Abstract
Background
Glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) play key roles in the early development of the central auditory pathway and the inner ear. Both have been successfully employed to treat experimental forms of hearing loss and are likely to operate in a broad spectrum of auditory phenotypes, including phantom perceptions of sound. We conducted a genetic association study addressing five biallelic candidate variants in 240 Caucasian subjects who had been diagnosed with tinnitus for more than 6 months.
Findings
Allele frequencies were determined for three GDNF and two BDNF markers, including a functional missense substitution (V66M). When data were compared to previously examined control populations, no significant allelic associations were noted after corrections for multiple testing. However, using a multiple regression approach and scores from a validated self-report questionnaire, GDNF and BDNF genotypes jointly predicted tinnitus severity in women (N=69, uncorrected p=0.04) but not in men (N=171, n.s.).
Conclusions
The present findings serve as an incentive for further explorations of neurotrophic factors' role in predicting clinical features of tinnitus. Possible implications of sexually dimorphic at-risk genotypes are discussed with regard to hearing and neural plasticity.
Keywords: BDNF, GDNF, tinnitus, sexual dimorphism, genetic variation
Introduction
The chronic phantom perception of sound, or tinnitus, places a significant burden on emotional well-being and disrupts daily living in a large proportion of the general population. Depending on the level of diagnostic stringency, in the U.S.A. between 8% and 25% of adults fulfill lifetime criteria for the disorder [1]. Tinnitus may be conceptualized as a state of central nervous hyperexcitability that is frequently precipitated and maintained by stressful environments, but can also induce adaptation processes and resolve under favourable circumstances [2]. The precise biological underpinnings of chronic tinnitus are unknown and current data suggest that only 3% of European cases are exclusively attributable to environmental noise exposure [3]. There is however, an increasing interest in genetic traits that may be associated with the phenotype [4]. Preliminary estimates of heritability range from 0.11 to 0.39 [5,6] and are best compatible with many causative genes exerting minor effects.
Evidence is accumulating that adaptation to tinnitus is highly dependent on neural plasticity and on the restoration of dysfunctional auditory circuits for tuning out the phantom noise signal [7-11]. In animal models, tinnitus is accompanied by peripheral signs of ongoing regeneration [12] and by the remodeling of entire neural circuits [13,14]. These responses to tinnitus correlate with the expression of neurotrophic factors [15], fuelling interests in the curative use of such factors [16]. An emerging role for glial cells in the restoration of auditory function [17] and the confirmed preventive effects of brain-derived neurotrophic factor (BDNF) on damage to the auditory pathway by tinnitus-inducing agents [18], or by acoustic trauma [19] further rationalize the search for related biomarkers.
The present investigation addresses genetic variants in the genes encoding glial cell-derived neurotrophic factor (GDNF) and BDNF in tinnitus, of which one missense substitution, BDNF Val66Met, has already been the object of extensive investigations in the sensitivity to stress [20]. Empirical data support additional roles in the adaptation to stress and noise [21,22], in the processing of auditory information, e.g., during acoustic startle [23], in auditory odball tasks [24], and in other auditory challenges [25]. It is noteworthy that positive associations have also been reported for auditory hallucinations in some settings [26]. Together, these findings argue in favor of a modulatory effect on sound perception, generation and processing beyond guiding early development of the auditory pathway.
Materials and methods
Subjects
In 240 German outpatients (171 men and 69 women, age 50.3 ±12.9 years, mean ± SD) consulting for chronic tinnitus, the diagnosis was confirmed by a detailed neurootological examination including otoscopy, stapedius reflexes, middle ear pressure measurements, and pure tone audiometry. The degree of hearing loss was calculated as the binaural pure tone average involving air conduction across seven test frequencies (0.125, 0.25, 0.5, 1, 2, 4, and 8kHz). For the present study, only patients with subjective tinnitus were included. Tinnitus severity was assessed by the German version of the Tinnitus Questionnnaire (TQ) [27]. All participants were Cauacasians and a majority originated from the Upper Palatinate region of Bavaria. All provided informed consent and the study was approved by the local ethics committee at the University of Regensburg.
Genotyping
Genomic DNA was extracted from lymphocytes using standard procedures prior to generating GDNF and BDNF amplicons for PCR-based RFLPs. Variants were selected based on functionality (rs6265), linkage disequilibrium (LD) tagging properties (rs2049046, rs1110149), and high heterozygosity estimates (remaining variants). Briefly, the following primer pairs were designed: 5'-CAT TTA CTG AGC TCT TAC TAC ATG TTC C-3' (rs3812047, forward), 5'-TCC TTG CAA GTC CCC AGT TC-3' (rs3812047, reverse), 5'-GCC CTT TCT ACA CAT CTT TTA CCT C-3' (rs1110149, forward), 5'-TGC TAA AGT GTG GAG AGT CTA AGT C-3' (rs1110149 and rs884344, reverse), 5'-GGC TCT CCT GAT CCA TTT TG-3' (rs884344, forward), 5'-GAA AAA CGC ACA CAC ACA GAA-3' (rs2049046, forward), and 5'-CCA AGA CCC TTG AAG AGA ACT TAT-3' (rs2049046, reverse). For rs6265, we used previously published primers [28]. Following incubation with restriction endonucleases (FokI for rs3812047, ApaI for rs1110149, NdeI for rs884344, Eco72I for rs6265, and HinfI for rs2049046) and electrophoretic separation on EtBr-stained agarose gels, genotypes were visualized under UV transillumination. Fragment lengths were 151+189+317bp (A) or 189+468bp (G) for rs3812047, 133+178bp (G) or 311bp (C) for rs1110149, 285+325bp (T) or 610bp (G) for rs884344, 72+236bp (G) or 308bp (A) for rs6265, and 58+196+206bp (A) or 58+402bp (T) for rs2049046. All alleles refer to the transcribed strand.
Statistical analysis
STATA 8.0 (Stata Corporation, College Station, TX, USA) was used for descriptive statistics, tests of allelic association and multiple regression. GDNF and BDNF allele frequencies from reference populations were retrieved from the literature and from human variation databases for comparison with the present data using Fisher's exact tests. The level of statistical significance was set at p<0.05 and a Bonferroni correction was applied to account for multiple comparisons. LD and conformity of genotype distributions with the Hardy-Weinberg equilibrium (HWE) was measured with HaploView 4.2 [29]. PS V2.1.15 [30] was used for power simulations. The Shapiro-Wilk statistic served to test the null hypothesis of normally distributed TQ scores. To minimize trade-offs in statistical power, heterozygous subjects and subjects homozygous for the minor alleles were collapsed into a single group under the assumption of dominant effects of the minor alleles [31]. Separate multiple regression models were calculated for male and female subjects based on epidemiological evidence of gender differences in presentation [32-34].
Results
All genotype distributions conformed to the Hardy-Weinberg Equilibrium. Overall, we noted only weak linkage disequilibrium between variants (Figure 1) and heterozygosities between 0.21 (rs 3812047) and 0.50 (rs1110149). With regard to single markers, the comparison of allele frequencies with reference data gave an uncorrected p value of <0.05 for two variants (rs6265 and rs1110149), but this finding did not survive corrections for multiple testing (Table 1). Still, a weak modulatory effect could not entirely be ruled out. According to power simulations based on the entire sample of tinnitus patients and all Caucasian control populations, we should require close to 15,000 affecteds and over 680,000 healthy controls in order to exclude a modifying role of rs6265 on allelic risk with a statistical power of 0.8.
Figure 1.
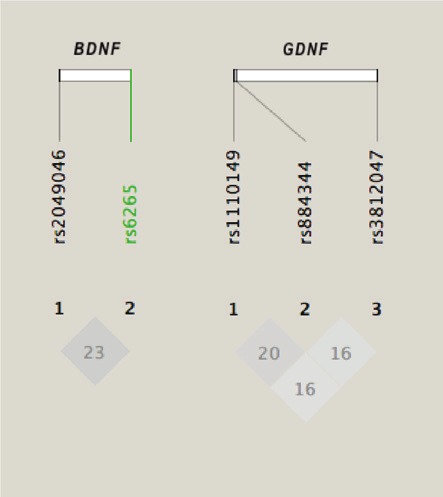
LD plots and R2x100 values for the variants investigated. A BDNF coding variant is indicated in green. Markers span 43.9kb on the BDNF gene and 11.4kb on the GDNF gene, corresponding to 65% and 42% of the respective genomic sequence.
Table 1.
Observed genotype and allele frequencies in subjects with chronic tinnitus (N=240) and tests for allelic association. Data from nine Caucasian control populations were used with Bonferroni corrections.
| dbSNP identifier | gene | 2N | major:minor allele (1:2) | predicted protein variant | genotypes (11:12:22) | MAF in chronic tinnitus | MAF in unrelated Caucasian controls (N) | p association (p corrected) | |
|---|---|---|---|---|---|---|---|---|---|
| rs2049046 | BDNF | 480 | A:T | - | 69:105:66 | 0.494 | 0.467 (379)a | n.s. | |
| 0.481 (128) | [51] | n.s. | |||||||
| 0.476 (287) | [52] | n.s. | |||||||
| rs6265 | BDNF | 480 | G:A | V66M | 155:76:9 | 0.196 | 0.191 (4299)b | n.s. | |
| 0.203 (379)a | n.s. | ||||||||
| 0.137 (128) | [51] | 0.05 (0.74) | |||||||
| 0.190 (2100) | [53] | n.s. | |||||||
| 0.213 (839) | [54] | n.s. | |||||||
| 0.185 (664) | [55] | n.s. | |||||||
| 0.218 (333) | [56] | n.s. | |||||||
| 0.175 (2184) | [57] | n.s. | |||||||
| rs1110149 | GDNF | 480 | G:C | - | 62:121:57 | 0.490 | 0.410 (379)a | 0.007 (0.10) | |
| rs884344 | GDNF | 480 | T:G | - | 125:101:14 | 0.269 | 0.236 (379)a | n.s. | |
| rs3812047 | GDNF | 476 | G:A | - | 178:52:8 | 0.143 | 0.144 (379)a | n.s. |
all alleles refer to the transcribed strand, MAF = minor allele frequency.
reference population of European ancestry from the 1000 Genomes Project, July 2010 data release (URL: http://www.1000genomes.org), accessed July 2012;
reference population of European ancestry from the NHLBI GO Exome Sequencing Project. Data retrieved with the Exome Variant Server (URL: http://evs.gs.washington.edu/EVS/), accessed July 2012.
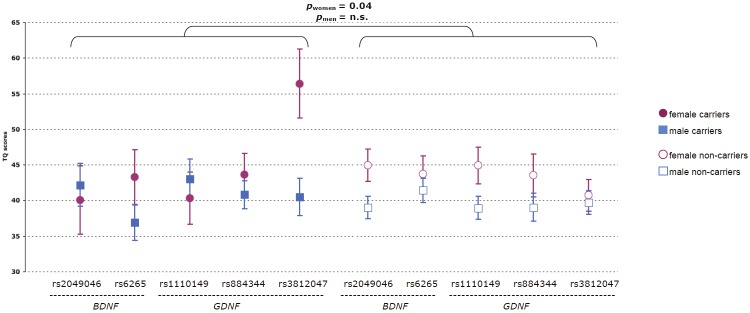
We then interrogated possible effects of BDNF and GDNF variants on tinnitus severity. TQ scores averaged 41.0 ± 1.2 (mean ± SEM) out of 84 points (N=236). By this measure, tinnitus was rated mild (0-30 points) in 73 subjects (30.9%), moderate (31-46 points) in 73 subjects (30.9%), severe (47-59 points) in 45 subjects (19.1%), and extreme (60-84 points) in 45 subjects (19.1%). There was no significant difference in mean TQ scores between carriers and non-carriers of the minor alleles (p>0.19) and neither the degree of concomitant hearing loss (uncorrected p>0.31, t-test) nor a positive family history of tinnitus in first-degree relatives predicted minor allele carrier status (uncorrected p>0.08, Fisher's exact test). However, when the joint effects of dichotomized GDNF and BDNF genotypes were examined in multiple regression models using log-transformed, normally distributed TQ scores as the dependent variable and correcting for the effect of age, tinnitus severity was predicted in women (F=2.32, uncorrected p=0.04), as opposed to men (F=1.16, n.s., Figure 2). Under these assumptions, minor allele carrier status explained 19.1% of the variance in tinnitus severity of the female subgroup.
Figure 2.
TQ scores (mean ±SEM) for men and women with chronic tinnitus stratified by minor allele carrier status (N=236). p values refer to multiple regression models using GDNF and BDNF genotypes as independent variables and correcting for the effect of age. For ease of interpretation, reported means and SEMs are not log-transformed.
Discussion
The present investigation provides original information on the contribution made by neurotrophic factor genes to predicting the intensity of tinnitus. Earlier research has suggested an inverse correlation of BDNF plasma levels with symptom severity in tinnitus sufferers [35] which could be explained by differences in secretion and is in line with neurotrophic support in adulthood [36]. While we failed to observe any major effects of individual BDNF or GDNF variants on susceptibility to the phenotype, the findings are compatible with minor, additive effects. Such effects would appear to be gender-sensitive according to subjective ratings of symptoms, offering a possible explanation for distinct patterns of central nervous activity in tinnitus [37].
If substantiated in larger studies, a differential role for GDNF and BDNF genotypes in predicting auditory symptoms of men and women could relate to the effects of estrogen on neurotrophic factor gene transcription [38,39]. Speculations on a specific role in tinnitus, however, will need to be postponed until the predictors have been evaluated in hearing sensitivity [40-42], in the cortical response to background noise [43], or more generally, in the subcortical and cortical processing of auditory information [44,45], all of which exhibit sex differences. Finally, developmental differences in the primary auditory cortex have long been noted [46] and distinct capabilities for the restoration of auditory function in men and women are emerging, e.g. in adult cochlear implants [47]. Exploitation of these phenotypes should promote the identification of genetic subgroups with high and low potentials for restoring the integrity of the auditory pathway in altered states of neural excitation or inhibition.
It also remains to be seen whether additional clinical features of tinnitus, e.g., the response to treatment, can be predicted from variation in neurotrophic factor genes. Preliminary data suggest that antidepressant-responsive forms of tinnitus may indeed be more common in women [48], but it is not yet known whether this dimorphism is due to the induction of neurotrophic factor signalling or downstream effects on synaptic plasticity [49].
We acknowledge three caveats. Firstly, with regard to the case-control-approach, it cannot be excluded that among the >11,200 external control subjects some suffered from mild forms of tinnitus that were not reported during recruitment. Possible heterogeneity of control samples was only addressed by verifying the conformity of these data with HWE. Future investigations will therefore need to ascertain auditory control status in more detail. Secondly, with regard to the quantitative trait analysis, we postulated a dominant mode of inheritance for all minor alleles. An alternative approach would have been to examine all possible modes of inheritance for each biallelic SNP, at the price of inflating the number of tests from 1 to 35=243. Thirdly, of the variants examined, only rs6265 is known to affect activity-dependent secretion of BDNF [50]. rs2049046, rs1110149, rs884344, and rs3812047 map to intronic regions of uncertain functionality. Effects could point at the existence of variants that affect gene transcription and are in tight LD with the SNPs genotyped here but validation is currently lacking.
With the above limitations in mind, and pending replication, BDNF and GDNF variants may prove helpful in assessing the potential for symptom control in well-defined tinnitus subpopulations. Longitudinal studies are invited to put these results into perspective and to specify the dynamics of explanatory variables in the context of neural plasticity.
Acknowledgments
We wish to thank Petra Stoertebecker for expert technical assistance and gratefully acknowledge support by the German Research Foundation and the Open Access Publishing Fund of the University of Regensburg.
Authors' contributions
PGS designed the study, wrote the protocol, conducted the experiments and drafted the manuscript. MS and BL assisted PGS with statistical analysis. BL and TK supervised the recruitment of subjects who took part in the study. MS, BL and TK managed the literature searches and contributed to draft the manuscript. All authors have approved the final manuscript.
References
- 1.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Ortmann AJ, Neely JG. Sudden sensorineural hearing loss and delayed complete sudden spontaneous recovery. J Am Acad Audiol. 2012;23:249–255. doi: 10.3766/jaaa.23.4.3. [DOI] [PubMed] [Google Scholar]
- 3.Deshaies P, Gonzales Z, Zenner HP, Plontke S, Paré L, Hébert S, Normandin N, Girard SA, Leroux T, Tyler R, Côté C. Environmental Noise and Tinnitus. In: Theakston F, editor. Burden of disease from environmental noise.Quantification of healthy life years lost in Europe. Copenhagen: WHO; 2011. pp. 71–90. [Google Scholar]
- 4.Sand PG, Langguth B, Kleinjung T, Eichhammer P. Genetics of chronic tinnitus. Prog Brain Res. 2007;166:159–168. doi: 10.1016/S0079-6123(07)66014-2. [DOI] [PubMed] [Google Scholar]
- 5.Petersen HC, Andersen T, Frederiksen H, Hoffman HJ, Christensen K, editors. The heritability of tinnitus: a twin study; Poster presented at: Nordic Epidemiology Congress; June 9-12, 2002; Aarhus, Denmark. [Google Scholar]
- 6.Kvestad E, Czajkowski N, Engdahl B, Hoffman HJ, Tambs K. Low heritability of tinnitus: results from the second Nord-Trøndelag health study. Arch Otolaryngol Head Neck Surg. 2010;136:178–182. doi: 10.1001/archoto.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;40:313–334. doi: 10.1016/j.jcomdis.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzounopoulos T. Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus. Am J Audiol. 2008;17:S170–175. doi: 10.1044/1059-0889(2008/07-0030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Møller AR. Plasticity diseases. Neurol Res. 2009;31:1023–1030. doi: 10.1179/174313209X383303. [DOI] [PubMed] [Google Scholar]
- 10.Dohrmann K, Elbert T, Schlee W, Weisz N. Tuning the tinnitus percept by modification of synchronous brain activity. Restor Neurol Neurosci. 2007;25:371–378. [PubMed] [Google Scholar]
- 11.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer W, Panford-Walsh R, Watermann D, Hendrich O, Zimmermann U, Köpschall I, Rohbock K, Knipper M. Salicylate alters the expression of calcium response transcription factor 1 in the cochlea: implications for brain-derived neurotrophic factor transcriptional regulation. Mol Pharmacol. 2008;73:1085–1091. doi: 10.1124/mol.107.041814. [DOI] [PubMed] [Google Scholar]
- 13.Weisz N, Wienbruch C, Dohrmann K, Elbert T. Neuromagnetic indicators of auditory cortical reorganization of tinnitus. Brain. 2005;128:2722–2731. doi: 10.1093/brain/awh588. [DOI] [PubMed] [Google Scholar]
- 14.Eggermont JJ. Role of auditory cortex in noise-and drug-induced tinnitus. Am J Audiol. 2008;17:S162–169. doi: 10.1044/1059-0889(2008/07-0025). [DOI] [PubMed] [Google Scholar]
- 15.Tan J, Rüttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Köpschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Martin DM, Raphael Y. Gene-based diagnostic and treatment methods for tinnitus. Int Tinnitus J. 2003;9:3–10. [PubMed] [Google Scholar]
- 17.Feng J, Bendiske J, Morest DK. Degeneration in the ventral cochlear nucleus after severe noise damage in mice. J Neurosci Res. 2012;90:831–841. doi: 10.1002/jnr.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabaizadeh R, Staecker H, Liu W, Van De Water TR. BDNF protection of auditory neurons from cisplatin involves changes in intracellular levels of both reactive oxygen species and glutathione. Brain Res Mol Brain Res. 1997;50:71–78. doi: 10.1016/s0169-328x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 19.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–142. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Nam YJ, Stöver T, Hartman SS, Altschuler RA. Upregulation of glial cell line-derived neurotrophic factor (GDNF) in the rat cochlea following noise. Hear Res. 2000;146:1–6. doi: 10.1016/s0378-5955(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 23.Hajcak G, Castille C, Olvet DM, Dunning JP, Roohi J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain Behav. 2009;8:80–85. doi: 10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, Cooper N, Grieve S, Dobson-Stone C, Morris C, Kuan SA, Gordon E. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2009;80:176–188. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Juckel G, Schumacher C, Giegling I, Assion HJ, Mavrogiorgou P, Pogarell O, Mulert C, Hegerl U, Norra C, Rujescu D. Serotonergic functioning as measured by the loudness dependence of auditory evoked potentials is related to a haplotype in the brain-derived neurotrophic factor (BDNF) gene. J Psychiatr Res. 2010;44:541–546. doi: 10.1016/j.jpsychires.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Zdanys KF, Kleiman TG, Zhang H, Ozbay F, MacAvoy MG, Gelernter J, van Dyck CH. BDNF variants, premorbid educational attainment, and disease characteristics in Alzheimer's disease: an exploratory study. J Alzheimers Dis. 2009;17:887–898. doi: 10.3233/JAD-2009-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goebel G, Hiller W. The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO. 1994;42:166–172. [PubMed] [Google Scholar]
- 28.Tsai SJ, Cheng CY, Yu YW, Chen TJ, Hong CJ. Association study of a brain-derived neurotrophic-factor genetic polymorphism and major depressive disorders, symptomatology, and antidepressant response. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:19–22. doi: 10.1002/ajmg.b.20026. [DOI] [PubMed] [Google Scholar]
- 29.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 30.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 31.Goodyer IM, Croudace T, Dudbridge F, Ban M, Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. Br J Psychiatry. 2010;197:365–371. doi: 10.1192/bjp.bp.110.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilgramm M, Rychlick R, Lebisch H, Siedentop H, Goebel G, Kirchhoff D. Tinnitus in the Federal Republic of Germany: A representative epidemiological study. In: Hazell JWP, editor. Proceedings of the Sixth Internantional Tinnitus Seminar. London: The Tinnitus and Hyperacusis Centre; 1999. pp. 64–67. [Google Scholar]
- 33.Scott B, Lindberg P. Psychological profile and somatic complaints between help-seeking and non-help-seeking tinnitus subjects. Psychosomatics. 2000;41:347–352. doi: 10.1176/appi.psy.41.4.347. [DOI] [PubMed] [Google Scholar]
- 34.Hébert S, Canlon B, Hasson D, Magnusson Hanson LL, Westerlund H, Theorell T. Tinnitus severity is reduced with reduction of depressive mood--a prospective population study in Sweden. PLoS One. 2012;7:e37733. doi: 10.1371/journal.pone.0037733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto F, Saruta J, Kanzaki S, To M, Tsutsumi T, Tsukinoki K, Ogawa K. Various levels of plasma brain-derived neurotrophic factor in patients with tinnitus. Neurosci Lett. 2012;510:73–77. doi: 10.1016/j.neulet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Stöver T, Nam Y, Gong TL, Lomax MI, Altschuler RA. Glial cell line-derived neurotrophic factor (GDNF) and its receptor complex are expressed in the auditory nerve of the mature rat cochlea. Hear Res. 2001;155:143–151. doi: 10.1016/s0378-5955(01)00227-1. [DOI] [PubMed] [Google Scholar]
- 37.Vanneste S, Joos K, De Ridder D. Prefrontal cortex based sex differences in tinnitus perception: same tinnitus intensity, same tinnitus distress, different mood. PLoS One. 2012;7:e31182. doi: 10.1371/journal.pone.0031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinaud R, Tremere LA. Control of central auditory processing by a brain-generated oestrogen. Nat Rev Neurosci. 2012 doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- 40.McFadden D. Sex differences in the auditory system. Dev Neuropsychol. 1998;14:261–298. [Google Scholar]
- 41.Rogers DS, Harkrider AW, Burchfield SB, Nabelek AK. The influence of listener's gender on the acceptance of background noise. J Am Acad Audiol. 2003;14:372–382. [PubMed] [Google Scholar]
- 42.Sagi E, D'Alessandro LM, Norwich KH. Identification variability as a measure of loudness: an application to gender differences. Can J Exp Psychol. 2007;61:64–70. doi: 10.1037/cjep2007007. [DOI] [PubMed] [Google Scholar]
- 43.Kocak M, Ulmer JL, Biswal BB, Aralasmak A, Daniels DL, Mark LP. The influence of gender on auditory and language cortical activation patterns: preliminary data. Am J Neuroradiol. 2005;26:2248–2255. [PMC free article] [PubMed] [Google Scholar]
- 44.Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46:1349–1362. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krizman J, Skoe E, Kraus N. Sex differences in auditory subcortical function. Clin Neurophysiol. 2012;123:590–597. doi: 10.1016/j.clinph.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rademacher J, Morosan P, Schleicher A, Freund HJ, Zilles K. Human primary auditory cortex in women and men. Neuroreport. 2001;12:1561–1565. doi: 10.1097/00001756-200106130-00010. [DOI] [PubMed] [Google Scholar]
- 47.Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Effect of gender on the hearing performance of adult cochlear implant patients. Laryngoscope. 2012;122:1126–1129. doi: 10.1002/lary.23214. [DOI] [PubMed] [Google Scholar]
- 48.Dobie RA, Sakai CS, Sullivan MD, Katon WJ, Russo J. Antidepressant treatment of tinnitus patients: report of a randomized clinical trial and clinical prediction of benefit. Am J Otol. 1993;14:18–23. [PubMed] [Google Scholar]
- 49.Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 50.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang R, Huang J, Cathcart H, Smith S, Poduslo SE. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer's disease. J Med Genet. 2007;44:e66. doi: 10.1136/jmg.2006.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemos C, Mendonça D, Pereira-Monteiro J, Barros J, Sequeiros J, Alonso I, Sousa A. BDNF and CGRP interaction: implications in migraine susceptibility. Cephalalgia. 2010;30:1375–1382. doi: 10.1177/0333102410368443. [DOI] [PubMed] [Google Scholar]
- 53.Green EK, Raybould R, Macgregor S, Hyde S, Young AH, O'Donovan MC, Owen MJ, Kirov G, Jones L, Jones I, Craddock N. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry. 2006;188:21–25. doi: 10.1192/bjp.bp.105.009969. [DOI] [PubMed] [Google Scholar]
- 54.Terracciano A, Tanaka T, Sutin AR, Deiana B, Balaci L, Sanna S, Olla N, Maschio A, Uda M, Ferrucci L, Schlessinger D, Costa PT Jr. BDNF Val66Met is associated with Introversion and interacts with 5-HTTLPR to influence Neuroticism. Neuropsychopharmacology. 2010;35:1083–1089. doi: 10.1038/npp.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter NT, Montag C, Markett SA, Reuter M. Interaction effect of functional variants of the BDNF and DRD2/ANKK1 gene is associated with alexithymia in healthy human subjects. Psychosom Med. 2011;73:23–28. doi: 10.1097/PSY.0b013e31820037c1. [DOI] [PubMed] [Google Scholar]
- 56.Cathomas F, Vogler C, Euler-Sigmund JC, de Quervain DJ, Papassotiropoulos A. Fine-mapping of the brain-derived neurotrophic factor (BDNF) gene supports an association of the Val66Met polymorphism with episodic memory. Int J Neuropsychopharmacol. 2010;13:975–980. doi: 10.1017/S1461145710000519. [DOI] [PubMed] [Google Scholar]
- 57.Lavebratt C, Aberg E, Sjöholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]