG] 2 channels), and electromyogram ([EMG] 1 channel) montage as described in the recent scoring manual of the American Academy of Sleep Medicine.16 Airflow and respiratory effort were monitored using oronasal thermocouple and nasal pressure transducer, respiratory inductive plethysmography (RIP) during the diagnostic study, and, during titration, using the flow channel from the CPAP and bilevel positive airway pressure (BPAP) plus RIP. Sleep staging and arousals were scored.17
An apnea was defined as cessation of inspiratory flow (> 90% reduction in airflow signal) ≥ 10 sec. An obstructive apnea was defined as the absence of airflow in the presence of rib cage and abdominal excursions. A central apnea was defined as the absence of airflow with absence of rib cage and abdominal excursions. Hypopnea was defined as a 30% reduction in airflow signal lasting ≥ 10 sec associated with ≥ 4% drop in oxygen saturation. We defined CSA to be present if the central apnea index (CAI) was > 5/h and the CAI was ≥ 50% of the AHI. CSA due to CSR was considered to be present based on the interpreting physician's report or consult note; CSR was defined by the presence of ≥ 3 consecutive cycles of cyclical crescendo and decrescendo breathing consisting of apneas, hypopneas, hyperpneas, and hypopneas, with each cycle lasting ≥ 45 sec, and a central apnea index ≥ 5/h. Complex sleep apnea (CompSAS) was considered present if the diagnostic PSG demonstrated findings consistent with obstructive sleep apnea, but CPAP titration sufficient to alleviate obstruction left residual central apneas and/or periodic breathing with hypopneas sufficient to produce an AHI ≥ 5/h.
Standard protocols for our sleep laboratory were used to titrate for CPAP and ASV. Different masks were used based on patient's preference, comfort, and fit. Mask leaks were monitored and corrections made accordingly. CPAP (Respironics Inc., Murrysville, PA) titration was performed using a uniform and standard approach. Titration usually began at CPAP of 5 cm of water and titrated to eliminate obstructive sleep disordered breathing events. If central apneas developed, the pressure was increased to monitor for improvement; if not, the pressure was brought back down to the pressure that eliminated the obstructive events. The development and persistence of central sleep apnea on CPAP as defined above led to ASV titration. ASV titration was performed using a ResMed VPAP Adapt SV (ResMed Ltd., NSW, Australia). The EEP was started at 5 cm of water or at the CPAP that eliminated OSA during the CPAP titration. Titration of EEP was performed by the technician to eliminate OSA, while the pressure support was on default settings (minimum inspiratory pressure support of 3 cm of water, maximum inspiratory pressure support of 10 cm of water above EEP that was controlled by the internal algorithm of the machine to target minute ventilation). The back-up rate was in the auto mode. The EEP could be adjusted to a maximum of 15 cm of water pressure.
Comparison and Outcomes
We assessed the effectiveness of ASV to treat CSA and CompSAS between the CHF group and chronic opioid group. For the primary analysis, we considered treatment successful if the ASV settings resulted in an AHI < 10/h. As a secondary analysis, we also looked at our data using an AHI threshold for control of < 5/h at the optimally prescribed EEP (that EEP that eliminated obstructive events while minimizing central events and sleep disruption, based upon sleep specialist's review of PSG). Finally, we assessed and compared sleep architecture, arousal index, periodic leg movements, and predictors for ASV success between the 2 groups.
Statistical Analyses
All analyses were done in Statistical Analysis System (SAS; version 9.2; SAS Institute, Cary, NC). The primary outcome was ASV success defined as AHI < 10. The secondary outcome was defined as AHI < 5 at the optimum EEP measured during ASV titration. Logistic regression was used to determine which variables predicted ASV success in the overall group as well as separately within the CHF and opioid groups. All p-values were 2-tailed, and statistical significance was defined with p < 0.05. Univariate analyses were first conducted on factors including BMI, age, and AHI during the diagnostic study, AHI on CPAP titration (CPAP AHI), bicarbonate levels (since most patients did not have arterial blood gas analysis to identify PaCO2 levels, we assessed bicarbonate levels close to the time of their PSGs), presence or absence of CSR, presence or absence of CSA, presence or absence of atrial fibrillation, gender, and EF from echocardiogram to identify factors that were independently associated with ASV success. Models were built with 3 different ASV success criteria, one defined as overall titration period AHI < 10/h, and the others with AHI < 5/h or 10/h at the optimum EEP. Based on the Wald test from logistic regression, predictors with p-value < 0.25 were included as candidates for multivariate analysis. This cutoff was selected rather than the traditional p-value cutpoint of 0.05 so as to include important co-variates in the model. Stepwise selection was performed in which the most nonsignificant (p-value > 0.05) and non-confounding co-variates (change in parameter estimate > 20%) were removed iteratively from the model. Hosmer-Lemeshow Goodness of fit test was used to assess model fit. All models were assessed at p < 0.05.
RESULTS
Demographics
Of 108 patients meeting our selection criteria, 61 (56.48%) had CSA during the diagnostic portion of the night and belonged to the CHF group. Of the 61 patients with CSA, 19 (31.1%) had CSR. Chronic opioid users comprised the remaining 47 of our 108 subjects; among them, 28 had CompSAS (59.6%), while the remaining 19 had CSA. The sample included 77 males (71.3%) and 31 females (28.7%) who ranged in age from 26 to 89 years. Among the males, 23 (29.9%) were in the opioid group and 54 (70.1%) were in the CHF group. Tables 1, 2, and 3 provide characteristics of all subjects, and comparisons between CHF and opioid groups. Forty-six of 61 patients (75%) had systolic congestive heart failure, as defined by an EF ≤ 45%. All 61 CHF patients were in a stable treatment phase at the time of their PSG.
Table 1.
Demographic characteristics
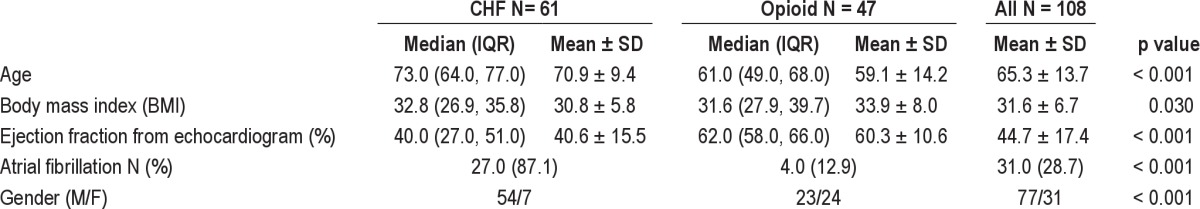
Table 2.
Polysomnographic respiratory measures
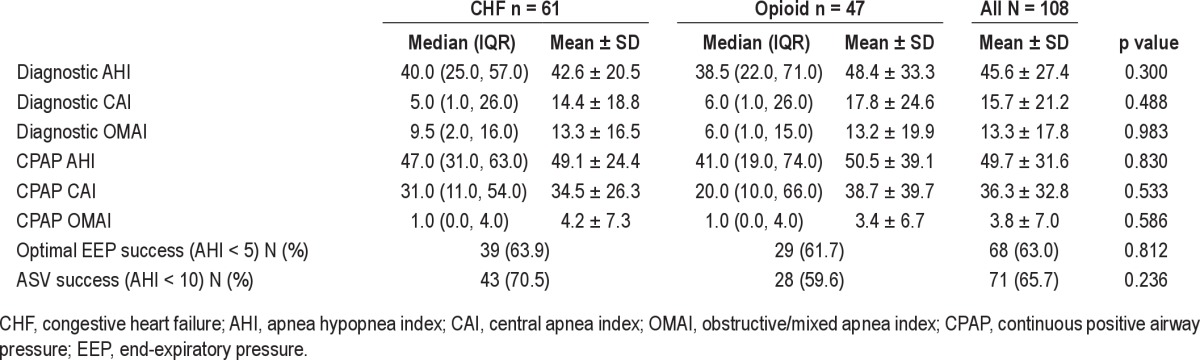
Table 3.
Polysomnographic sleep characteristics
Overall, the subjects had severe sleep apnea at baseline (AHI 45.6 ± 27.4) and had significantly abnormal breathing on CPAP (AHI 50.0 ± 32.2, with CAI 36.6 ± 32). Compared with the opioid group, the CHF group had a significantly higher proportion of men, were older in age, had lower LVEF, and had higher residual CAI on CPAP (Table 1). The 2 groups were not otherwise significantly different with respect to measures of sleep disordered breathing
Efficacy
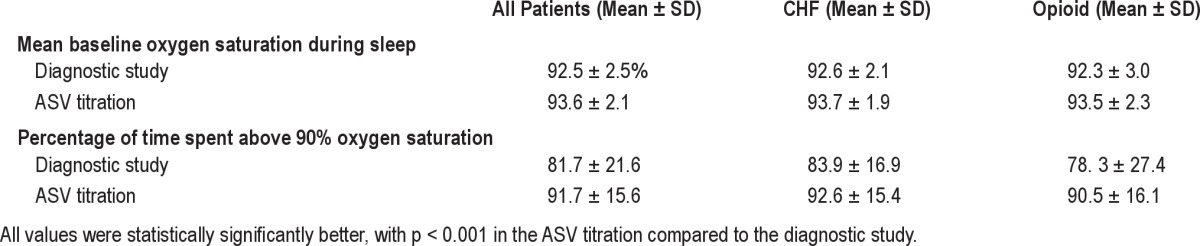
There was no significant difference between the groups in overall ASV success defined as AHI < 10/h (p = 0.236). Of those in the opioid group, 28 (59.6%) found success with ASV, compared to 43 (70.5%) of those in the CHF group. When success was defined as AHI < 5/h at the optimum EEP, there was again no significant difference between the groups having ASV success (χ2 p-value = 0.812). Of those in the opioid group, 29 (61.7%) found success with ASV, compared to 39 (63.9%) of those in the CHF group. Similarly, no significant differences were noted in ASV success (as defined by an AHI < 10/h) between overall CSA patients (irrespective of whether they belonged to the CHF or chronic opioid group) and CompSAS patients (64.2% vs 65.7%, p = 0.8826). The average EEP was 8 (± 2) cm of water at the end of titration. The mean baseline oxygen saturation during sleep was 92.5% ± 2.5%, and improved during ASV titration to 93.6% ± 2.1% (p < 0.001). Similarly, the percentage of time spent above 90% during the diagnostic portion of the study improved from 81.7 ± 21.6 to 91.7 ± 15.6 (p < 0.001) (Table 5).
Table 5.
Oxygen saturation during the diagnostic study vs. ASV titration
The men made up a significantly greater proportion of the CHF group than the opioid group (88.5% vs. 48.9%), however gender was not associated with treatment success as defined by an AHI < 5/h at the optimum prescribed EEP (p = 0.274), or by an AHI < 10/h on ASV titration (p = 0.781). There was also a significant difference in age between the 2 groups (p < 0.001); the CHF group had a median age of 73 (range 47-89), while the opioid group had a median age of 61 (range 26-84). However, age was also not significantly associated with ASV success using AHI < 10/h (p = 0.647), or ASV success using an AHI < 5/h at optimum prescribed EEP (p = 0.733). The overall mean BMI was 31.6 (range 16.3-53.5); mean BMI did vary significantly between the opioid and CHF groups (32.8, range 16.3-53.5 and 31.6, range 18.8-43.6, respectively, p = 0.038) and was a significant predictor of ASV success in both groups as discussed below. There was also a significant difference in mean EF between the groups (60.3 vs. 40.6, p < 0.001) and in the presence of atrial fibrillation (4% vs. 27%, p < 0.001) in the opioid vs. CHF group, respectively.
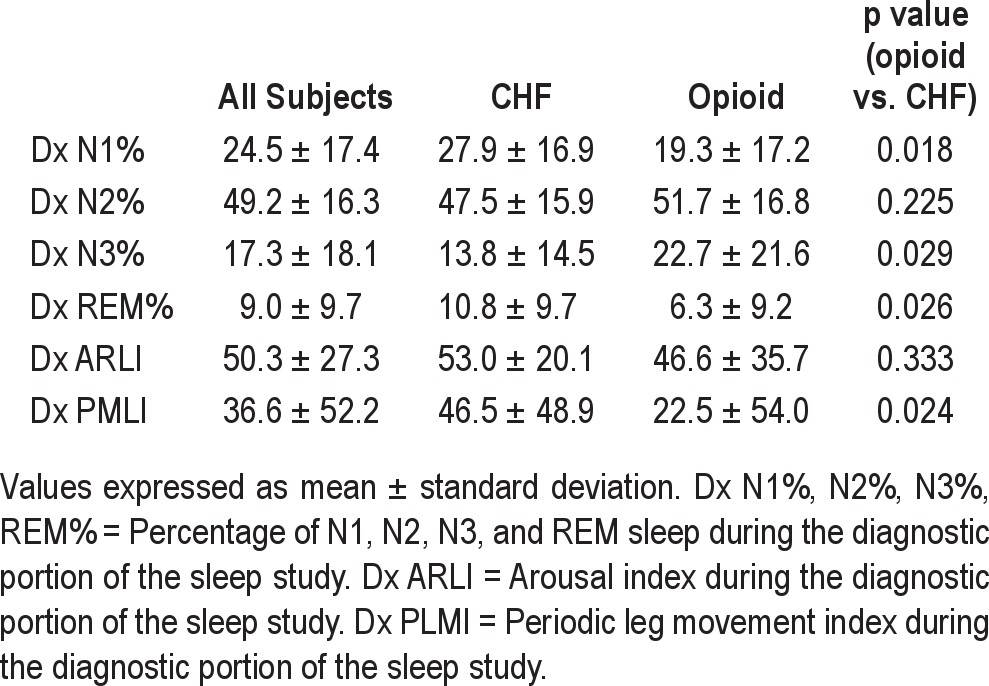
Summary distributions of sleep architecture, arousal index, and periodic leg movements in our subjects are found in Table 3. Overall, there was a decrease in stage N1, increase in stages N2 and N3, and decrease in stage R in the opioid group compared to the CHF group. The periodic leg movement index (PLMI) and arousal index was lower in the opioid group than in the CHF group.
Predictors of ASV Success
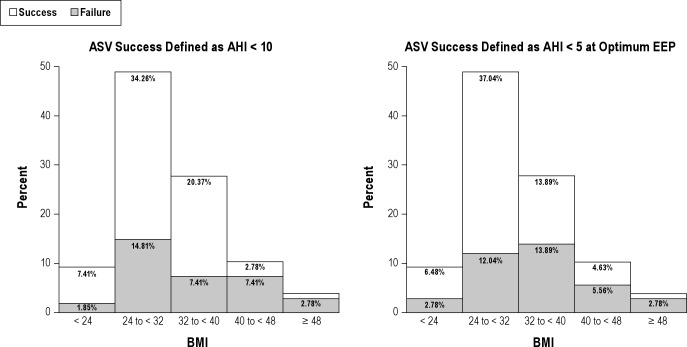
Logistic regression showed that BMI and CPAP AHI were inversely predictive of overall ASV success using the AHI < 10/h criterion (OR 0.920; CI 0.861, 0.983 and OR 0.984: CI 0.970, 0.998, respectively; Table 4). When ASV success was defined by AHI < 5/h at the optimum EEP, BMI, CSR, bicarbonate levels were all significant predictors of ASV success (OR 0.851: CI 0.775, 0.934; OR 0.213: CI 0.062, 0.727; OR 1.218: CI 1.008, 1.471, respectively). Unit increases in BMI, unit increases in HCO3, and presence of CSR were each associated with decreased likelihood of ASV success (Figure 1). Of note, in the last model, CSR and HCO3 were not significant in univariate analysis; however, they were forced in to the multivariate model due to biologic relevance and came out as significant predictors of ASV success. Overall, higher measures of BMI, especially in the overweight class, correlated with lower probability of ASV success with AHI < 10/h (p = 0.0005) and AHI < 5/h at optimum EEP (p = 0.0048). Interestingly, the highest proportion of those who had failures at AHI < 10/h (37%) had a BMI in the range of 30-36 (Figure 1).
Table 4.
Univariate and multivariate correlates of ASV success
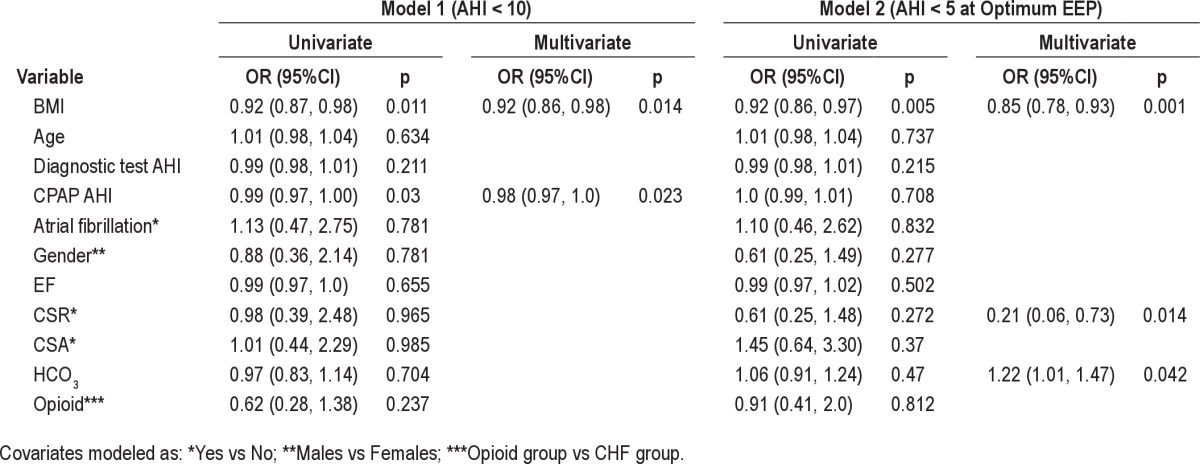
Figure 1. Overall percent success and failure by BMI.
Proportions of overall success and failure by body mass index (BMI). Unit increases in BMI were associated with decreased likelihood of ASV success. The greatest difference in proportion of successes compared to failures at AHI < 10/h (19%) occurred among those with a BMI in the range of 24 to < 32. The difference between proportion of success compared to failures in this BMI range was even more pronounced (25%) when success was defined as AHI < 5/h at optimum EEP.
DISCUSSION
The findings of this study indicate that ASV is as effective in the treatment of central and complex sleep apnea in chronic opioid users as it is in patients with CHF. Overall success rates using more liberal criteria (AHI < 10 on ASV titration) or more stringent criteria (AHI < 5/h at best EEP), were statistically not different. The predictors for success with ASV in both groups were lower BMI, higher bicarbonate values, and absence of CSR. This is not the result we expected. We had hypothesized that ASV would be more likely to succeed in patients with CHF, especially if they had CSR. Although not specifically part of our initial hypothesis, we had considered it likely that since patients with CHF and CSR often have lower PaCO2 and bicarbonate levels, that bicarbonate levels would inversely correlate with likelihood of success. Again, this proved to not be the case.
Of the 108 patients, ASV was successful in reaching an AHI < 5 in 63% and AHI < 10 in 73.2%. These rates are consistent with findings of most other studies. Success rates for ASV (using an AHI < 10) in patients with CompSAS have been reported in as low as 18%14 and as high as 100%15 associated with chronic opioid use, and in more mixed populations as 70% to 100%.8,18–20 Published success rates for patients with CSR have ranged from 73% to 100%.8,21–25 Although our overall success rates were not dissimilar to prior reports, the reason why ASV succeeds in controlling some, but not all types of central sleep apnea remains elusive. Possible mechanisms for ASV failure might include inadequate stabilization of the upper airway, high interface leak, ineffective pressure support, patient-ventilator dyssynchrony due to poorly timed pressure support, or unstable sleep leading to oscillation between ventilatory control patterns.
Role of Upper Airway Stabilization, Interface Leak, and Ineffective Pressure Support
CPAP provides a pneumatic splint for the upper airway throughout the breathing cycle and successfully treats obstructive sleep apnea (OSA). The expiratory pressure on ASV is titrated to eliminate OSA, while the variable inspiratory pressure support along with the back-up rate tries to eliminate the central sleep disordered breathing events. CPAP has some success in studies to treat CSA in the setting of heart failure,26–28 though overall effectiveness is inferior to ASV.5–7,29 In contrast, CPAP alone is most often ineffective in controlling sleep disordered breathing in patients with chronic opioid use.12,14 In our own study, though it helped to control OSA, CPAP overall was unsuccessful in controlling central sleep disordered breathing in opioid users, and in fact worsened the CSA, similar to findings in other studies. When using ASV, it appears that we achieved adequate EEP, since on ASV the OMAI was only 3.4 ± 6.7/h. Therefore, we do not think the ASV failures were due to inadequate stabilization of the upper airway. It also seems likely that leak was not a major contributor, since titration was performed manually under direct sleep technologist observation; the technologists promptly adjust interfaces when leaking is detected. However, we did not measure leak, and this could be an opportunity for improvement in our practice and outcomes. The fact that higher BMI was associated with lower likelihood of ASV success may speculatively reflect an expected decreased effectiveness of pressure support in enhancing tidal volumes when the respiratory compliance is reduced by obesity. We did not measure tidal volumes in a calibrated fashion, and thus were not able to compare volumes between ASV success and failures.
Patient-Ventilator Dyssynchrony
Failure via ventilatory-patient dyssynchrony might be expected when ventilatory patterns being generated by the ASV do not match or compensate adequately for the patient's pattern. We had postulated that since different underlying mechanisms result in central sleep apnea patterns in patients with chronic opioid use vs. CHF that the interaction of the central respiratory controller with ASV algorithms would be characteristically different. The central sleep apnea patterns noted with CSR (in association mostly with CHF) are most pronounced during NREM sleep and clearly represent the manifestation of a respiratory system with high loop gain and delayed feedback. The pattern is typically reduced during REM sleep because ventilation is less reliant on feedback mechanisms. The resultant ventilatory pattern is predictable and we thought would result in better synchrony with the ASV algorithm. In contrast, most opioid induced sleep disordered breathing patterns are less periodic, poorly attenuated in REM, and the pathophysiology is complex and less well worked out. Opioids appear to exert differential effects on central and peripheral chemoreceptors.30 In addition, opioids inhibit the inspiratory rhythm generating neurons located in the pre-Bötzinger complex neurons, but do not appear to affect the expiratory motor neurons located in the retrotrapezoid nucleus/parafacial respiratory group.31 Thus opioids interfere with normal ventilatory rhythmogenesis and regulation, resulting in less predictable patterns of ventilation than in CSR. Some of the breathing patterns in chronic opioid users are similar to those in heart failure patients, such as the presence of OSA and the cluster breathing pattern, though the cycle lengths in the periodic breathing pattern are considerably shorter in the cluster breathing than in the CSR pattern seen in heart failure patients. In contrast, ataxic/Biot breathing pattern is a non-periodic irregular breathing pattern seen in chronic opioid users but not in CHF.
There were differences in the proportion of sleep stages between the CHF and opioid group during the diagnostic study (Table 3), with a higher percentage of stage N3 and lower REM in the chronic opioid group than the CHF group. Decrease in REM has been reported in chronic opioid users32; however, results are conflicting for NREM sleep. Most studies show a decrease in stage N1 and an increase in stage N2 compared to controls not on opioids, but the results are conflicting with stage N3.12,32 We found a higher percentage of stage N3 in chronic opioid group than the CHF group. Our reported percentage of stage N3 is higher than the previous reported literature in chronic opioid users.12,32 Whether this has any effect on the efficacy of the PAP therapy is unclear, though we believe it not to be the case.
We postulated that the ASV algorithm would synchronize ventilation more poorly with opioid-related SDB than with CHF-associated patterns. We did not demonstrate this. In fact, when we analyzed the influence of CSR pattern on ASV success by forcing CSR into the regression model, its presence correlated negatively. This may be reflective of imprecise characterization of the breathing patterns. Patients might have experienced CSR for only relatively short percentages of the study (for example, 3-5 min out of an entire night) and yet would be characterized as having demonstrated CSR. Our hypothesis regarding a positive correlation between a regular cyclic breathing pattern and ASV success might be better studied by using a more global measure of periodicity across the entire study, such as percent of time spent in a periodic pattern; we did not design our study to accommodate such measures. CSR associated with CHF is most often accompanied by hypocapnia, which we thought might be reflected in serum bicarbonate levels, thus hypothesizing that lower serum bicarbonate levels would be associated with a greater likelihood of ASV success. Again, our hypothesis was proven incorrect. This may be influenced by the fact that not all of our subjects had bicarbonate levels immediately prior to ASV titration. Some of the levels were obtained shortly afterward as a result of other clinical needs. It is possible the bicarbonate levels do not represent in any way the PaCO2 levels at the time of ASV titration. Therefore, the contribution of this mechanism for ASV failure cannot be reliably inferred from our data.
Although our study identified BMI, CSR, and HCO3 levels as significant predictors of ASV success, there may be other factors that might determine success with ASV in chronic opioid users that we did not discern. The type and duration of opioids used, the predominant breathing pattern, i.e., cluster vs. ataxic/Biot breathing pattern, comorbid conditions, and co-ingestion of other medications such as benzodiazepines may be other factors that should be assessed and possibly controlled for, in future studies.
The main limitation of our study is the retrospective nature of our assessment, with no prospective randomization or blinding performed. Referral bias is also likely, and this may have affected the overall effectiveness of ASV, as patients referred to our center were the ones who likely failed therapies elsewhere. While our sample size was small and could have resulted in a type II error, post hoc power calculation of 77% suggests that a type II error is not unlikely with this sample size. Another limitation of our study was the use of bicarbonate levels to indirectly assess pCO2 as most patients did not have arterial blood gas analysis around the time of their PSG. Though our assumption was that bicarbonate values would inversely correlate with pCO2, it is possible that bicarbonate levels may have been affected by various conditions such as the use of diuretics. Not reporting the exact dose of opioids consumed around the time of the PSG, or whether the patients were concomitantly taking benzodiazepines, is another limitation of our study. Due to the retrospective nature of our study, however, we knew that the patients were on opioids based on the medication list; it was difficult to assess the exact dose at the time of the PSG or the type of chronic pain syndrome, as this information was not available on the consult or progress note. We compared two patient cohorts with different underlying diseases. However, both CHF and chronic opioid use are associated with central sleep apnea, and ASV is currently being used to treat varied types of central sleep apnea.
The main intent of this study was to assess whether ASV would be an effective treatment for central sleep apnea, inclusive of CSR and CompSAS, and irrespective of the underlying cause or disorder that triggers it. CompSAS that occurs in the setting of chronic opioids may be different in pathophysiology (probably a “dulling” of the loop gain mechanism) than that due to CSA/CSR (where the loop gain is high with increased chemosensitivity). Therefore, we hypothesized that ASV as a treatment option would not work well in patients on chronic opioids. Although previous data suggest that both CSA and CompSAS improve over time in some patients undergoing treatment with CPAP,33 the retrospective nature of our study design did not allow us to address this, as all patients in our sample with persistent CSA on CPAP immediately underwent ASV titration. A future prospective and randomized study could be designed to specifically address this question. The main purpose of this study was to assess which patients are most likely to acutely succeed with ASV, rather than determining whether ASV would succeed better than CPAP of chronic use. Also, most studies in our center are performed based on the split-night protocol.34 Though there might have been differences in the proportion of obstructive and central sleep disordered breathing between the first and second half of the night, it would be difficult to speculate whether it would have made a difference, as all our patients in this study were selected based on the split night protocol. Additionally, we used only the VPAP Adapt SV, which is a ResMed device and not the Respironics BiPAP AutoSV device during our ASV titration. There are differences between these two machines in how they address the underlying central sleep disordered breathing events. The AutoSV uses a different target (peak flow) in its servomechanism, while the VPAP Adapt SV targets the minute ventilation. Consequently, our findings may not apply to patients using the Respironics BiPAP autoSV. Despite these limitations, our data are the most comprehensive regarding the use and comparing the effectiveness of ASV between CHF and chronic opioid groups. Also, our sample size is the largest on opioid users using ASV to treat their sleep disordered breathing.
CONCLUSION
In conclusion, we did not find a statistically significant difference in the effectiveness of ASV between the CHF patients and chronic opioid users, with the overall success rate approaching around 70%, as defined by an AHI of less than 10/h. With the increasing use of chronic opioids and increasing prevalence of congestive heart failure and their associated breathing disorders, further studies are needed to assess predictors for success in this group of patients.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Morgenthaler is the principal investigator in a multicenter trial for which his institution as received funding form ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Ramar acknowledges funding via an internal faculty development grant from the Mayo Clinic Department of Internal Medicine to prepare and submit this manuscript. Work for this study was performed at Mayo Clinic, Rochester, MN, USA.
REFERENCES
- 1.Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810–33. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 2.Caravati EM, Grey T, Nangle B, et al. Increase in poisoning deaths caused by non-illicit drugs--Utah, 1991-2003. MMWR Morb Mortal Wkly Rep. 2005;54:33–6. [PubMed] [Google Scholar]
- 3.Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998-2005. Arch Intern Med. 2007;167:1752–9. doi: 10.1001/archinte.167.16.1752. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 6.Philippe C, Stoica-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–42. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 8.Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–46. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 10.Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–9. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- 11.Teichtahl H, Prodromidis A, Miller B, Cherry G, Kronborg I. Sleep-disordered breathing in stable methadone programme patients: a pilot study. Addiction. 2001;96:395–403. doi: 10.1046/j.1360-0443.2001.9633954.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3:455–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Teichtahl H, Wang D, Cunnington D, et al. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest. 2005;128:1339–47. doi: 10.1378/chest.128.3.1339. [DOI] [PubMed] [Google Scholar]
- 14.Farney RJ, Walker JM, Boyle KM, Cloward TV, Shilling KC. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4:311–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4:305–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specification. [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized terminology techniques and scoring system of sleep stages of human subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 18.Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7:187–95. [PMC free article] [PubMed] [Google Scholar]
- 19.Randerath WJ, Galetke W, Kenter M, Richter K, Schafer T. Combined adaptive servo-ventilation and automatic positive airway pressure (anticyclic modulated ventilation) in co-existing obstructive and central sleep apnea syndrome and periodic breathing. Sleep Med. 2009;10:898–903. doi: 10.1016/j.sleep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Randerath WJ, Galetke W, Stieglitz S, Laumanns C, Schafer T. Adaptive servo-ventilation in patients with coexisting obstructive sleep apnoea/hypopnoea and Cheyne-Stokes respiration. Sleep Med. 2008;9:823–30. doi: 10.1016/j.sleep.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Banno K, Okamura K, Kryger MH. Adaptive servo-ventilation in patients with idiopathic Cheyne-Stokes breathing. J Clin Sleep Med. 2006;2:181–6. [PubMed] [Google Scholar]
- 22.Bitter T, Westerheide N, Faber L, et al. Adaptive servoventilation in diastolic heart failure and Cheyne-Stokes respiration. Eur Respir J. 2010;36:385–92. doi: 10.1183/09031936.00045609. [DOI] [PubMed] [Google Scholar]
- 23.Bitter T, Westerheide N, Hossain MS, et al. Complex sleep apnoea in congestive heart failure. Thorax. 2011;66:402–7. doi: 10.1136/thx.2010.146522. [DOI] [PubMed] [Google Scholar]
- 24.Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3:140–8. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XL, Yin KS, Jiang SS, Li XL, Jia EZ, Su M. [Efficacy of adaptive pressure support servo-ventilation in patients with congestive heart failure and Cheyne-Stokes respiration] Zhonghua Yi Xue Za Zhi. 2006;86:1620–3. [PubMed] [Google Scholar]
- 26.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 27.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 28.Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:92–7. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 29.Szollosi I, O'Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 30.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90:1329–38. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–6. [PubMed] [Google Scholar]
- 33.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Khawaja IS, Olson EJ, van der Walt C, et al. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med. 2010;6:357–62. [PMC free article] [PubMed] [Google Scholar]