Abstract
Study Objectives:
We sought to provide an updated systematic review and meta-analysis of studies investigating the effect of positive airway pressure (PAP) treatment for obstructive sleep apnea (OSA) on systolic and diastolic blood pressure (SBP, DBP).
Methods:
Two independent investigators undertook a systematic search of the PubMed database (1980-2012) to identify randomized controlled trials comparing therapeutic PAP to sham-PAP, pill placebo, or standard care over at least one week in adult OSA patients without major comorbidities. The mean, variance, and sample size for diurnal and nocturnal SBP and DBP data were also extracted independently from each study. Random effects meta-analyses were conducted, followed by pre-specified subgroup and meta-regression analyses.
Results:
32 studies were identified, with data available from 28 studies representing n = 1,948 patients. The weighted mean difference in diurnal SBP (−2.58 mm Hg, 95% CI −3.57 to −1.59 mm Hg) and DBP (−2.01 mm Hg, 95% CI −2.84 to −1.18 mm Hg) both significantly favored PAP treatment over control arms, with similar results seen in nocturnal readings. Statistically significant reductions in BP were seen in studies whose patients were younger, sleepier, had more severe OSA, and exhibited greater PAP adherence. Meta-regression indicated that the reductions in DBP with PAP were predicted by mean baseline BP (β = −0.22, p = 0.02) and Epworth Sleepiness Scale scores (β = −0.27, p = 0.04).
Conclusions:
PAP treatment for OSA is associated with modest but significant reductions in diurnal and nocturnal SBP and DBP. Future research should be directed towards identifying subgroups likely to reap greater treatment benefits as well as other therapeutic benefits provided by PAP therapy.
Citation:
Montesi SB; Edwards BA; Malhotra A; Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8(5):587-596.
Keywords: Systematic review, meta-analysis, obstructive sleep apnea, positive airway pressure, hypertension, blood pressure, lung
Obstructive sleep apnea (OSA) is a disorder characterized by recurrent upper airway collapse that occurs during sleep. The repeated airway obstructions often expose sufferers to intermittent hypoxemia/hypercapnia, and in many untreated individuals the only way to reopen the airway and restore normal blood gases is to arouse from sleep. Accumulating evidence has suggested that such exposures to repetitive arousals and periods of hypoxemia are associated with important deleterious consequences such as cardiovascular disease, metabolic disorders, and impaired neurocognition.
The gold standard for treatment of moderate/severe OSA is positive airway pressure (PAP), which works by holding the airway open using a pneumatic splint. There is evidence to suggest that compliant PAP use can have beneficial effects on some of the deleterious consequences of this disorder; in terms of the cardiovascular effects of OSA, much of the existing interventional literature has focused on the role of PAP in mitigating OSA-induced hypertension. There are a number of published randomized controlled trials comparing blood pressure (BP) in patients receiving PAP versus a control arm; however, the extent to which PAP leads to alterations in BP values remains an area of debate based on evidence from single studies.
In 2007, a number of meta-analyses were published investigating the effect of PAP on BP, each adopting different study selection criteria, with conflicting results. An analysis of ten studies did not find any significant difference in either systolic or diastolic BP (SBP, DBP) between PAP and control groups when 24-h ambulatory blood pressure monitoring (ABPM) and office BP measurements were combined.1 Twenty-four hour mean arterial BP declined significantly by 1.7 mm Hg in a meta-analysis of 12 trials, and meta-regression analyses of these data found that a greater reduction in BP occurred with increasing OSA severity, greater frequency of arousals during diagnosis of OSA, and greater adherence to treatment, with no significant effect of subjective sleepiness reported at baseline.2 In the largest meta-analysis to date which included 16 studies representing 818 subjects, PAP use was associated with mean decreases in SBP of 2.5 mm Hg, DBP of 1.8 mm Hg, and mean arterial pressure of 2.2 mm Hg, all of which reached statistical significance.3 Thus, these previously published meta-analyses suggest that a small but significant reduction in SBP and DBP can result from PAP treatment, but it is unclear which patient subgroups may benefit most from treatment.
Many additional large randomized controlled trials have been published since 2007. We therefore aimed to perform an updated systematic review and meta-analysis of randomized controlled trials comparing therapeutic PAP to sham-PAP, pill placebo, or standard care over at least one week in adult OSA patients without major comorbidity. A secondary aim was to investigate the influence of treatment duration, age, daytime sleepiness, OSA severity, baseline BP, and adherence to treatment, using both subgroup analyses and meta-regression techniques.
METHODS
Being a review of published literature, this investigation did not require ethical review.
Literature Search
The PubMed database was searched for randomized controlled trials of PAP among OSA patients using the following search string in PubMed format: ((((((((((“Sleep Apnea Syndromes”[Mesh])) OR (apn*)) OR (hypopn*)) OR (sleep apn*)) OR (obstructive sleep apn*)) OR (OSA)) OR (OSAS)) OR (OSAHS)) OR (SAHS)) AND (((((((“Continuous Positive Airway Pressure”[Mesh])) OR (Continuous positive airway pressure)) OR (positive airway pressure)) OR (positive pressure)) OR (CPAP)) OR (PAP)) AND (((((“Randomized Controlled Trial”[Publication Type])) OR (“Clinical Trial”[Publication Type])) OR (randomi*)) OR (clinical trial)). The search was limited to the dates 1 January 1980 to 1 January 2012.
The reference lists of previous meta-analyses on this topic identified during the PubMed search were scanned for additional suitable studies. Any papers or peer-reviewed abstracts that were referenced in any of the identified studies (see literature exclusion criteria below) were also eligible for potential inclusion. Finally, our list of identified studies was forwarded to the first, last and/or corresponding author/s of these studies, who were asked to suggest further suitable publications if known to them.
Literature Exclusion Criteria
Authors SBM and JPB independently identified and excluded all qualitative reviews, commentaries, letters, and meta-analyses from the pooled literature set. The same authors then independently applied the following exclusion criteria to the abstracts of all identified publications in the order of “PICOS” (patient/intervention/comparator/outcome/study design)4: (a) patients did not have a diagnosis of OSA made either during full polysomnography, cardiorespiratory monitoring, or oximetry; (b) patients had significant comorbidity; (c) patients were aged < 18 years; (d) the study investigated an intervention other than auto-PAP, flexible PAP, or standard continuous PAP applied at therapeutic pressure; (e) the study included a comparator other than sham-PAP, pill placebo, or standard care; (f) the study did not include office BP and/or ABPM measured at ≥ 2 time points; (g) the study was not randomized; (h) the treatment arms were < 1 week long. We anticipated that many publications would report BP as a secondary outcome measure, and therefore all studies reaching point (f) were inspected in full rather than relying on the information contained in the abstract. If any studies reported duplicate data, only the latest publication was retained. Publication in a language other than English was not grounds for exclusion. Any disagreements as to study eligibility were discussed until consensus was reached.
Data Extraction
Data extraction was also conducted independently by authors BAE and JPB. BP data were extracted as systolic and diastolic values, measured diurnally (primary analyses) and nocturnally. When studies reported both 24-h ABPM and office BP, the ABPM data were extracted. If studies measuring 24-h ABPM did not split the data into diurnal/nocturnal, the data were classified as diurnal. Studies that measured office BP did not contribute to the nocturnal data; if office BP was measured throughout the day, morning (preferable) or midday data were extracted. If BP was measured at more than one follow-up time point, data from the final end point were extracted.
The mean difference in BP between PAP and control arms in crossover trials was calculated as [end-trial BP in PAP arm minus end-trial BP in control arm]. The mean difference in BP between PAP and control arms in parallel trials was calculated as [(end-trial BP in PAP arm minus baseline BP in PAP arm) minus (end-trial BP in control arm minus baseline BP in control arm)]. If a measure of variance (either standard deviation, standard error of the mean, or confidence interval) for the within-arm change in BP was not reported, it was calculated using standard methods for paired data assuming a correlation of r = 0.5.2,5 If any descriptive or BP data were missing or unclear, we attempted to email the first, last, and/or corresponding author and allowed up to 8 weeks response time.
Statistical Analysis
Statistical analyses were conducted by author JPB using Stata Version 12 (StataCorp; TX, USA). Forest plots were produced using Review Manager (RevMan) Version 5.1 (Nordic Cochrane Center, Copenhagen, Denmark). The following Stata commands were used: metan (Version 3.04; September 2010), metaninf, metafunnel, metabias, and metareg.
It was decided a priori that due to the diverse patient characteristics and experimental protocols anticipated in the final literature set, random effects models using DerSimonian and Laird methodology5 would be applied during all meta-analyses. A random effects model assumes that the true effect size varies between studies due to clinical and methodological diversity; the alternative fixed effect model assumes that the true effect size is the same for all studies and any between-studies variance is due solely to sampling error. If there is zero heterogeneity between studies, both models will yield the same result; when heterogeneity is present the confidence interval of the summary effect will be wider with the more conservative random effects model.5
The pooled effect and 95% confidence interval (CI) for each meta-analysis was calculated using the weighted effects of contributing individual studies and summarized graphically in a forest plot. Sensitivity analysis (the process by which one study at a time is excluded from the pooled analysis to determine if any studies exert undue influence on the model) was then conducted. The Q-statistic for each analysis—a statistical test of heterogeneity—was considered significant at p ≤ 0.10 by convention.5 The I2 statistic—the percentage of observed variance attributed to between-studies heterogeneity rather than chance—was calculated for each analysis. A funnel plot was produced for each meta-analysis in order to assess visually publication bias, which was also assessed statistically using Egger's and Begg's tests.
For the primary outcomes only (diurnal SBP and DBP), pre-specified subgroup analyses of trial structure (parallel versus crossover), comparator type (sham-PAP versus all other types), treatment duration (≥ 4 weeks versus < 4 weeks), age (≥ 50 years versus < 40 years), Epworth Sleepiness Scale (ESS) score (' 11/24 versus < 11/24), apnea-hypopnea index (AHI ≥ 30 events/h vs < 30 events/h), baseline hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg versus SBP < 140 mm Hg or DBP < 90 mm Hg), and PAP adherence (≥ 4 h/night vs < 4 h/night) were performed. Each p-value obtained in sub-group analyses was corrected using a Bonferroni approach. Separate pre-specified random effects meta-regression analyses were performed using treatment duration, mean age at baseline, mean ESS at baseline, mean AHI at baseline, PAP adherence, and mean BP at baseline (SBP or DBP as appropriate) as continuous predictors. Subgroup and meta-regression analyses were performed using the diurnal BP data only, in order to avoid inflating the type I error rate.
RESULTS
Identification and Description of Included Studies
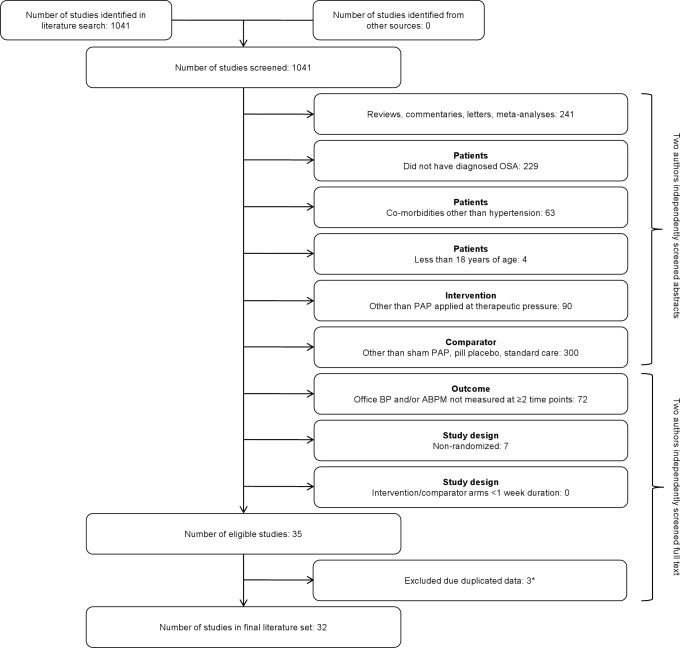
A total of 1,041 suitable studies were identified through the PubMed search. Of these publications, the authors screened the abstracts of 927 and the full text of 114. The reasons for excluding 1,006 of these studies are shown in Figure 1. Of the remaining 35 studies (all published as complete papers), 4 reported duplicate BP data, and the most recently-published of these was retained in the final literature set of 32 studies with 2,303 patients (see Figure 1).
Figure 1. Literature screening flowchart, with exclusion criteria listed in “PICOS” order4.
*Of the papers reporting identical data, the most recently published was included. ABPM, ambulatory blood pressure monitoring; BP, blood pressure; OSA, obstructive sleep apnea; PAP, positive airway pressure; PICOS, patient/intervention/comparator/outcome/study design.
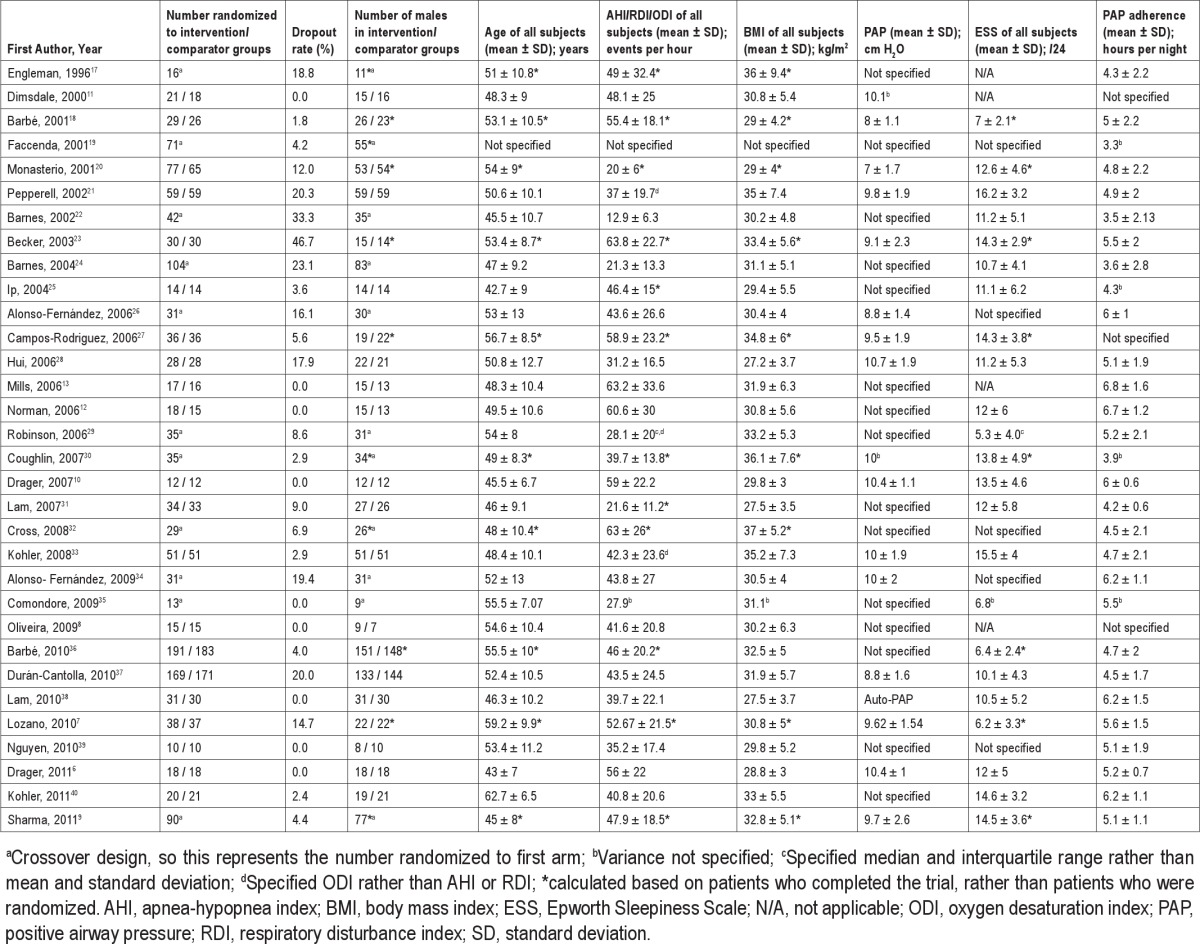
Table 1 summarizes the studies in terms of design characteristics, while Table 2 summarizes the descriptive information of patients in each study. Twenty-one studies adopted a parallel structure; all but one used fixed continuous PAP as the intervention, and 20 used sham-PAP as the comparator. Treatment arm durations ranged from one week to one year. Most studies recruited predominantly males, with the mean age and body mass index (BMI) of the studies falling in a reasonably narrow range (43-63 years, and 27-37 kg/m2 respectively). The mean ESS scores ranged from 5-16/24, while the mean PAP adherence ranged from 3-7 hours per night. Information as to the authors' judgment of risk of bias is available from the corresponding author.
Table 1.
Design characteristics of identified studies
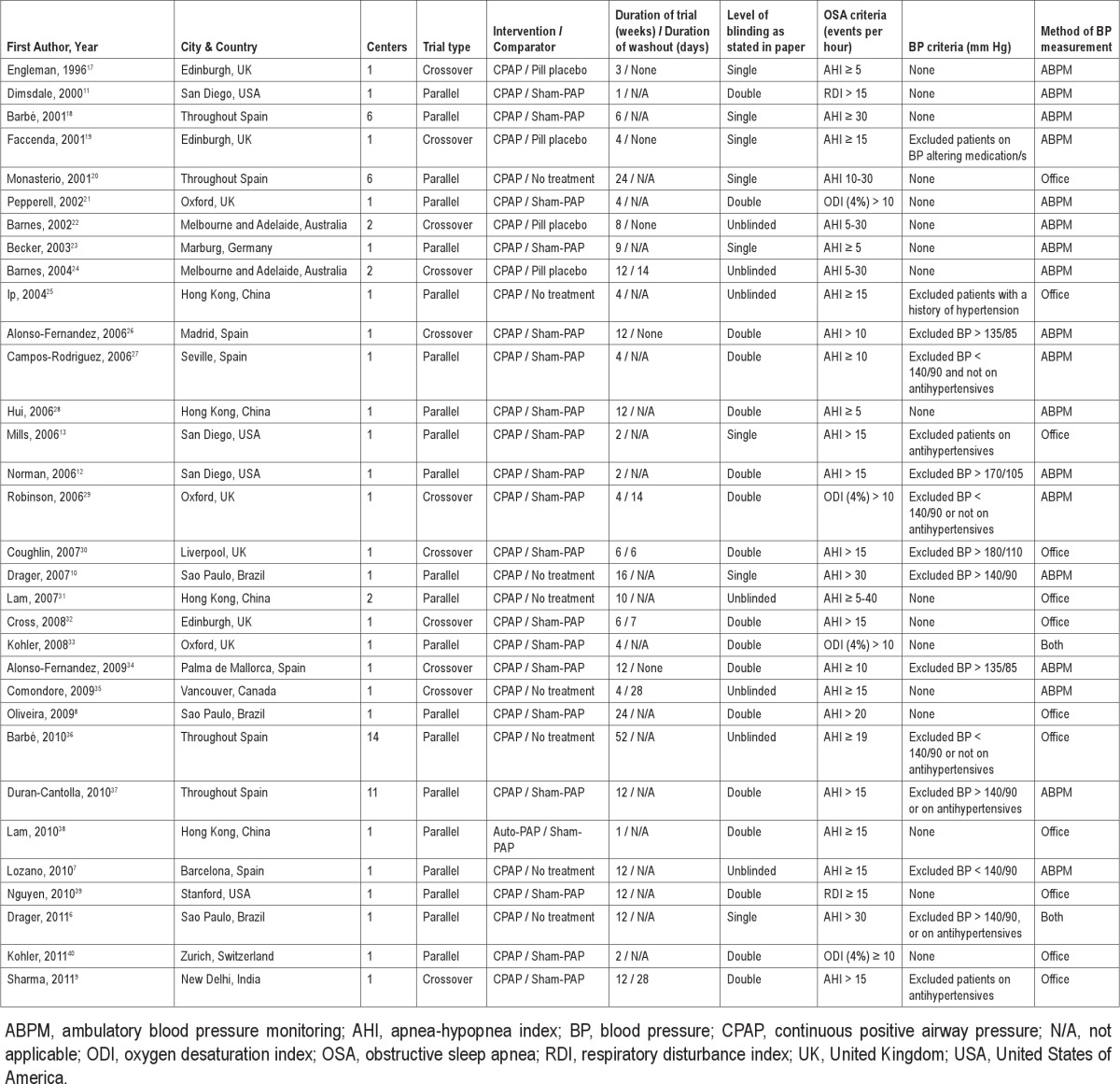
Table 2.
Descriptive characteristics of patients in identified studies
Additional Data Obtained
We are grateful to Drs. Dimsdale, Drager, Lloberes, Lozano, Oliveira, Poyares, Sharma and Tomfohr for supplying additional data and/or information on study design.6–13
Meta-Analyses
Diurnal BP
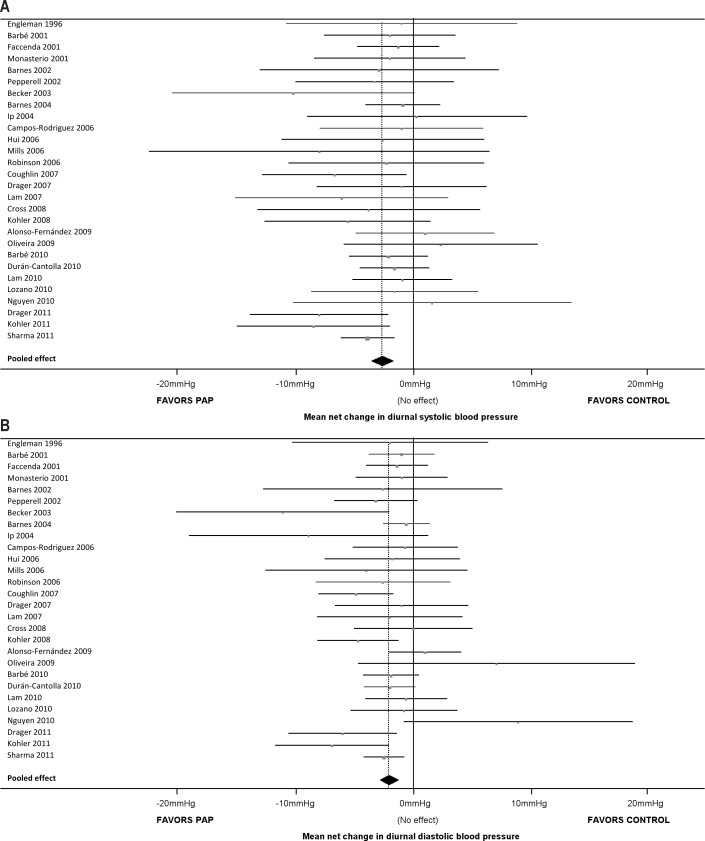
For our primary analyses (diurnal SBP and DBP), we were able to extract analyzable data for 1,948 patients from 28 studies (87.5% of the 32 studies identified). As shown in Figure 2, only 4 studies showed significant differences in SBP and DBP between PAP and control arms, with a further 2 studies showing significant differences in DBP only. In the pooled analyses, diurnal SBP and DBP were both significantly reduced by PAP treatment compared with control (pooled SBP change −2.58 mm Hg, 95% CI −3.57 mm Hg to −1.59 mm Hg, p ≤ 0.001; pooled DBP change −2.01 mm Hg, 95% CI −2.84 to −1.18, p ≤ 0.001). The Q-tests for SBP (Q(27) = 20.51, p = 0.81) and DBP (Q(27) = 34.90, p = 0.14) were both nonsignificant, and the I2 values for SBP and DBP did not indicate substantial heterogeneity (0% for SBP and 22.6% for DBP).
Figure 2. Forest plots comparing the effects of PAP and control on (A) diurnal SBP, and (B) diurnal DBP.
For each study, the square represents the mean difference in blood pressure between PAP and control arms and the width of the line represents the 95% confidence interval; a line crossing the 0 mm Hg vertical line indicates no significant difference at α = 0.05. The diamond summarizes the pooled effect; the apex represents the overall mean difference in diurnal SBP between PAP and control arms, and the width represents the 95% confidence interval. In both plots, the diamond does not cross the 0 mm Hg vertical line, hence there is a significant difference between PAP and control in terms of the change in diurnal SBP (−2.58 mm Hg, p ≤ 0.001) and diurnal DBP (−2.01 mm Hg, p ≤ 0.001). DBP, diastolic blood pressure; PAP, positive airway pressure; SBP, systolic blood pressure.
The results of the planned subgroup analyses are shown in Table 3. Diurnal SBP was significantly reduced by PAP in both parallel and crossover studies, whereas diurnal DBP was only significantly reduced by PAP in parallel studies. Both diurnal SBP and DBP were significantly reduced only in studies utilizing a sham-PAP control, studies with treatment duration ≥ 4 weeks, studies with mean age at baseline < 50 years, studies with mean ESS at baseline ≥ 11/24, studies with mean AHI at baseline ≥ 30 events/h, studies with mean PAP adherence ≥ 4 h/night, and studies with patients who were not hypertensive at baseline on average (all p ≤ 0.05 using a Bonferroni correction).
Table 3.
Results of subgroup and meta-regression analyses
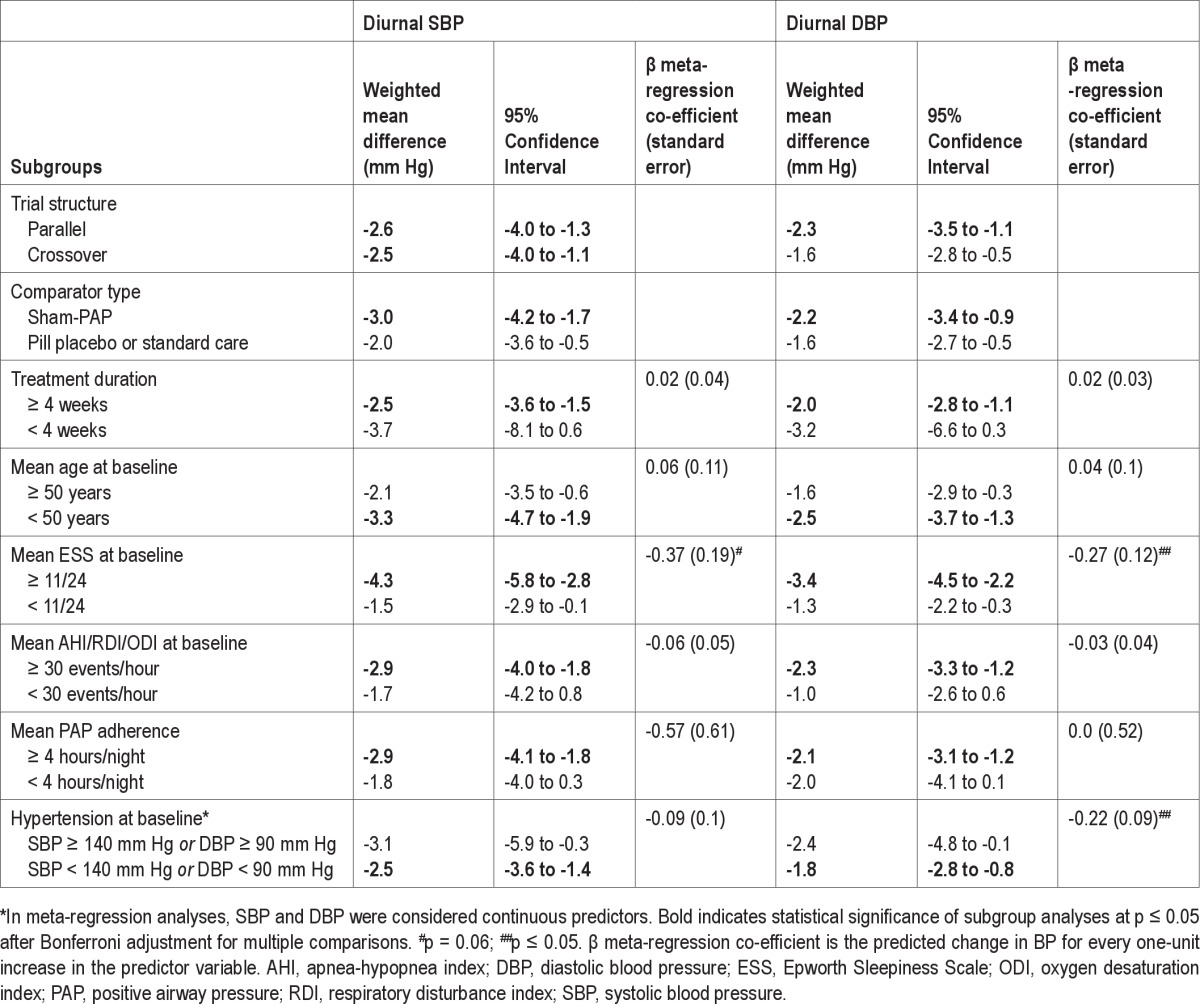
Meta-regression indicated no significant effect of AHI, age, treatment duration, or adherence to PAP, on diurnal SBP or DBP (all p > 0.05). Baseline diurnal SBP was not a significant predictor of the weighted mean difference in diurnal SBP between PAP and control, however as shown in Figure 3A, baseline diurnal DBP was a significant predictor of the weighted mean difference in diurnal DBP (β = −0.22, standard error of the mean 0.09, p = 0.02). Baseline ESS was a significant predictor of the weighted mean difference in diurnal DBP (β = −0.27, standard error of the mean 0.12, p = 0.04; see Figure 3B), and this association approached significance for diurnal SBP (β = −0.37, standard error of the mean 0.19, p = 0.06).
Figure 3. Scatterplots of the weighted mean difference between PAP and control for each study against (A) mean diurnal DBP at baseline, and (B) mean ESS score at baseline.
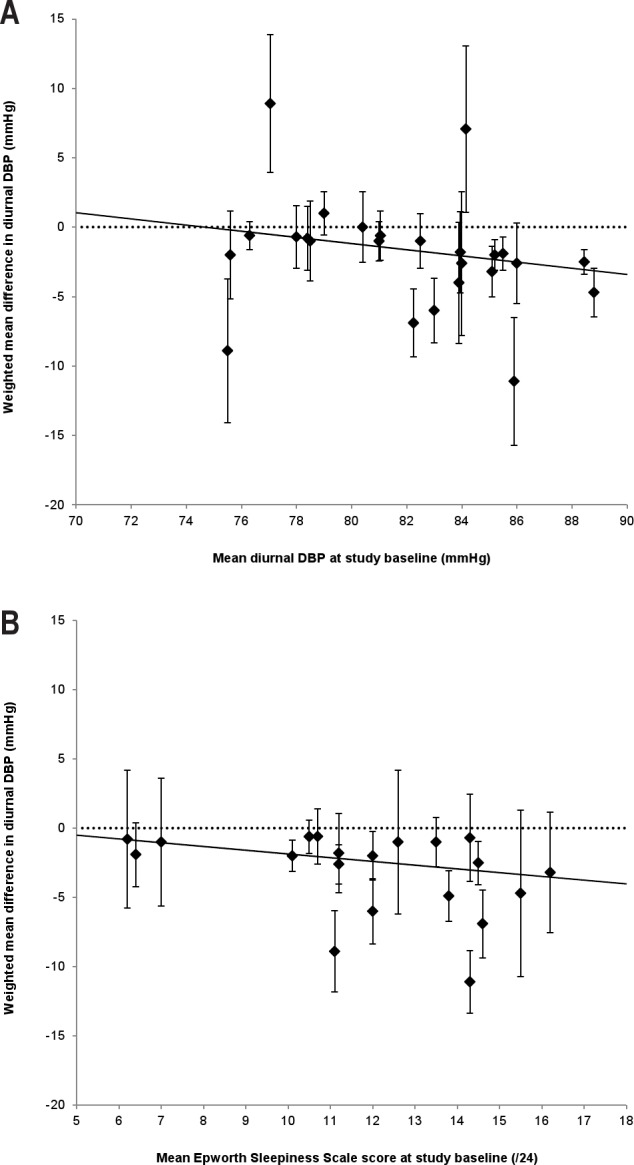
In each plot, the error bars represent the standard error of the mean for each weighted mean difference, the solid lines represent the random effects meta-regression lines, and the dotted lines represent the null effect (0 mm Hg difference between PAP and control). The analysis in (A) suggests that the mean difference in diurnal DBP between PAP and control would decrease by 2.22 mm Hg with every increase of 10 mm Hg diurnal DBP at baseline (β = −0.22, p = 0.02), while the analysis in (B) suggests that the mean difference in diurnal DBP between PAP and control would decrease by 1.35 mm Hg with every 5-point increase in ESS score at baseline (β = −0.27, p = 0.04). DBP, diurnal blood pressure; ESS, Epworth Sleepiness Scale.
Finally, sensitivity analyses found that no study, when removed individually, changed the statistical significance of the pooled result.
Nocturnal BP
Nocturnal SBP and DBP data were available for 661 patients from 10 studies. Both were both significantly reduced by PAP treatment compared with control (pooled nocturnal SBP change −4.09 mm Hg, 95% CI −6.24 mm Hg to −1.94 mm Hg, p ≤ 0.001; pooled nocturnal DBP change −1.85 mm Hg, 95% CI −3.53 mm Hg to −0.17 mm Hg, p = 0.03). The Q-tests for SBP (Q(9) = 9.55, p = 0.39) and DBP (Q(9) = 12.21, p = 0.20) were both nonsignificant, and the I2 values for SBP and DBP (5.7% and 26.3%, respectively) did not indicate substantial heterogeneity. No subgroup or meta-regression analyses were planned for the nocturnal BP data. During sensitivity analysis, no study changed the statistical significance of the pooled result when removed from each meta-analysis individually.
Assessment of Publication Bias
We found no evidence of publication bias when Funnel Plots were inspected for each meta-analysis (not shown), although there is a possibility that further unpublished studies exist which we were unable to identify. The results of the Egger's and Begg's tests for both diurnal SBP and DBP, and nocturnal SBP and DBP, were all nonsignificant (all p > 0.05).
DISCUSSION
Our meta-analyses show that PAP treatment significantly reduces diurnal and nocturnal SBP and DBP compared with non-therapeutic comparators (sham-PAP, pill placebo, or standard care), despite only a few trials reporting statistically significant results individually. The weighted mean reductions in diurnal SBP (−2.58 mm Hg) and DBP (−2.01 mm Hg), and nocturnal SBP (−4.09 mm Hg) and DBP (−1.85 mm Hg), were reasonably small and would be unlikely to be considered clinically significant for an individual without additional treatment benefits occurring alongside. These results are in agreement with existing meta-analyses, despite the fact that more than double the number of studies were available to us in our updated literature search. Along with our quantitative results indicating minimal heterogeneity between studies, this provides evidence of a consistent effect across studies despite diverse locations and methodological approaches.
In terms of study design, planned subgroup analyses indicated that the reductions in diurnal SBP and DBP were larger in parallel studies, studies with at least four weeks treatment duration, and studies that used a sham-PAP control arm and hence were able to blind investigators and/or patients to treatment allocation.
We investigated the influence of descriptive covariates using both subgroup analyses and meta-regression. As anticipated, significant reductions in diurnal SBP and DBP were evident only in studies whose patients were younger, reported a greater degree of daytime hypersomnolence, had more severe OSA, and who exhibited greater PAP adherence. Our results concerning age are in agreement with data from the Sleep Heart Health Study, which did not detect a significant association between hypertension and OSA in those aged over 60 years.14 The subgroup of studies whose patients were (on average) hypertensive at baseline showed a greater reduction in BP with PAP compared with studies whose patients were normotensive, and yet this analysis did not reach values with statistical significance. Few trials targeted hypertensive patients during recruitment, suggesting that this analysis was underpowered particularly after Bonferroni adjustment for multiple comparisons.
Meta-regression analyses indicated that with every 10 mm Hg increase of diurnal DBP at baseline, a drop in diurnal DBP of 2.22 mm Hg with PAP compared with control could be expected (p = 0.02). Similarly, with every 5-point increase in ESS score at baseline, a drop in diurnal SBP of 1.86 mm Hg (p = 0.06) and a drop in diurnal DBP of 1.35 mm Hg (p = 0.04) could be expected. The mechanisms by which BP changes relate to daytime sleepiness remain unclear but may relate to cortical or subcortical arousals occurring at the termination of respiratory events coinciding with repeated surges in BP. There is some evidence that this cardiovascular response to arousal is attenuated in the elderly, which may partly explain our finding that age was not predictive of the BP reduction with PAP.15
The results of all other meta-regression analyses were nonsignificant—most likely due to the fact that significant heterogeneity between studies was not found—which highlights a deficiency in the literature. Despite the fact that studies recruited a heterogeneous sample of OSA patients (see Table 1) such that the within-study variances of baseline age, AHI, and adherence were wide, the means of these covariates were similar across studies. Hence little between-studies heterogeneity was evident, making statistical investigation of study-level co-variates impractical. The efficacy and effectiveness of PAP in the prevention and treatment of cardiovascular disease including hypertension is still an area of debate, making the identification of OSA subsamples likely to reap greater benefits from PAP treatment particularly important. Although this question could be addressed by performing further clinical trials targeting specific patient subgroups, a more practical and efficient approach would be to perform a patient-level meta-analysis using existing data from the identified literature. The availability of such a large pooled sample would also allow for the inclusion of a number of other important co-variates that were not investigated here, such as the arousal index and the influence of antihypertensive medications, the latter of which would be particularly useful in interpreting our meta-regression results suggesting greater reductions in BP when patients were hypertensive at baseline.
This meta-analysis builds on previous analyses by including data from an additional 1,170 patients from 13 studies—more than double the sample that was available to Bazzano et al. in 2007.3 Further strengths include the fact that we chose to identify only randomized controlled trials and separately extracted both diurnal and nocturnal BP data. We addressed several potential confounders of the effect of PAP on BP which have not been investigated using meta-regression to date, namely treatment duration, age, and baseline BP. Our thorough search for suitable studies outside of the PubMed database did not lead to the identification of further publications, and as such we believe our search terms were sensitive enough to capture all available literature; hence it is unlikely that our results have been heavily influenced by publication bias. Furthermore, by performing sensitivity analyses, we are confident that no single trial exerted undue influence on any of the meta-analyses presented here.
Some may consider the overall observed effect of PAP on BP to be minor and may question the benefits of treating OSA. We would offer several lines of logic in response. Firstly, although a 2-3 mm Hg change in BP could be considered trivial for an individual, at a population level the impact of OSA treatment on BP could have substantial benefits (for example, decreasing the risk of stroke). Secondly, we would agree that if the sole purpose of PAP were to lower BP, we would favor antihypertensive medications. However, the other benefits of PAP on daytime symptoms, driving risk, and possible cardiovascular benefits are noteworthy. Third, profound surges in BP are well known to occur in the physiological response to apnea, although such surges are typically not assessed using noninvasive technology with intermittent readings in clinical trials. Given that these BP surges may be a substrate for plaque rupture, we would encourage focus on hard cardiovascular outcomes, rather than surrogate outcome measures such as daytime BP. Finally, Guyenet et al. have regarded hypertension as a central disorder of autonomic control given that counter-regulatory mechanisms tend to maintain BP within a relatively narrow range based on changes in vasoconstriction or vasodilation.16 As such, one might predict modest changes in BP based on reduced catecholamine-mediated vasoconstriction. Thus, we favor further research into the benefits of CPAP and the role of BP on the causal pathway from OSA to hard cardiovascular outcomes.
Our meta-analysis has a number of limitations that need to be considered. Despite attempts to gather unpublished data from study authors, our data set was not complete. However, we were able to analyze diurnal BP data from almost 90% of identified trials and as such believe that our synthesized results are an accurate summary of the literature. Secondly, we analyzed BP data across trials with substantial methodological diversity (for example, differing equipment, timing and number of BP measurements), which is an unfortunate but unavoidable limitation of meta-analyses of this kind. We were also forced to calculate estimated differences in paired BP data based on the assumption of an r = 0.5 correlation when these data were not reported. This requirement undoubtedly introduced a minor degree of error into our data set.
In conclusion, the use of PAP in the treatment of OSA results in a modest yet significant reduction in BP, with the greatest effect seen with nocturnal SBP values. As more is learned of the cardiovascular effects of OSA, further testing will be needed to assess the role of PAP in mitigating such effects. Patient level meta-analysis may prove beneficial, especially in terms of defining the patient subgroups which would sustain the largest benefit from PAP.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Malhotra is a consultant for Philips Respironics, SHC, SGS, Apnicure, Apnex, and Pfizer. Dr. Bakker has received funding from Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs. Dimsdale, Drager, Lloberes, Lozano, Oliveira, Poyares, Sharma, and Tomfohr for their help in providing missing data for these analyses. Dr. Edwards is supported by the National Health and Medical Research Council of Australia's CJ Martin Overseas Biomedical Fellowship (1035115). Dr. Malhotra is supported by NIH (5R01HL085188-04, 5R01HL090897-03, 5K24HL093218-03, 1P01HL095491-01A1) and AHA (0840159N) grants. No financial support was obtained for this investigation. All work performed at Sleep Disorders Research Program, Brigham & Women's Hospital & Harvard Medical School, Boston MA.
ABBREVIATIONS
- ABPM
ambulatory blood pressure monitoring
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- ESS
Epworth Sleepiness Scale
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PICOS
patient/intervention/comparator/outcome/study design
- RDI
respiratory disturbance index
- SBP
systolic blood pressure
REFERENCES
- 1.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 2.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 3.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 5.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 6.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–55. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 7.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira W, Campos O, Cintra F, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95:1872–8. doi: 10.1136/hrt.2009.173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 10.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 11.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35:144–7. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 12.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 13.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 14.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 15.Goff EA, O'Driscoll DM, Simonds AK, Trinder J, Morrell MJ. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep. 2008;31:1009–17. [PMC free article] [PubMed] [Google Scholar]
- 16.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 17.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep. 1996;19:378–81. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- 18.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 19.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 20.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–43. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 21.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 22.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 23.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 24.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 25.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 27.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–67. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 28.Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. 2006;61:1083–90. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–35. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 30.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 31.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax. 2008;63:578–83. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 33.Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32:1488–96. doi: 10.1183/09031936.00026608. [DOI] [PubMed] [Google Scholar]
- 34.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64:581–6. doi: 10.1136/thx.2008.100537. [DOI] [PubMed] [Google Scholar]
- 35.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung. 2009;187:17–22. doi: 10.1007/s00408-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 36.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 37.Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 38.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–45. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson. 2010;12:50. doi: 10.1186/1532-429X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohler M, Stoewhas AC, Ayers L, et al. The effects of CPAP therapy withdrawal in patients with obstructive sleep apnea: a randomised controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]