Abstract
Total motile count (TMC) is a useful tool for sperm evaluation, comprising both quantitative and motility parameters. Although frequently used, TMC has not yet been evaluated as a contributory variable for intracytoplasmic sperm injection (ICSI) cycles. In this study we evaluate the possible role of TMC as a prognostic parameter in cycles designated for ICSI. We also test the existence of a possible TMC-threshold value that might be predictive for ICSI cycle outcome in the everyday practice. This is a retrospective cohort study in which the research question is addressed by a locally weighted regression (LOESS) analysis. Primary outcome measures are fertilization rate, good quality embryos rate and implantation rate. A total of 666 patients were included, contributing 1456 cycles. The effect of TMC over the fertilization rate was significant, depicting an inverted U-shaped curve: with up to approximately 10 million motile sperm, fertilization rates increased as TMC increased, but from this point on decreased. A slight increment in the rate of good embryo formation with increasing value of TMC was noted, but this did not reach a statistical significance. TMC values demonstrated no effect in the case of implantation rates. ICSI may offer an advantage related to fertilization rates for the sub-fertile male population, with a motile sperm count up to 10 million.
Keywords: Male infertility, sperm motility, ICSI, fertilization, embryo Implantation
Introduction
Total motile count (TMC) is a useful tool for sperm evaluation, composing both quantitative and motility parameters. The combination of three critical sperm parameters into a single indicator, which does not concern the more time consuming sperm morphology, turns it into a handy instrument in the practice of fertility treatments, available in the very same day of the procedure. After being established as a crucial parameter influencing the success of intrauterine insemination [1,2], TMC was also tested as a predictor for fertilization failure and for pregnancy rates during in vitro fertilization treatment, yielding conflicting results [3-5]. Total motile sperm count was also described as a general predictor of sperm quality [6].
To our best of knowledge, TMC has not been evaluated as a contributory factor in intracytoplasmic sperm injection (ICSI) cycles, once this specific procedure had been chosen; ICSI is a commonly used assisted reproduction technology [7] that was developed in order to enable the sperm to bypass the zona pellucida. This technique presents fertilization rates comparable to those observed with conventional IVF in the absence of male factors [8], although the main indication for ICSI has been male infertility [9]. Though very common, conclusive data about which semen parameters truly determine the results of ICSI are still lacking [10-12].
We performed a retrospective cohort study aiming to evaluate the possible role of TMC as a prognostic parameter in cycles designated for ICSI. We also wished to test the existence of a possible threshold value that might be predictive for the cycle outcome in the everyday practice. Such value may also serve as guidance in the discussion regarding whether or not to postpone IVF-ICSI until male related medical or surgical treatment is being accomplished. For the purpose of this analysis, we collaborated with statisticians, thus treating the research question in a novel fashion comprising input from both disciplines: medicine and mathematics.
Materials and methods
Patients
We reviewed the medical records from all IVF/ICSI cycles performed at a university-affiliated reproductive unit (Meir Medical Center, Kfar Saba, Israel), from January 1, 2000 to December 31, 2008. All data were obtained from a computerized database. Cycles in which testicular extracted sperm (TESE/TESA) was used were excluded. All cycles were initially designated to undergo ICSI. Results regarding frozen embryos were not included in implantation and pregnancy rate calculations. Primary outcome measures were fertilization rate, good quality embryo rate and implantation rate.
Ovarian stimulation protocols
During the study period, patients were treated by one of two protocols for induction of ovulation; the long protocol, "GnRH agonist" or the “GnRh antagonist protocol.” The suitable protocol for each patient was determined by the clinical judgment of the treating physician and if possible with regard to performance exhibited in previous cycles. Patients on the long protocol received Triptorelin (Ferring, Hoofddorp, the Netherlands) and those on the short antagonist protocol, Cetrotide (Serono, Israel). The medications for induction of ovulation were the recombinant FSH (Gonal F Merck Serono SA, Aubunne the Switzerland or Purigon, NV Organon Oss the Netherlands) or the purified urinary FSH+LH (Menogon Ferring SA, Sainet-Prex, Switzerland). Cumulus-oocyte complexes were recovered by transvaginal ultrasound 36 hours after injection of 10,000 IU of purified urinary hCG (Pregnyl Organon Oss) or recombinant hCG (Ovitrelle, Merck Serono SA, Bari, Italy). Embryo transfer (ET) took place on day 2 or 3 after ovum pick-up. A pregnancy test was performed 12 days after the embryo transfer. For this study, we analyzed only clinical pregnancies confirmed by the presence of intrauterine gestational sacs on transvaginal US performed 26-32 days after ET.
Laboratory procedures
The culture media used in our routine laboratory work was P1 supplemented with 10% human serum albumin (HSA) (Irvine Scientific, St. Louis, MO, USA) combined with either Cook Culture System (Cook, Brisbane, Australia) until 2006, or with SAGE (SAGE, Cooper Surgical, Inc., SAGE, Trumbull, CT, USA), supplemented with 10% synthetic serum substitute (SSS), from 2007 onwards.
Sperm evaluation criteria were consistent through the entire study period. On the day of oocyte retrieval, concentration, motility and volume of fresh sperm were evaluated and the total motile sperm count (TMC) was calculated. The samples were washed in sperm wash medium, separated on a gradient and adjusted for insemination.
The oocytes were denuded using hyaluronidase (Irvine Scientific). Metaphase II (MII) oocytes, selected by the presence of the first polar body, were considered suitable for injection. ICSI was performed according to the procedure described by Van Steirteghem et al. [13]. The injected oocytes were cultured individually in 25 μl medium drops under mineral oil at 37°C, in 5% CO2 humidified air.
Assessment of fertilization was performed 16-18 hours after injection, using a high power phase microscope. Oocytes with two visible pronuclei were cultured further to the cleavage stages, day 2 or day 3, until the embryo transfer (ET) procedure.
Embryo grading was performed on an inverted microscope as described previously [14]. In brief, the embryos were assessed at 200-400X magnification on an inverted microscope and classified by an embryologist according to the following morphological criteria: grade 4, equal sized, symmetrical blastomers; grade 3, uneven blastomers with <10% fragmentation; grade 2, 10-15% blastomeric fragmentation; and grade 1, >50% blastomeric fragmentation or pronucleated single cell embryos.
Statistical analysis
The statistical analysis was performed after receiving the approval of the local Institutional Review Board, addressed by one of the co-authors as part of a Masters Degree in Statistics. The computerized database (Microsoft Excel spreadsheet) was checked for accuracy by a second person and then transformed and processed by the statistical software R, version 2.11. A P value < 0.05 was considered statistically significant.
The three response variables were as following: (i) Good Fertilization Rate - the number of good fertilizations divided by the number of total fertilization trials (within a subject), (ii) Good Embryo Rate - the number of good quality embryos divided by the number of successful fertilizations and (iii) Implantation Rate - the number of implanted embryonal sacs divided by the number of embryos transferred.
The TMC distribution was found to be strongly skewed to the right (presenting a distribution which is not normal), and therefore was logarithmically transformed. Several potential explanatory variables to the response variables were considered before constructing the final model: female partner age, number of ampoules used for induction, number of induction days, coasting, maximal estradiol (E2) level, TMC, and number of retrieved oocytes. After testing inter-variable correlations, both number of ampoules and the number of retrieved oocytes were removed due to high correlation coefficients with the other variables and for the purpose of composing the most appropriate model.
In our statistical analysis, we fitted a locally weighted regression (LOESS) which does not use any parametric assumptions to estimate the effect of TMC over the success rates [15-20]. This model refrains from linearity assumptions that do not necessarily fit the data, and integrates observations located in the extremities. In addition, while a logistic regression model addresses the research question from a yes/no point of view, LOESS analysis enables processing the data as a rate, meaning that each success rate is analyzed according to a certain value of TMC. The disadvantage of LOESS analysis rises from its inability to provide a marginal significance level for the explanatory variables in the model.
Thus, for determining the explanatory variables that enter the three LOESS models, we fitted generalized linear models (GLM), specifically logistic regressions, for the probability of successful fertilization, good quality embryos, or implantations rates. This model assumed a linear effect in female partner age, maximal E2 level, number of induction days, and coasting and a polynomial effect of the transformed TMC level. We then computed the log likelihood difference between the three full models to models from which each of the explanatory variables was dropped, one at a time. If the log likelihood change was significant, we kept the variable in the model (Table 1). For modeling Good Fertilization Rate, we used only TMC; for modeling Good Embryos we used Number of induction days, female partner age, maximal E2 level and TMC; for modeling Implantation, we used female partner age, maximal E2 level and TMC.
Table 1.
Explanatory variables for outcome measures: related significance level (P value) according to log likelihood differences
| Model | Variable | ||
|---|---|---|---|
|
| |||
| Good fertilizations | Good quality embryos | Implantations | |
| TMC | <0.01 | <0.01 | 0.24 |
| Age | 0.69 | <0.01 | <0.01 |
| Maximal E2 level | 0.37 | <0.01 | <0.01 |
| Number of induction days | 0.55 | 0.02 | 0.07 |
| Presence of coasting | 0.15 | 0.32 | 0.07 |
The LOESS and GLM analysis results are presented in separate scatter plots. The abscissa is the TMC and the ordinates are the different success rates. The blue horizontal line stands for the mean success rate, while the LOESS estimates of the success rate and the corresponding 95% confidence intervals are drawn in red. The estimated success rate produced by the GLM is drawn in green and presented only when the effect of the TMC was significant.
Results
A total of 666 patients were included, contributing 1456 cycles, with a mean of 2.2 cycles per patient. Basic patient characteristics are presented in Table 2. Of the total cycles, 1205 (83%) ended in embryo transfer. A total of 11,602 oocytes were picked-up and 8352 (73%) were fertilized. Of the fertilizations, 7,671 (92%) were normal at the first evaluation and 3,532 (46%) resulted in a good quality embryo, graded 3-4. Of the good quality embryos, 2,517 were transferred resulting in 476 implantations (18.9% implantation rate) and 392 pregnancies (excluding extra-uterine pregnancies and chemical pregnancies). Though not included in this analysis, 1,039 of the good quality embryos were frozen.
Table 2.
Basic cycle characteristics presented by median, mean and standard deviation.
| Age (years) | Maximal E2 levels (pm/ml) | Sperm volume (ml) | Sperm count (millions) | TMCa | Picked ova (N) | Fertilizations (N) | Transferred embryos (N) | |
|---|---|---|---|---|---|---|---|---|
| Median | 30.00 | 2254.4 | 2.5 | 5 | 3.60 | 7.00 | 5.00 | 2.00 |
| Mean | 30.95 | 2440 | 3.42 | 12.68 | 15.87 | 7.97 | 5.75 | 1.94 |
| SDb | 5.54 | 1251 | 6.17 | 20.43 | 37.25 | 4.76 | 3.85 | 1.21 |
TMC is not normally distributed.
SD: Standard Deviation.
The affect of TMC over fertilization rate was significant; both the LOESS and the logistic regression depicted the same inverted U-shaped affect of TMC on this success rate (Figure 1).Up to the value of approximately 10 million motile sperm, fertilization rates increased as TMC increased. From this point though, the fertilization rates decreased although TMC increased.
Figure 1.
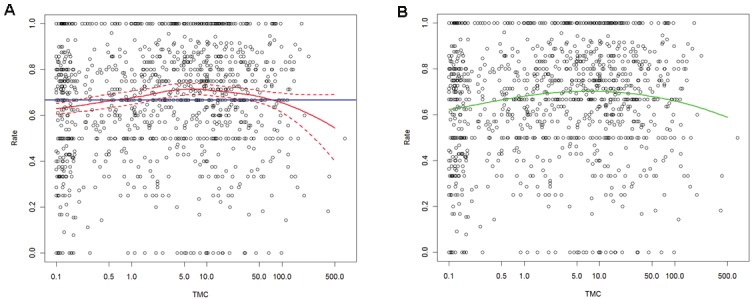
The effect of sperm total motile count (TMC) over fertilization rate in IVF-ICSI cycles. A. LOESS model. Blue horizontal line stands for the mean success rate and the LOESS estimates of the success rate and the corresponding 95% confidence intervals are drawn in red. B. generalized linear model (GLM). The estimated success rate produced by the GLM (significant).
The TMC influence on good quality embryo rate and on implantation rates (Figures 2 and 3) is presented only in the LOESS analysis, since the GLM model was not found to be significant. Although we noted a slight increase in the rate of good embryo formation with increasing TMC values, this trend did not reach statistical significance (Figure 2). Moreover, the TMC values were clearly non significant in terms of implantation rate (Figure 3).
Figure 2.
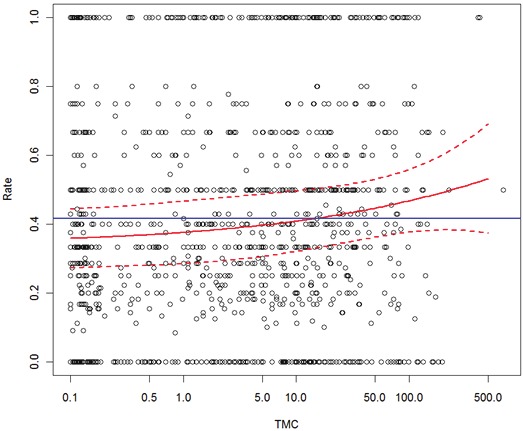
The effect of sperm total motile count (TMC) over good quality embryo rate in IVF-ICSI cycles. Blue horizontal line stands for the mean success rate and the LOESS estimates of the success rate and the corresponding 95% confidence intervals are drawn in red.
Figure 3.
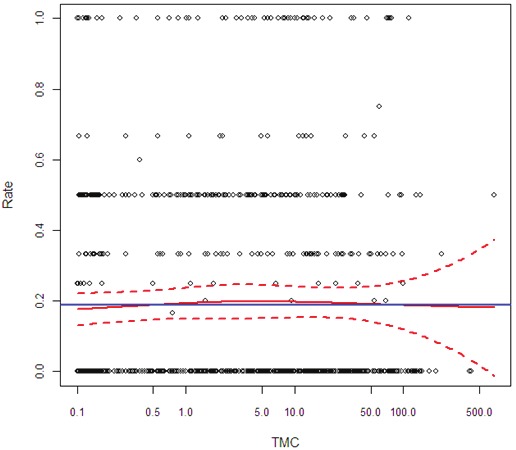
The effect of sperm total motile count (TMC) on implantation rate inIVF-ICSI cycles. Blue horizontal line stands for the mean success rate. TheLOESS estimates of the success rate and the corresponding 95% confidenceintervals are drawn in red.
Discussion
Assessing the contribution of a certain variable can have a great value in the success of a general treatment plan. Previous literature has focused mainly on ICSI results regarding sperm morphology; severe teratozoospermia treated with ICSI, as compared to standard IVF, was previously reported [21-23] to yield better fertilization rates. Though previously doubted in the literature concerning IVF cycles [11], we decided to conduct a study testing the effect of TMC on ICSI outcomes, thus combining sperm's motility, count and volume un a single handy parameter. We hypothesized that although intracytoplasmic injection bypasses the zona pellucida, the basic sperm characteristics, as reflected by the TMC, might still influence the final outcome.
Sperm motility was tested before as an independent factor for ICSI results in a statistical model [24] and reported to be directly correlated to the 2PN rate. Profound cytoskeletal deformities serve as a hypothetical factor that could potentially explain such a correlation [25], while others suggested that the presence of aneuploidies could also be associated with reduced sperm motility and therefore with a compromised result [26]. A very low sperm count (<1 x 104) was also described as a negative predictor for ICSI outcome [27]. In fact, when sperm's count was confronted with its motility, the latter was the one to be most related to a good result in the ICSI cycle [28].
According to our cohort, there does not seem to be a substantial correlation between TMC and ICSI outcome, though TMC was demonstrated as having a significant influence over normal fertilization rates. The approximate value of 10 million TMC served as a threshold for fertilization success in our cohort. As mentioned before, up to this value a positive relation exists between TMC and fertilization rate, while for TMC greater than 10 million, fertilization rates decreased. Stating it differently, this might serve as a guiding tool for determining the advantage of using ICSI procedure and may possibly reflect the existence of sub-populations in our study group. We did not track a different discriminatory value even though the TMC range included counts as low as a few motile spermatozoa.
Our ICSI population was composed mainly from male patients diagnosed with sub-fertility, presenting a rather heterogeneous sperm count. There were 461 observations of TMC above 10 million in our cohort. In cases of unexplained infertility, ICSI was reported to yield significantly higher fertilization rates vs. conventional insemination, but significantly lower implantation rates (17.8% vs. 24.9%) and lower pregnancy rates than with ICSI (33.6% vs. 52.7%) [29]. In another study, concerned with mild male-factor subfertility (defined by the presence of sperm concentrations <20 × 106 per milliliter and/or <40% motility according to the World Health Organization), fertilization failures were less likely to happen after ICSI, though the good quality embryo rate and implantation rate did not differ [30]. Others have found no advantage in any laboratory or clinical field test, meaning there was no statistically significant difference in fertilization rate, implantation rate, clinical pregnancy, live-birth rate, or embryo quality between IVF and IVF-ICSI in cases of unexplained infertility [31]. The literature also presents supportive evidence for worse results when ICSI, rather than IVF, is used for non-male factor infertility [32]. In our cohort, fertilization rates declined in the range of TMC >10 million, but implantation rates did not, consistent with these previous reports.
The lack of correlation between TMC and either good embryo rate or implantation rate may reflect the dominance of the technique over sperm quality even in its worst performance. Though sperm count and sperm motility were separately evaluated and reported to correlate with ICSI outcome, as described above, the reports are scarce. In our model, the combination of the two was tested, which might explain the difference. As for the higher TMC range, which probably reflects indications other than male factor, in these cases, ICSI results are similar to those with very low counts. Recalling the reports demonstrating no advantage of ICSI vs. standard IVF for unexplained infertility, this lack of advantage becomes clearer.
Though our analysis did not control for the protocol type or for female diagnosis, we assume that they were equally distributed across the different sperm categories. We found that the strength of this analysis rose from regarding TMC as a continuum, thus refraining from composing any subgroups for the purpose of both logistic and LOESS regression models. We also believe that the special regression model chosen (i.e. LOESS) provides a visual tool fitting remarkably well for the biological behavior of TMC, our main point of interest.
To conclude: ICSI may offer an advantage related to fertilization rates for the sub-fertile male population, with a motile sperm count up to 10 million. In light of our current findings regarding good embryo rate and implantation rate, we recommend a careful consideration of postponing IVF-ICSI for the sake of male directed medical or surgical procedures; presented data do not support a better outcome to be expected due to rising TMC values in ICSI cycles.
References
- 1.Nyboe Andersen A, Carlsen E, Loft A. Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update. 2008;14:593–604. doi: 10.1093/humupd/dmn032. [DOI] [PubMed] [Google Scholar]
- 2.Kim HH, Bundorf MK, Behr B, McCallum SW. Use and outcomes of intracytoplasmic sperm injection for non-male factor infertility. Fertil Steril. 2007;88:622–628. doi: 10.1016/j.fertnstert.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 3.van Rumste MM, Evers JL, Farquhar CM. Intracytoplasmic sperm injection versus partial zona dissection, subzonal insemination and conventional techniques for oocyte insemination during in vitro fertilisation. Cochrane Database Syst Rev. 2000;CD001301 doi: 10.1002/14651858.CD001301. [DOI] [PubMed] [Google Scholar]
- 4.Devroey P, Van Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- 5.ESHRE Capri Workshop Group. Intracytoplasmic sperm injection (ICSI) in 2006: evi dence and evolution. Hum Reprod Update. 2007;13:515–526. doi: 10.1093/humupd/dmm024. [DOI] [PubMed] [Google Scholar]
- 6.van der Westerlaken L, Naaktgeboren N, Verburg H, Dieben S, Helmerhorst FM. Conventional in vitro fertilization versus intracytoplasmic sperm injection in patients with borderline semen: a randomized study using sibling oocytes. Fertil Steril. 2006;85:395–400. doi: 10.1016/j.fertnstert.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 7.van Weert JM, Repping S, Van Voorhis BJ, van der Veen F, Bossuyt PM, Mol BW. Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta-analysis. Fertil Steril. 2004;82:612–620. doi: 10.1016/j.fertnstert.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Van Voorhis BJ, Barnett M, Sparks AE, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001;75:661–668. doi: 10.1016/s0015-0282(00)01783-0. [DOI] [PubMed] [Google Scholar]
- 9.Repping S, van Weert JM, Mol BW, de Vries JW, van der Veen F. Use of the total motile sperm count to predict total fertilization failure in in vitro fertilization. Fertil Steril. 2002;78:22–29. doi: 10.1016/s0015-0282(02)03178-3. [DOI] [PubMed] [Google Scholar]
- 10.Rhemrev JP, Lens JW, McDonnell J, Schoemaker J, Vermeiden JP. The postwash total progressively motile sperm cell count is a reliable predictor of total fertilization failure during in vitro fertilization treatment. Fertil Steril. 2001;76:884–891. doi: 10.1016/s0015-0282(01)02826-6. [DOI] [PubMed] [Google Scholar]
- 11.van Weert JM, Repping S, van der Steeg JW, Steures P, van der Veen F, Mol BW. A prediction model for ongoing pregnancy after in vitro fertilization in couples with male subfertility. J Reprod Med. 2008;53:250–256. [PubMed] [Google Scholar]
- 12.Schulte RT, Keller LM, Hiner MR, Ohl DA, Smith GD. Temporal decreases in sperm motility: which patients should have motility checked at both 1 and 2 hours after collection? J Androl. 2008;29:558–563. doi: 10.2164/jandrol.107.004002. [DOI] [PubMed] [Google Scholar]
- 13.Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H, Derde MP, Van Assche E, Devroey P. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod. 1993;8:1055–1060. doi: 10.1093/oxfordjournals.humrep.a138191. [DOI] [PubMed] [Google Scholar]
- 14.Shulman A, Ben-Nun I, Ghetler Y, Kaneti H, Shilon M, Beyth Y. Relationship between embryo morphology and implantation rate after in vitro fertilization treatment in conception cycles. Fertil Steril. 1993;60:123–126. doi: 10.1016/s0015-0282(16)56048-8. [DOI] [PubMed] [Google Scholar]
- 15.Beck N, Jackman S. Beyond linearity by default: generalized additive models. Am J Polit Sci. 1998;42:596–627. [Google Scholar]
- 16.Becker R, Cleveland WS, Schyu M. The visual design and control of trellis displays. J Comput Graph Stat. 1996;5:123–155. [Google Scholar]
- 17.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 18.Cleveland WS, McGill R. Graphical perception: theory, experimentation, and application to the development of graphical methods. J Am Stat Assoc. 1984;79:531–553. [Google Scholar]
- 19.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 20.Cleveland WS, Devlin SJ, Grosse E. Regression by local fitting: methods, properties, and computational algorithms. J Econometrics. 1988;37:87–114. [Google Scholar]
- 21.Pisarska MD, Casson PR, Cisneros PL, Lamb DJ, Lipshultz LI, Buster JE, Carson SA. Fertilization after standard in vitro fertilization versus intracytoplasmic sperm injection in subfertile males using sibling oocytes. Fertil Steril. 1999;71:627–632. doi: 10.1016/s0015-0282(98)00538-x. [DOI] [PubMed] [Google Scholar]
- 22.Kihaile PE, Misumi J, Hirotsuru K, Kumasako Y, Kisanga RE, Utsunomiya T. Comparison of sibling oocyte outcomes after intracytoplasmic sperm injection and in vitro fertilization in severe teratozoospermic patients in the first cycle. Int J Androl. 2003;26:57–62. doi: 10.1046/j.1365-2605.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie LJ, Kovanci E, Amato P, Cisneros P, Lamb D, Carson SA. Pregnancy outcome of in vitro fertilization/intracytoplasmic sperm injection with profound teratospermia. Fertil Steril. 2004;82:847–849. doi: 10.1016/j.fertnstert.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 24.Shen S, Khabani A, Klein N, Battaglia D. Statistical analysis of factors affecting fertilization rates and clinical outcome associated with intracytoplasmic sperm injection. Fertil Steril. 2003;79:355–360. doi: 10.1016/s0015-0282(02)04675-7. [DOI] [PubMed] [Google Scholar]
- 25.Chatzimeletiou K, Rutherford AJ, Griffin DK, Handyside AH. Is the sperm centrosome to blame for the complex polyploid chromosome patterns observed in cleavage stage embryos from an OAT patient? Zygote. 2007;15:81–90. doi: 10.1017/S0967199406004059. [DOI] [PubMed] [Google Scholar]
- 26.Collodel G, Capitani S, Baccetti B, Pammolli A, Moretti E. Sperm aneuploidies and low progressive motility. Hum Reprod. 2007;22:1893–1898. doi: 10.1093/humrep/dem099. [DOI] [PubMed] [Google Scholar]
- 27.Strassburger D, Friedler S, Raziel A, Schachter M, Kasterstein E, Ron-el R. Very low sperm count affects the result of intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:431–436. doi: 10.1023/A:1009413201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy ZP, Verheyen G, Tournaye H, Van Steirteghem AC. Special applications of intracytoplasmic sperm injection: the influence of sperm count, motility, morphology, source and sperm antibody on the outcome of ICSI. Hum Reprod. 1998;13:143–154. doi: 10.1093/humrep/13.suppl_1.143. [DOI] [PubMed] [Google Scholar]
- 29.Check JH, Bollendorf A, Summers-Chase D, Horwath D, Hourani W. Conventional oocyte insemination may result in a better pregnancy outcome than intracytoplasmic sperm injection (ICSI) for unexplained infertility. Clin Exp Obstet Gynecol. 2009;36:150–151. [PubMed] [Google Scholar]
- 30.van der Westerlaken L, Naaktgeboren N, Verburg H, Dieben S, Helmerhorst FM. Conventional in vitro fertilization versus intracytoplas mic sperm injection in patients with borderline semen: a randomized study using sibling oocytes. Fertil Steril. 2006;85:395–400. doi: 10.1016/j.fertnstert.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 31.Foong SC, Fleetham JA, O'Keane JA, Scott SG, Tough SC, Greene CA. A prospective randomized trial of conventional in vitro fertilization versus intracytoplasmic sperm injection in unexplained infertility. J Assist Reprod Genet. 2006;23:137–140. doi: 10.1007/s10815-005-9008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, Braude P, Kennedy R, Rutherford A, Hartshorne G, Templeton A. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]


